Abstract
Abstract 3903
Poster Board III-839
A peripheral blood (PB) absolute eosinophil count (AEC) of ≥ 1.5 × 109/L is operationally referred to as “hypereosinophilia”. In general, one can classify hypereosinophilia into 3 major categories: i) secondary, ii) clonal, and iii) idiopathic. The 2008 WHO proposal provides a modern-era framework for the classification of clonal eosinophilia and subcategories include (i) myeloid/lymphoid neoplasms associated with eosinophilia and PDGFRA-, PDGFRB-, or FGFR1-rearrangement; (ii) clonal eosinophilia associated with an otherwise WHO-defined myeloid malignancy such as systemic mastocytosis (MN-eo), and (iii) chronic eosinophilic leukemia, otherwise not specified (CEL-NOS). Idiopathic hypereosinophilia (IH) with organ damage and without clonal/abnormal lymphocytes is referred to as hypereosinophilic syndrome (HES). In the current study, we define the spectrum of disease phenotypes and survival in 357 consecutive patients with hypereosinophilia seen at a single institution.
Mayo Clinic patient records from January 1994 to October 2008 were reviewed, and those with persistent eosinophilia ≥1.5 × 109/L identified. Clinical data and bone marrow (BM) histology were reviewed, and the eosinophilic disorder classified as above. The aims of the current study were to: (i) study the spectrum of eosinophilic disorders within the context of the WHO proposal; (ii) describe the clinical, laboratory, and molecular/cytogenetic characteristics of patients with primary eosinophilia (i.e. clonal and idiopathic); and (iii) assess the prognostic relevance of the WHO proposal from the standpoint of life expectancy and risk of leukemic transformation.
(i) Disease spectrum and clinical characteristics: A total of 357 cases (median age=52 years) were identified: (i) secondary eosinophilia 165 (46%; male ∼52%) (autoimmune 33%, non-myeloid malignancy 19%, drugs 12%, allergies 10%, parasitosis 2%, others 24%); (ii) PDGFRA- or PDGFRB-rearranged myeloid neoplasm 17 (5%; male 100%); (iii) CEL-NOS 6 (2%; male 67%); (iv) MN/eo 36 (10%; male 69%) (systemic mastocytosis 50%, chronic myeloproliferative neoplasm 33%, AML 6%, others 11%); (v) IH 115 (32%; male 58%); and (vi) eosinophilia associated with clonal/abnormal lymphocytes 14 (4%; male 57%). Of the 115 patients with IH, 72 (63%) had evidence of end-organ damage, and could be classified as having HES: 36 patients had 1 organ involved, 21 had 2, and 15 had 3-4. In HES patients, the major organs involved were: lung 23%, skin/mucosa 22%, cardiac 14%, nervous system 10%, and thrombosis 9%.
(ii) Molecular and cytogenetic abnormalities: Complete karyotype information was available for 144 (77%) patients; 21 (15%) patients had an abnormal karyotype; MN/eo 12 (35%), PDGFRA- or PDGFRB-rearranged myeloid neoplasm 4 (31%), CEL-NOS 5 (83%), IH and eosinophilia associated with clonal/abnormal lymphocytes 0 (p<0.01). Of the 126 (67%) patients screened for FIP1L1-PDGFRA, 16 (13%) tested positive. Two patients (1%) harbored t(5;12). Of the 108 (57%) patients screened for presence of an occult T-cell clone (PCR for TCR gene rearrangement), 14 (13%) tested positive.
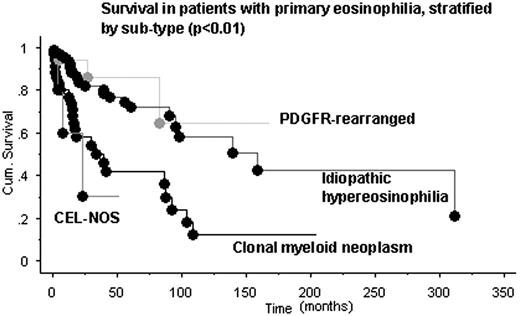
(iii) Survival and risk of disease transformation: At a median follow of 21 months (range <1-174), 56 (30%) deaths were recorded; MN/eo 23 (64%), IH 25 (22%), PDGFRA- or PDGFRB-rearranged myeloid neoplasm 3 (18%), CEL-NOS 3 (50%), and T-cell/eo 2 (14%). The combined median survival was 95 months: MN/eo 34 months, CEL-NOS 13 months, IH 151 months, PDGFRA- or PDGFRB-rearranged myeloid neoplasm and eosinophilia associated with an abnormal lymphocyte clone (not reached) (p<0.01; Figure). Transformation to acute leukemia was seen in 4 patients (2 with PDGFRA- or PDGFRB-rearranged myeloid neoplasm, and 1 each with CEL-NOS and IH) (p<0.01).
In a major tertiary center in the United States, the two major causes of hypereosinophilia were secondary (46%) and idiopathic (32%). Regarding the former, autoimmune diseases and parasitic infections were the most and least frequent causes, respectively. Cases with clonal eosinophilia were predominantly male while the sex ratio was more balanced in the other subcategories. Among primary hypereosinophilia, survival was the worst in clonal eosinophilia not associated with the imatinib-sensitive PDGFR mutations. Additional data regarding clinical characteristics, laboratory findings, and treatment outcome of idiopathic hypereosinophilia patients will be presented at the meeting.
Pardanani:TargeGen: Research Funding; Cytopia: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.