Abstract
In humans, the kinetics of the appearance of memory B cells and plasma cells during primary immunization are not well defined. In this study, we assessed the primary B-cell response of rabies-antigen naive volunteers during a 3-dose course of rabies vaccine compared with the B-cell response to a booster dose of rabies vaccine given to previously immunized volunteers. After a single dose of vaccine, in the naive group plasma and memory B cells appeared later (peak at day 10) than in the primed group (peak at day 7) and were at lower frequency. The most rapid responses (day 4) were detected after a third immunization in the naive group. This is the first study to document the detailed kinetics of the plasma cell and memory B-cell responses to immunization in adult humans and to demonstrate differences in the responses that relate to the preexisting immune status of the persons.
Introduction
Long-term humoral immunity may be represented by the size of the B-cell pool from which long-lived plasma cells and memory B cells are derived,1 but the pool is not readily accessible in humans, residing in lymphoid organs. Antigen (Ag)–specific plasma cells are not detectable in peripheral blood at steady state, but these cells are thought to use the circulation to reach the bone marrow and they therefore appear transiently in peripheral blood after immunization.2-7 Memory B cells can be detected in peripheral blood at steady state, but the majority probably reside in lymphoid tissues.8-10 An increase in memory B-cell frequency is consistently observed shortly after immunization2,3,5,11,12 and might represent newly generated memory B cells transiting through the circulation to other lymphoid tissues from germinal centers (GCs). The definition of the kinetics of the appearance of plasma cells and memory B cells in peripheral blood in the primary and secondary immune response will inform design of studies to assess the relationship between B-cell responses and the persistence of humoral immunity.
In this study, the inactivated rabies vaccine was used to study the primary B-cell response in healthy adults, as a novel Ag for all unimmunized persons in the United Kingdom.
Methods
Study population
Two groups of 10 healthy adult volunteers 18 to 50 years of age, with no history of allergy to a vaccine component, were enrolled in 2007 after written informed consent. The study was approved by Oxfordshire's Research Ethics Committee (BO7/Q1605/29) with informed consent in accordance with the Declaration of Helsinki. Group 1 included 10 volunteers who had not previously received rabies vaccine, and group 2 included 10 volunteers who had received at least a primary course of 3 doses of rabies vaccine with or without booster immunization 2 to 10 years previously.
Immunization and sampling protocol
A total of 1 mL of human diploid cell vaccine (rabies vaccine BP; Sanofi Pasteur MSD Ltd) was given by intramuscular injection into the deltoid region at days 0, 28, and 56 for group 1, and a single dose was administered at day 0 for group 2. Blood samples (20 mL) were taken before immunization and at days 2, 4, 7, 10, 14, and 28 after the first and third dose for group 1 and after the single dose for group 2.
Antibody, plasma cell, and memory B-cell responses
Statistical analysis
Stata Version 9.1 (Stata Corp) was used to calculate geometric means for the ELISA immunoglobulin concentrations and medians for the B-cell numbers. Comparison of the magnitude of the B-cell and Ab responses between the naive and immune groups were made using the Mann-Whitney U test. Spearman rank correlation was used to compare variables (log-transformed IgG concentration and untransformed B-cell numbers).
Results and discussion
This is the first study to document the detailed kinetics of the plasma cell and memory B-cell responses to immunization in adult humans and to demonstrate differences in the responses that relate to the preexisting immune status of the persons (Figures 1–2).
