Abstract
Abstract 3349
Poster Board III-237
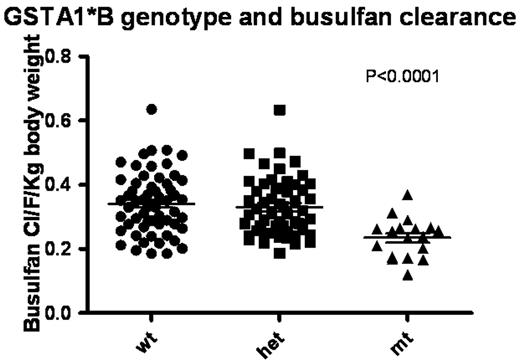
Busulfan in combination with cyclophosphamide is the commonly used conditioning regimen for hematopoietic stem cell transplantation (HSCT). We have studied the pharmacokinetics (PK) of oral busulfan in children with beta thalassaemia major undergoing BMT and observed up to 12 fold variation in PK. Since PK parameters of Bu have been shown to be influencing BMT outcome and regimen related toxicity, we attempted to understand factors influencing Bu PK using a population PK based model. The aim of this study was to explore the effect of several demographic, biological, and pharmacogenetic covariates on the disposition of busulfan in children with beta thalassaemia major undergoing hematopoietic stem cell transplant. One hundred and fifty children with beta thalassaemia major received 16 mg/kg/day of busulfan as part of the pre-BMT conditioning regimen. Plasma busulfan concentrations were measured after first and 13th doses using HPLC based method with UV detection. Non-linear mixed effects modeling analysis was performed with Monolix (version 2.4, www.monolix.org) to evaluate the effect of age, body weight, sex, Lucarelli class, serum ferritin levels, pre-transplant liver function and ten polymorphisms corresponding to GSTA1, GSTM1, GSTP1, GSTT1, CYP2B6, CYP2C9, CYP2C19, CYP3A4 (pharmacogenetic data was available for 133 of 150 patients). A one-compartment pharmacokinetic model with first-order oral absorption was used to describe the data. The pharmacokinetic parameters estimated included apparent clearance and volume (CL/F (L/hr or L/hr/kg), V/F (L or L/kg)), and the absorption parameter (ka (1/hr)). The bioavailability, F, was not identifiable since only oral drug was used. The distribution of the parameters was assumed log-normal. The main covariate which explained the largest portion of the inter-individual variation in busulfan kinetic parameters (45%, 22%, and 15% of clearance (CL), volume of distribution (V), and absorption rate constant (ka), respectively) was body weight. The next most significant covariate was GSTA1 promoter polymorphism. In particular, clearance decreased 124% and 148% and the oral absorption increased 16% and 15% in GSTA1 heterozygous and homozygous patients, respectively, compared to wild type. GSTA1 explained an additional 17% and 1% of inter-individual variation in the CL and ka, respectively, compared to the weight normalized model. By combining these two covariates, the interindividual variability on busulfan CL/F decreased from 45% to17%. We have developed a population pharmacokinetic model of oral busulfan in children with beta thalassaemia major undergoing BMT which explains the inter-individual variation in Bu PK, considering demographic and biological covariates.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.