Abstract
Abstract 682
Eltrombopag (PROMACTA; GlaxoSmithKline, Collegeville, PA) is the first oral, small molecule, thrombopoietin receptor agonist approved in the US for the treatment of chronic immune thrombocytopenic purpura (ITP). Eltrombopag is also being evaluated for the treatment of thrombocytopenia due to other causes (eg, hepatitis C, MDS). Chronic ITP is characterized by autoantibody-induced platelet destruction and reduced platelet production, leading to chronically low platelet counts. Eltrombopag has been shown to significantly increase platelet counts and reduce clinically relevant bleeding symptoms in 3 placebo-controlled ITP trials evaluating a total of 429 patients. EXTEND is an ongoing open-label, phase 3 extension study to assess the long-term safety and efficacy of eltrombopag in chronic ITP.
Patients with previously treated, chronic ITP who completed a prior eltrombopag study were eligible to participate in EXTEND. Eltrombopag treatment was initiated at 50 mg once daily and then adjusted to maintain platelet counts between ≥50,000/μL and <200,000/μL, with doses between 75 mg and 25 mg once daily (or less often if necessary). Patients who achieved platelet counts ≥50,000/μL were considered responders. Bleeding events were prospectively evaluated using the World Health Organization (WHO) Bleeding Scale: grade 0 = no bleeding, grade 1 = mild bleeding, grade 2 = moderate bleeding, grade 3 = gross bleeding, and grade 4 = debilitating blood loss. Bone marrow (BM) biopsy was required after 1 year on treatment.
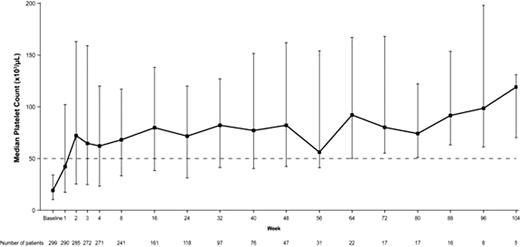
At the time of this analysis, 299 patients (median age 50 years; 66% female) had received eltrombopag (240, 126, 48, and 17 patients exposed for ≥6, 12, 18, and 24 months, respectively). The median duration of eltrombopag treatment was 204 days and ranged from 2–861 days. At baseline, 33% were receiving concomitant ITP medication and 38% had been splenectomized. The majority of patients (70%) had baseline platelet counts <30,000/μL, followed by 17% and 13% with baseline platelet counts from μ30,000/μL to <50,000/μL, and μ50,000/μL, respectively; all had baseline platelet counts <50,000/μL at the time of entry into their previous study. Overall, 86% of patients (257/299) achieved a platelet count μ50,000/μL. Splenectomized and non-splenectomized patients responded equally well (89% and 82%, respectively). Patients responded to eltrombopag regardless of baseline use of concomitant ITP medications (no baseline ITP medications and baseline ITP medications: 86% each). Median platelet counts increased to μ50,000/μL by week 2, and remained μ50,000/μL throughout the observation period of the study (Figure 1). Patients on treatment for μ6 months or μ12 months achieved platelet counts of μ50,000/μL and 2x baseline for 69% (18/26 weeks) and 71% (37/52 weeks) of the time on treatment, respectively. At baseline, 56% of patients reported bleeding symptoms (WHO grades 1–4) compared to 27%, 21%, 40%, and 25% at 6, 12, 18, and 24 months, respectively. Adverse events (AEs) were reported in 248 patients (83%) while on therapy, the majority being mild to moderate. The most common AEs reported were headache (23%), upper respiratory tract infection (17%), nasopharyngitis (17%), fatigue (13%), arthralgia (12%), and diarrhea (11%). Five deaths were reported: 2 occurred on therapy and 3 occurred more than 30 days posttherapy; none considered related to study medication. A total of 24 patients (8%) met any of the hepatobiliary laboratory abnormality screening criteria (ALT ≥3x ULN, AST ≥3x ULN, total bilirubin >1.5x ULN, or alkaline phosphatase >1.5x ULN). Thirteen patients (4%) experienced 16 thromboembolic events (TEEs); 11/13 (85%) experienced the event at a platelet count lower than the maximum platelet count achieved during eltrombopag treatment. Platelet counts proximal to the TEEs ranged from 14,000–407,000/μL. Eighty-six BM biopsies were performed. No clinically relevant effects of eltrombopag on BM were detected. CONCLUSION: Oral eltrombopag treatment for up to 2 years effectively raised platelet counts, decreased bleeding symptoms, and was generally well-tolerated in chronic ITP.
Median Platelet Countsa,b
aUpper and lower bars represent 75th percentile and 25th percentile respectively
bMedian platelet counts remained μ50,000/μL with the exception of weeks 86, 99, and 103 in which the median platelet counts remained >40,000/μL.
Median Platelet Countsa,b
aUpper and lower bars represent 75th percentile and 25th percentile respectively
bMedian platelet counts remained μ50,000/μL with the exception of weeks 86, 99, and 103 in which the median platelet counts remained >40,000/μL.
Saleh:GlaxoSmithKline: Speakers Bureau; Amgen: Speakers Bureau. Bussel:Genzyme: Research Funding; GlaxoSmithKline: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cangene: Research Funding; Amgen: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding; Immunomedics: Research Funding; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai, Inc: Research Funding; Sysmex: Research Funding; Scienta: Speakers Bureau; Shionogi: Membership on an entity's Board of Directors or advisory committees. Cheng:GlaxoSmithKline: Research Funding. Mayer:GlaxoSmithKline: Employment. Bailey:GlaxoSmithKline: Employment. Aivado:GlaxoSmithKline: Employment.
Author notes
Asterisk with author names denotes non-ASH members.