Abstract
Abstract 3789
Poster Board III-725
Imatinib mesylate is effective therapy against Philadelphia chromosome-positive leukemia, but the resistance develops in all phases of the disease. The identification of new proteins induced by imatinib may lead to find the novel potent molecular targets in imatinib-resistant CML.
K562 cells were treated with or without 1 mM imatinib for 24 hours, and then differential display between them was performed. TRIM68 expression was examined by RT-PCR, and in vivo ubiquitination or sumoylation assay was performed by transfection experiment and Western blot analysis. The substrates for TRIM68 were analyzed by mass spectorometry.
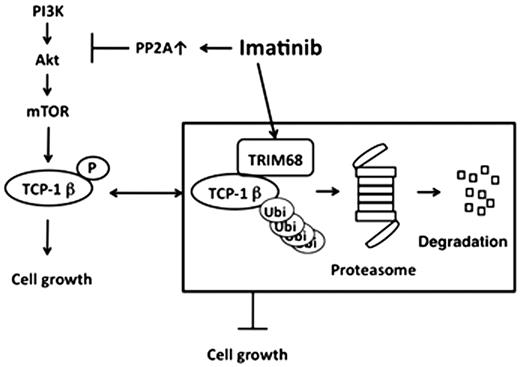
As the results of RNA differential display, we found that the expression of TRIM68 mRNA was increased when the K562 cells were treated with 1 mM imatinib for 24 hours. TRIM68 protein possesses a RING finger domain at its N-terminal site. Since many RING-finger proteins have been identified as E3 ligases for ubiquitination or sumoylation (Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of esingle protein RING finger' E3 ubiquitin ligases. Bioessays 2005; 27: 1147-57.), we examined whether TRIM68 functions as an E3 ligase for ubiquitination or sumoylation. To examine the function of TRIM68 as an E3 ligase, wild type TRIM68 and a RING domain deletion mutant of TRIM68 (TRIM68/¢R) genes were constructed into a mammalian expression vector and they were transfected into MCF7 cells. TRIM68 had auto-ubiquitination activity but not auto-sumoylation activity on the in vivo assays, suggesting that TRIM68 can be an ubiquitin E3 ligase but not sumo ligase. Moreover, wild type TRIM68 promoted the whole ubiqutination in the cells, whereas TRIM68/¢R prevented the ubiquitination inside of the cells. To identify the TRIM68-interacting proteins, we transfected FLAG-tagged wild type TRIM68 gene or B30.2/SPRY domain of TRIM68 gene into MCF7 cells, and immunoprecipitation with FLAG-M2 agarose was performed and mass spectrometric analysis was performed. As the results, we revealed that the members of molecular chaperone T-complex polypeptide 1 (TCP-1) complex, TCP-1 b and heat shock protein 70 (HSP70) interacted with TRIM68 at the B30.2/SPRY domain. Then, we examined whether TCP-1 b is one of the substrates for TRIM68-related ubiqutination. TCP-1 b was ubiquitinated by wild type TRIM68, but not by TRIM68/¢R. Furthermore, the ubiquitination of TCP-1 b was accumulated by the treatment with a proteasome inhibitor MG132. These suggested that TCP-1 b is one of the substrates for TRIM68.
We found that TRIM68 is induced by the treatment with imatinib and functions as an ubiquitin E3 ligase. Furthermore, we identified that TCP-1 b is a substrate of TRIM68. TRIM68 may inhibit the function of TCP-1 b as a chaperone by ubiquitination and proteasome-mediated degradation. TRIM68 is possible for a new target in the imatinib-resistant CML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.