Abstract
Abstract 63
Anti-tumor effects of allogeneic hematopoietic cell transplantation have been attributed to engrafted donor lymphocytes and long-term donor engraftment is thought to be essential to cure hematological malignancies. However, following non-myeloablative hematopoietic cell transplantation (HCT), we have observed that some patients are cured of advanced hematologic malignancies despite rejection of their donor hematopoietic cell graft. This led us to hypothesize that host-versus-graft (HVG) reactions may induce host-derived anti-tumor effects.
In a murine model, we established that HVG responses can indeed promote host-anti-tumor responses that are dependent on host-type CD8 T cells, host-type NKT cells, IFN-g, and initial donor engraftment (Rubio et al, Blood 2003 102:2300 and J Immunol 175:665,2005; Saito et al. manuscript submitted). Host CD8 T cells in the bone marrow secrete high levels of IFN-g even after peripheral donor chimerism has totally disappeared (Saito et al. manuscript submitted). Thus, we hypothesized that alloresponses may persist in the bone marrow, driven by the presence of alloantigens, either due to sustained engraftment of the donor cells (direct alloantigen presentation) or to presentation of alloantigens by host antigen-presenting cells (APC) (indirect alloantigen presentation) in the bone marrow.
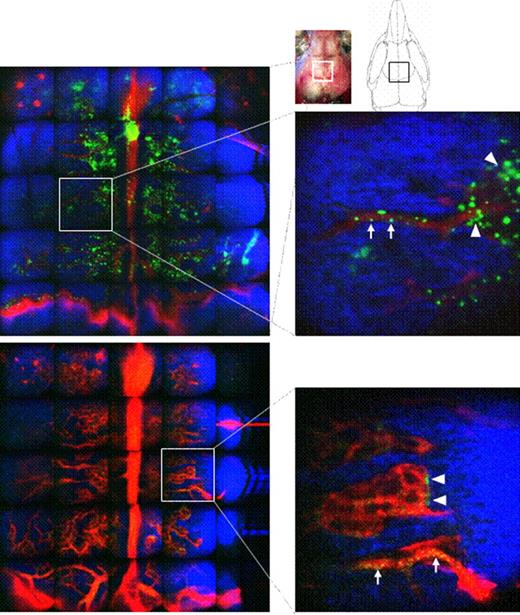
To assess this hypothesis, we used dynamic in vivo microscopy to directly visualize events in living animals. Two-photon microscopy allowed visualization of the bone marrow environment 150 mm below the skull bone surface of anesthetized mice. With these tools, we were able to track fluorescently labeled donor protein together with bone and blood vessels in real time.
We established stable mixed allogeneic bone marrow chimeras using GFP+ C57BL/6 mice as donors and BALB/c mice as recipients using non-myeloablative conditioning. This system allows tracking of GFP as a model donor antigen and discrimination of donor cells from host cells engulfing donor proteins by examining both GFP staining and expression of specific major histocompatibility complex (MHC) alleles. Recipient leukocyte infusion (RLI) was performed to induce HVG reactions 7 to 10 weeks after initial allogeneic BMT in order to induce rejection of engrafted donor cells. GFP expression in the bone marrow was examined in stable mixed chimeras and in recipients of RLI.
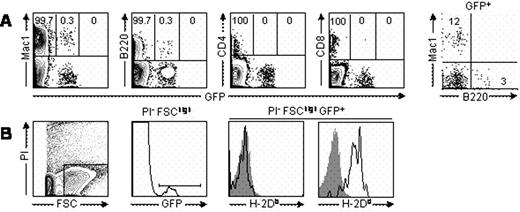
In stable mixed chimeras, clusters of GFPhigh donor cells were detected as green areas that were evenly distributed and located mainly around the blood vessels in the bone marrow. Fast-moving and rolling GFPhigh cells were observed inside the vessels. Even 11 to 22 days after RLI administration, we still observed static GFPdim cells surrounding the vessels, when donor chimerism in peripheral blood had become undetectable. When we gated out most of the RBCs on the basis of forward scatter, the majority of GFPdim cells in the bone marrow at 22 days post-RLI were Mac1+ cells, while a smaller proportion were of the B cell lineage. When we examined the origin of the Mac1+ GFPdim cells using donor and recipient MHC-specific antibodies, they were found to be recipient-derived Mac1+ cells.
Our studies revealed the persistence of host-derived myeloid cells containing donor protein and residing around the bone marrow blood vessels after peripheral blood chimerism was lost. The results suggest that persistent presentation of donor-derived antigens on host APC may maintain alloreactive host CD8 T cell activation in the bone marrow, promoting the anti-tumor effect induced by HVG reactions.
Engraftment of donor bone marrow cells and remaining cells after rejection. GFP+ cells are shown in green, bone in blue and blood vessels in red. GFP+ cells are visualized in vivo inside the skull bone of chimeras (A) and of chimeras that received RLI 11 days earlier (B). Arrowheads point to GFP+ cells around the blood vessels and arrows point to GFP+ cells in the blood vessels. Data from one representative mouse of two mice are shown.
(A) GFP expression among Mac1+, B220+, CD4+, and CD8+ bone marrow cells in RLI recipient. (B) GFP positive leukocytes in the bone marrow are stained for H-2Db and H-2Dd. Data from representative mouse from two mice are shown.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.