Abstract
Abstract 2467
Poster Board II-444
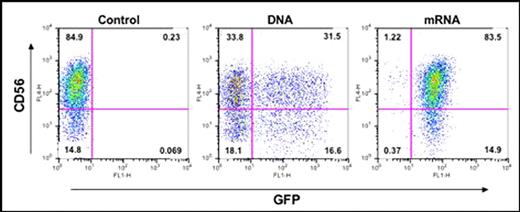
Natural killer (NK) cells play an important role in immune surveillance against a variety of infectious microorganisms and tumors. The main restrictions to developing NK cells for immunotherapy are the limited quantity of cells available for adoptive transfer and their relative resistance to gene transfer by any method. We have developed an efficient method to expand CD3-CD56+ primary NK cells in vitro using K562 artificial APCs expressing membrane-bound IL21. Here we have investigated the potential of these expanded human NK cells to be gene modified through electroporation of DNA and mRNA. Expanded NK cells were electroporated (Amaxa Nucleofector device, program X-01) with DNA or mRNA coding for the GFP reporter gene, and expression of the transgene was evaluated by flow cytometry. Analysis at 48 hours post electroporation revealed that the viability of NK cells electroporated with GFP mRNA was 78% and those electroporated with GFP DNA was 69%. When electroporated with DNA, 32% of the viable NK cells were positive for GFP but had heterogeneous expression level, whereas 98% of viable cells were positive for GFP following mRNA electroporation, with much more homogeneous GFP expression (Figure).
Based on this success we further investigated the potential of expanded NK cells to be gene modified with a Sleeping Beauty transposon/transposase vector system carrying the transgene for a second-generation Chimeric Antigen Receptor (CAR) against the GD2 ganglioside antigen, with signaling via the CD28 and CD3z endodomains. The GD2 antigen is abundantly expressed in neuroblastoma and melanoma and is therefore a relevant target for adoptive immunotherapy. Electroporation of expanded NK cells with the GD2-CAR transposon alone yielded 25% electroporation efficiency, with a viability of 55%. Electroporation of expanded NK cells with the GD2-CAR transposon and the transposase plasmid decreased the transfection efficiency to 14%. Nonetheless, expanded NK cells modified with the GD2-CAR showed improved killing of the target cell CHP-134 using the calcein AM release assay, as compared to unelectroporated expanded NK cells from the same donor. While freshly isolated human NK cells are highly resistant to gene modification, in this study we show that expanded human NK cells can be efficiently electroporated with both DNA and mRNA. NK cells modified with DNA to express CAR gain improved cytotoxic function against target cells, but viability and gene transfer efficiency are low. Since electroporation of GFP mRNA resulted in increased transduction efficiency and viability, we are now evaluating electroporation of expanded NK cells with GD2-CAR mRNA.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.