Abstract
Abstract 4124
We reported our experience on the antifungal prophylaxis by posaconazole in patients with acute myeloblastic leukaemia (AML) who were exposed to induction chemotherapy. To validate the benefit of an antifungal prophylaxis in this kind of population, we conducted a prospective study giving posaconazole (200mg per os x 3/24h) to all AML patients hospitalized for induction in the period 2007-2008 (group I) and to compare the observed results (incidence of severe invasive aspergillosis and overall survival) with a control group (group II) which was represented by all AML patients hospitalized for induction during the period 2006-2007 and who did not received any antifungal prophylaxis. There was in total 143 AML patients and after matching on age, gender, FAB classification and molecular markers, we got 121 patients (59 males and 62 females with a median age of 55 years): 55 patients in group I and 66 patients in group II. There were 91 AML de novo and 30 secondary AML, 18 patients had good cytogenetic markers, 43 intermediate and 58 poor (2 non evaluated). According to cytogenetics plus molecular markers, we distinguished 2 groups: a good prognostic group (n=29) associating favourable cytogenetics and intermediate 1 (normal cytogenetics+ Flt3 ITD, CEBPA,NMP1) and a poor prognosis group (n=75) with unfavourable cytogenetics and intermediate 2 (17 patients were not determined). As induction chemotherapy, patients received different combinations according to protocols, age and AML characteristics. The median interval between AML diagnosis and hospitalization was 0 day (-41 – +7), 92 patients (76%) were placed in laminar air flow rooms. After induction, 100 patients achieved a CR and 2 patients died during induction period. The median duration of aplasia and of hospitalization were 28 days (7 – 91) and 37 days (22 – 101) respectively. There was no significant difference for all the above characteristics between group I and group II. Concerning posaconazole prophylaxis, the treatment was started the 1st day of chemotherapy with a median duration of 27 days (8-94): 35 patients (64%) received their prophylaxis until their discharge, 7 (13%) discontinued due to toxicity [hepatic (n=3), renal (n=1), transfer to intensive care unit (n=3)] and 13 (23%) switched to another fungal treatment because of IA suspicion (n=5), probable IA (n=2) and invasive candidosis (n=5).
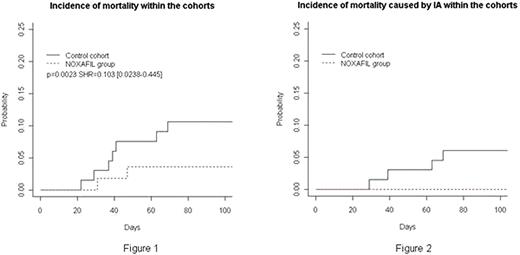
At Day32 post induction, we observed 2 probable IA (3.6%) in group I and 8 IA [possible (n=4) + probable IA (n=4)] (12%) in group II (p = 0.085). The cumulative incidence of IA in group I and II was: at day 100, 7.27% vs 15.5%, at 1 year 12.72% vs 22.72% and at 18 months 14.54% vs 24.24% respectively. The Kaplan-Myer analysis on time to death from any cause at Day 100, showed a significant survival benefit in favour of the Pozaconazole group (group I) over the control group (group II) (p=0.0023) (figure1), this difference was also significant when we adjusted the analysis only on deaths caused by IA (figure2). After a median follow-up of 8.6 months, the probability of overall survival was 92.3% at day 100, 83.5% at 6 months, 70% at 1 year, 58% at 18 months and 36% at 2 years with a significant difference between groups I and II(p=0.02). At the last follow-up, 39 patients died in the group II and 10 in the group I. Concerning the other risk factors, the multivariate analysis showed a significant impact on long term OS of age [HR= 0.103 (0.02 – 0.5) (p= 0.00029) ], cytogenetics [HR=2.524 (1.172-5.44) (p=0.0018)] and response to induction [HR=7.73 (3.579-16.7) (p=1.9e-7)].
We showed a very important impact of anti-fungal prophylaxis in patients undergoing chemotherapy for AML especially for invasive aspergillosis, which is a risk factor to take into account in addition to age, cytogenetics and response to treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.