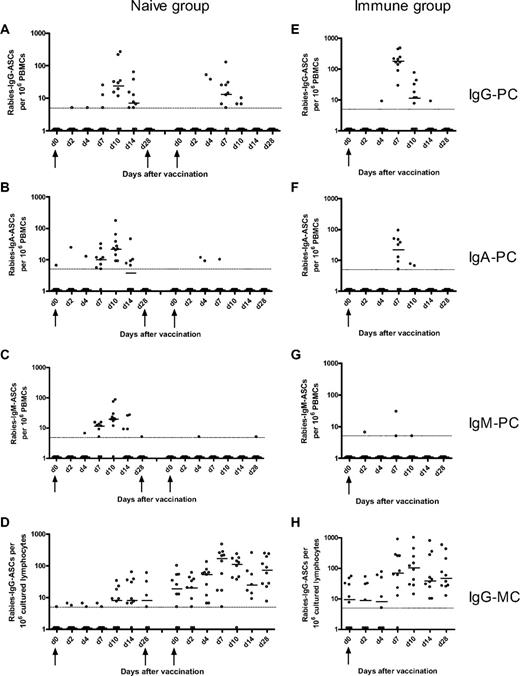
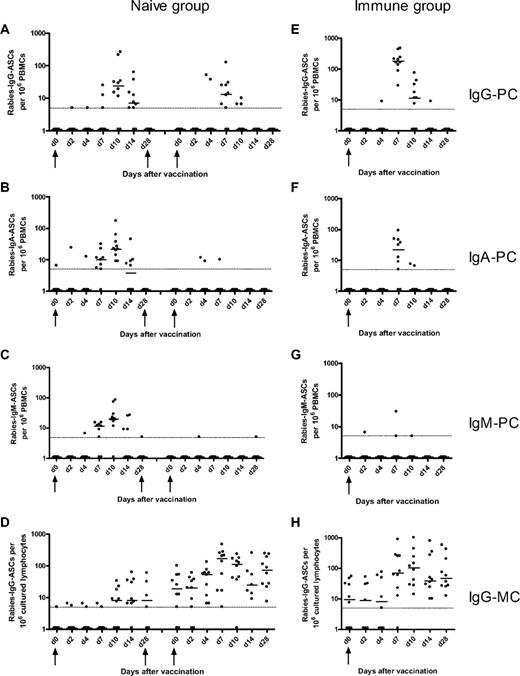
Rabies-specific IgG, IgA, IgM plasma-cell and IgG memory B-cell responses after immunization of naive and immune volunteers. The naive volunteers (left) were immunized at days 0, 28, and 56, and the frequencies of rabies IgG- (A), IgA- (B), and IgM-specific (C) plasma cells (PCs) and IgG memory B-cells (MCs; D) were measured at various days after the first and third doses of immunization. The immune volunteers (right) were immunized at day 0, and the frequencies of rabies IgG- (E), IgA- (F), IgM-specific PCs (G), and IgG MCs (H) were measured at various days after immunization. The horizontal bars represent the median number of specific antibody-secreting cells at each time point. The minimum sensitivity of the assay is plotted as a broken line on the graphs for each figure. The zero values were assigned a value of 1 for illustrative purposes. The magnitude of the IgG PC response after booster immunization in the immune group was greater than after primary and tertiary immunization in the naive group. However, the magnitude of the IgG MC response after booster immunization in the immune group was greater than after primary and secondary immunization but not tertiary immunization.
Rabies-specific IgG, IgA, IgM plasma-cell and IgG memory B-cell responses after immunization of naive and immune volunteers. The naive volunteers (left) were immunized at days 0, 28, and 56, and the frequencies of rabies IgG- (A), IgA- (B), and IgM-specific (C) plasma cells (PCs) and IgG memory B-cells (MCs; D) were measured at various days after the first and third doses of immunization. The immune volunteers (right) were immunized at day 0, and the frequencies of rabies IgG- (E), IgA- (F), IgM-specific PCs (G), and IgG MCs (H) were measured at various days after immunization. The horizontal bars represent the median number of specific antibody-secreting cells at each time point. The minimum sensitivity of the assay is plotted as a broken line on the graphs for each figure. The zero values were assigned a value of 1 for illustrative purposes. The magnitude of the IgG PC response after booster immunization in the immune group was greater than after primary and tertiary immunization in the naive group. However, the magnitude of the IgG MC response after booster immunization in the immune group was greater than after primary and secondary immunization but not tertiary immunization.
