Abstract
Abstract 3806
Poster Board III-742
Although iron overload in patients with myelodysplastic syndromes (MDS) is a negative prognostic factor for survival, many chronically transfused patients are not regularly evaluated for iron overload and remain unchelated. The once-daily oral iron chelator deferasirox (Exjade®) maintains or reduces body iron in patients with MDS, however, it has not previously been assessed based on chelation history. This analysis therefore evaluates the efficacy and safety of deferasirox in a large population of chelation-naïve and previously chelated patients with MDS.
This analysis is based on data from iron overloaded MDS patients aged ≥2 years who were enrolled in the 1-year multicenter EPIC study; patients with a life expectancy <1 year were excluded. Patients initially received deferasirox 10–30 mg/kg/day, depending on the frequency of blood transfusions; 5–10 mg/kg/day dose adjustments (range 0–40 mg/kg/day) were made based on 3-month serum ferritin trends and safety markers. Efficacy was assessed by monthly measurement of serum ferritin. Safety assessments included adverse event (AE) and laboratory parameter monitoring.
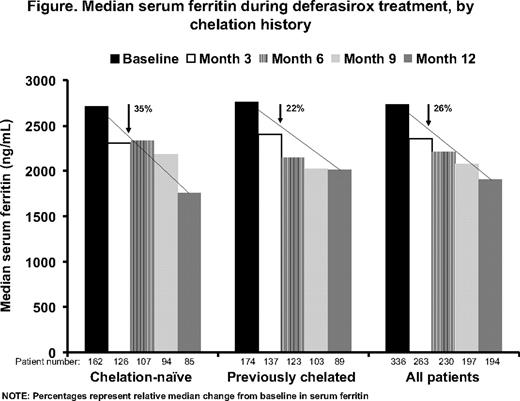
In total, 341 MDS patients were enrolled; 165 (48.4%) were chelation-naïve, 176 (51.6%) previously chelated with deferoxamine and/or deferiprone. Overall, 326 patients (95.6%) were aged ≥50 years. Baseline demographics, including median serum ferritin levels, were comparable between the two cohorts. Median serum ferritin decreased significantly in the overall population from baseline to month 12 (P=0.002; Figure). Serum ferritin levels also decreased in chelation-naïve (P=0.004) and in previously chelated (P=0.19) patients; median change was not significantly different between the two cohorts (P=0.107). Chelation-naïve and previously chelated patients who had a relatively low mean iron intake and baseline iron burden achieved a median reduction in serum ferritin with an average actual dose of <20 mg/kg/day. Patients in both cohorts who received a mean actual dose of ≥20–<30 mg/kg/day had higher baseline serum ferritin and iron intake, therefore required these higher doses to achieve a similar decrease in serum ferritin. Patients who had the highest baseline serum ferritin and iron intake achieved a substantial serum ferritin reduction with an average actual dose of ≥30 mg/kg/day. In total, 78 (47.3%) chelation-naïve and 88 (50.0%) previously chelated patients discontinued, most commonly due to AEs (n=78, 23%; 44 [13%] for drug-related AEs). Most (95%) AEs were mild-to-moderate in nature. The most common drug-related AEs were diarrhea (33%), nausea (13%), vomiting (8%) and abdominal pain (8%); there was no substantial difference in AE profile between the two cohorts. Overall, 48 chelation-naïve (29.1%) and 37 previously chelated (21.0%) patients had two consecutive serum creatinine increases >33% above baseline and >ULN; there were no progressive increases. One chelation-naïve patient (0.3%) who had normal baseline alanine aminotransferase levels had an increase >10 × ULN on two consecutive visits.
Although baseline serum ferritin levels were >2500 ng/mL, almost 50% of patients were chelation-naive, suggesting that the importance to treat iron overload continues to be underestimated in the MDS population. For previously chelated patients these data suggest that iron burden was not adequately managed with previous regimens. Irrespective of chelation history, with appropriate dosing based on transfusion requirements, serum ferritin levels and safety markers, deferasirox effectively decreased serum ferritin to <2500 ng/mL and had a manageable safety profile in MDS patients. These data highlight the need to monitor iron load in MDS and initiate effective chelation therapy to reduce body iron burden.
Habr:Novartis Pharmaceuticals: Employment. Domokos:Novartis Pharma AG: Employment. Roubert:Novartis Pharma AG: Employment. Rose:Novartis: Research Funding. Gattermann:Novartis: Honoraria, Participation in Advisory Boards on deferasirox clinical trials.
Author notes
Asterisk with author names denotes non-ASH members.