Abstract
Abstract 4408
Severe thrombocytopenia resulting from packed marrow, splenomegaly, toxicity of therapy, or autoimmunity/idiopathic thrombocytopenic purpura (ITP) is frequent in CLL. Eltrombopag is a small molecule TPO receptor (TPO-R) agonist being developed as a treatment for thrombocytopenia. Eltrombopag is used and approved for the treatment of chronic ITP and is under investigation for thrombocytopenia of chronic liver disease. In order to provide the basis for the investigational clinical use of eltrombopag (with and without chemotherapy) in CLL, we have studied eltrombopag effects in vitro on primary CLL cells in order to provide evidence that eltrombopag does not stimulate CLL cells by untoward or off-target effects.
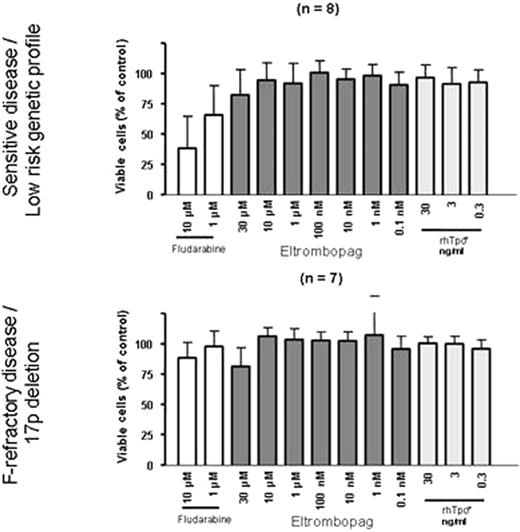
We analyzed cell number and apoptosis induction in a set of CLL patient samples (n=15) after 48h incubation with eltrombopag, thrombopoietin or fludarabine. CLL cases were characterized with respect to genetics (genomic aberrations, TP53 mutation, IGHV mutation status), as well as clinical course and immunophenotype. To study the effect on CLL cells, we used in vitro treatment with eltrombopag (0.1nM-30μM), thrombopoietin (0.3ng-30ng/ml) and Fludarabine (1-10μM). We assessed the effect using FACS (7-AAD) and CellTiterGlo for viability. We studied 8 CLL patients' cells with low risk disease and 7 with high risk disease (17p-, TP53 mutation or F-refractory CLL).
In spite of increasing concentrations up to 30μM we did not observe significant changes in cell number suggesting that there is no growth stimulation or apoptosis induction in primary CLL cells by eltrombopag in vitro. In contrast, Fludarabine caused a dose dependent decrease in cell viability (mean 60-80% (1, 10μM) viable cells at 48 hours). As shown in Fig. 1 the response to fludarabine but not to eltrombopag was dependent on the risk profile. Cases with F-refractory disease or 17p deletion did not respond to Fludarabine. The effect of eltrombopag on the CLL marker profile (CD5, 19, 20, 23, 38) and CD41/CD61 expression was also investigated, but no differences were observed. In order to more broadly explore the possibility of (untoward) effects of eltrombopag in CLL we are currently generating gene expression profiles on eltrombopag treated CLL cells to assess potential cellular responses and pathway activation.
Our results suggest that eltrombopag is unlikely to stimulate the growth of CLL cells. Our data therefore suggest that clinical trials investigating its effect on platelet counts in CLL are warranted.
Zenz:GSK: Membership on an entity's Board of Directors or advisory committees. Erickson-Miller:GSK: Employment. Mostafa Kamel:GSK: Employment. Stilgenbauer:GSK: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.