Abstract
Abstract 2162
Poster Board II-139
Cytoplasmic Bcr-Abl kinase evokes a diverse array of transforming signals and is responsible for development of Philadelphia chromosome-positive (Ph+) leukemias. In this fusion protein, disruption or deletion of a N-terminal coiled-coil (CC) domain of Bcr results in substantially decreased or absent tyrosine kinase activity and defective cellular transformation, indicating the crucial role of Abl oligomerization in constitutive activation of its intrinsic kinase activity. Fusion of the estrogen receptor (ER) ligand binding domain (LBD) to the C-terminus of Abl generates a ligand-activatable and transformation-competent tyrosine kinase dimer. However, additional sequences in Bcr may also be required for cellular transformation since Bcr-Abl mutants containing just the extreme CC domain cannot transform fibroblasts.
To revisit the mechanism of Bcr-Abl-induced leukemogenesis and especially to delineate early events upon Bcr-Abl activation, we applied this fusion technology to construct DCCp190ER, a p190Bcr-Abl mutant including ER-LBD at the C-terminus but not CC domain at the N-terminus. Cytokine-dependent murine Ba/F3 and human TF-1 cells were retrovirally transduced with DCCp190ER as well as wild-type p190 (wt), DCCp190 and vector control, respectively. Such a series of transformants were subjected to not only biological assays but also biochemical analysis such as anti- phosphotyrosine immunoblot and NF-kB assay.
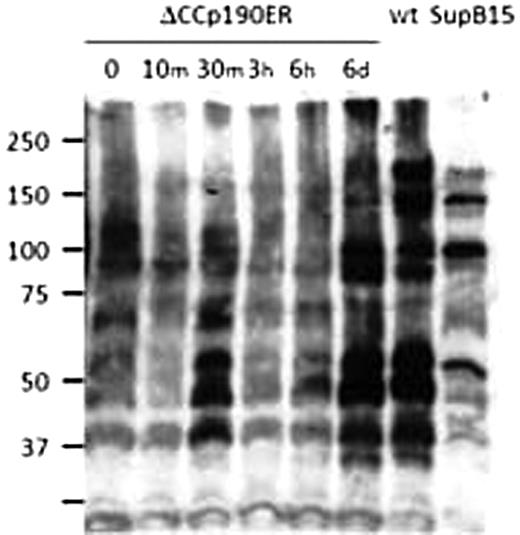
DCCp190ER, but not DCCp190, can support cytokine-independent growth of both Ba/F3 and TF-1 cells in a dose-dependent manner on 4-HT, a synthetic ER-ligand, although excess of 4-HT is rather toxic to these cell lines. Optimal concentration of 4-HT has been determined as 0.5mM. The growth stimulatory effect of 4-HT on DCCp190ER -transduced cells was efficiently canceled by imatinib. Expectedly, upon 4-HT binding, a number of phosphotyrosil proteins could be detected using 4G10 MAb. Protein tyrosine phosphorylation was induced within 10 min after ligand stimulation and a part of proteins revealed a biphasic phosphorylation pattern during the time course. The overall protein profiles of DCCp190ER-induced tyrosine phosphorylation including CrkL and Stat5 resembled p190wt- induced those, but a significant time-interval beyond a day was required for the former to be comparable to the latter in signal intensity (Figure 1). Curiously this holds true for autophosphorylated DCCp190ER.
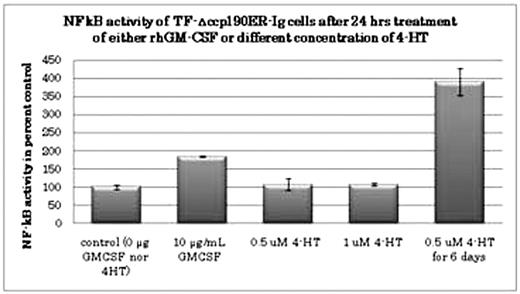
It is evidenced that Bcr-Abl constitutively activates NF-kB signaling pathway via unknown mechanism. TF-1/DCCp190ER cells were lentivirally transduced with NF-kB/luciferase (kB/Luc) reporter construct and were subjected to monitoring Luc activity upon ligand stimulation. Unstimulated TF-1/DCCp190ER-kB/Luc cells revealed a weak but significant Luc activity over the background. GM-CSF, but not 4-HT, moderately enhanced their Luc activities after 24 hrs of stimulation. On the contrary, 4-fold up-regulation of Luc activity was observed after 6 days culture with 4-HT (Figure 2), suggesting the delayed cellular transformation by DCCp190ER, which is compatible with delayed enhancement of tyrosine phosphorylation. Finally, we investigated gene expression profiling of TF-1 cells induced by DCCp190ER upon 4-HT binding. As a result, expression of a number of genes including TRAIL, GATA2 and endoglin were altered in response to Bcr-Abl activation by treatment with 4-HT for 4 days. The present results suggested that Bcr-Abl kinase may evoke early transforming signals in a unique time-dependent manner, leading to established transformed phenotype.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.