Abstract
Abstract 2947
Poster Board II-923
DLBCL occasionally presents in leukemic phase, and the prognostic significance of circulating lymphoma cells is unknown. We herein report characteristics and outcomes of newly diagnosed DLBCL presenting in leukemic phase at 2 Institutions.
Flow cytometry database analysis and retrospective chart reviews were carried out with IRB-approval for cases accrued between 2001 and 2008. Leukemic phase DLBCL patients were matched on a 3:1 basis with control DLBCL with no circulating lymphoma cells based on IPI, year of diagnosis, and age ± 10 years.
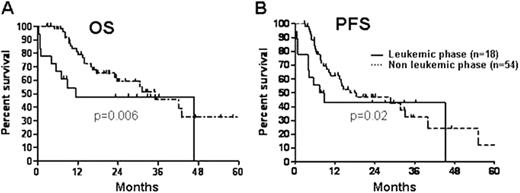
18 patients, median age 48 years (range 34-80), ECOG PS-1 (22%), 2(38%) and 3(40%), and IPI - 3(56%), 4(40%) and 5(4%) presented in leukemic phase. Extranodal sites included bone marrow (100%), spleen (83%), pleura (61%) and CSF (22%). 61% had B symptoms, and LDH was 6xULN (range, 1-56). WBC was 13,000/microL (range, 7,100-127,400), with 50% lymphoma cells (range, 2-92); these cells were immunophenotypically similar to those in the histologically confirmed DLBCL node, and co-expressed CD19, CD20, CD22, CD38, CD45, HLA-DR and FMC7 in >90% of cases, and kappa or lambda light chain restriction in > 50%. Karyotype was abnormal and complex in 61%. One patient expired before treatment began. Treatment consisted of R-CHOP (10), R-HCVAD (6), and single agent rituximab (1). 8 (44%) achieved CR (5 R-HCVAD and 3 R-CHOP), 5 (28%) PR, and 4 (22%) had resistant disease. 1 patient was autografted in CR1 and remains in remission. With a median follow-up of 32 months, 2 relapsed in leukemic phase, 1 of whom achieved CR2, but relapsed at the time of conditioning for a consolidative allograft. 10 (56%) patients died from progressive disease, 2 (11%) were lost to follow-up and 6 (33%) remain alive in remission. Overall (Panel A) and progression-free (Panel B) survival curves the 18 leukemic (solid line) and 54 non-leukemic phase (dashed line) DLBCL are depicted in the Figure.
DLBCL presenting with circulating lymphoma cells is associated with chemo-resistance (44% CR) and poor outcomes with the exception of those who achieve complete remission. These patients are candidates for alternative therapies.
Probability of overall (Panel A) and progression-free (Panel B) survival
Kaufman:Millenium: Consultancy; Genzyme: Consultancy; Celgene: Consultancy, Research Funding; Merck: Research Funding. Lonial:Millennium: Consultancy, Research Funding; Celgene: Consultancy; BMS: Consultancy; Novartis: Consultancy; Gloucester: Research Funding. Armitage:Eisa: Consultancy; Allo: Consultancy; Ziopharm: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.