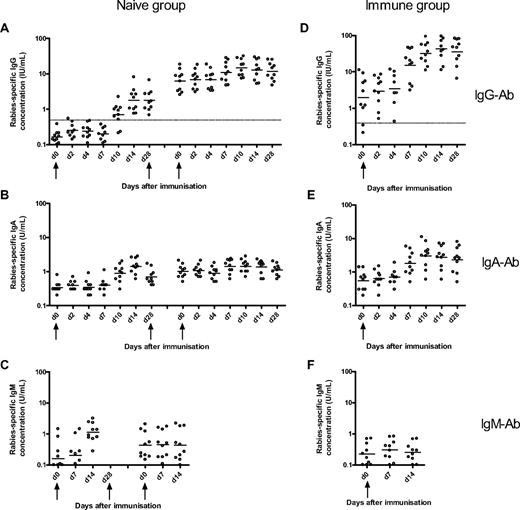
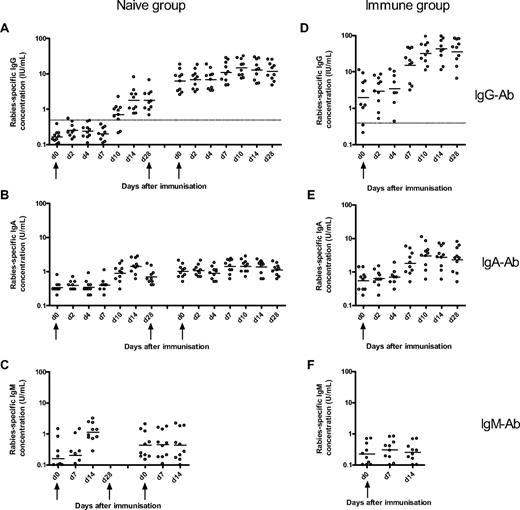
Rabies-specific IgG, IgA, and IgM Ab responses to immunization with rabies vaccine in naive and immune volunteers. The naive volunteers (left) were immunized at days 0, 28, and 56, and the concentration of rabies-specific IgG- (A), IgA- (B), and IgM-specific Ab (C) concentration was measured at various days after the first and third doses of immunization. The immune volunteers (right) were immunized at day 0, and the frequencies of rabies IgG- (D), IgA- (E), and IgM-specific (F) Ab were measured at various days after immunization. The horizontal bars represent the geometric mean concentration at each time point. The broken line represents the World Health Organization IgG seroconversion level of 0.5 IU/mL. The magnitude of the IgG Ab response after booster immunization in the immune group was greater than after primary, secondary, and tertiary immunization in the naive group.
Rabies-specific IgG, IgA, and IgM Ab responses to immunization with rabies vaccine in naive and immune volunteers. The naive volunteers (left) were immunized at days 0, 28, and 56, and the concentration of rabies-specific IgG- (A), IgA- (B), and IgM-specific Ab (C) concentration was measured at various days after the first and third doses of immunization. The immune volunteers (right) were immunized at day 0, and the frequencies of rabies IgG- (D), IgA- (E), and IgM-specific (F) Ab were measured at various days after immunization. The horizontal bars represent the geometric mean concentration at each time point. The broken line represents the World Health Organization IgG seroconversion level of 0.5 IU/mL. The magnitude of the IgG Ab response after booster immunization in the immune group was greater than after primary, secondary, and tertiary immunization in the naive group.
After primary immunization in the naive group, the plasma cell, memory B-cell, and Ab responses were delayed and of lower magnitude compared with the response to booster immunization in the immune group. Plasma cells were observed in peripheral blood from day 7 to day 14 with a peak of the response by day 10. The primary memory B-cell response was delayed but more sustained compared with the primary plasma cell response, with memory B cells being apparent in peripheral blood from day 10 onward and remaining at a constant frequency 1 month after immunization. The primary B-cell response observed in this study is similar to the response observed in a study assessing the B-cell response to primary immunization with serogroup C meningococcal conjugate vaccine in 2-month-old infants. In that study, there was a small increase in IgG plasma cells between days 8 and 16 with a return to baseline by day 30 and a small increase in IgG memory B cells between days 14 and 30 after the primary immunization.4 These observations are consistent with murine data showing that both memory B cells and plasma cells appear in peripheral blood by 1 week of immunization14 and that GCs in spleen and lymph nodes can be detected from day 4 after a primary Ag exposure with peak activity between days 12 and 14, involution occurring after 3 to 4 weeks.15,16 As in the present study, the frequency of murine Ag-specific memory B cells was constant for several weeks after immunization with selection of high-affinity clones.14,16
In mice, an increase in the affinity maturation of plasma cells in bone marrow and blood between day 7 and day 14 after primary immunization is observed.14 The increase in plasma cell numbers observed in peripheral blood between day 7 and day 14 after primary immunization in the present study might represent long-lived plasma cells produced in GCs, and their appearance might correspond to migration to the bone marrow. Of note, the area under the curve of the IgG plasma cell response was strongly correlated with the IgG Ab response after the primary and tertiary immunization in the naive group (r = 0.71, P = .002). Although the existence of long-lived plasma cells has only been observed directly in mice, preliminary studies in humans depleted of CD20 provide support for their existence in humans.17,18
The Ab and B-cell responses after booster immunization in the immune group (from day 7 onward) were more rapid and of greater magnitude than the naive group, suggesting that these cells might originate from previously formed memory B cells, which have a lower threshold of activation19 and might not need to reenter GCs to differentiate into plasma cells. However, GC formation has been documented during the rapid booster response after secondary and higher degree immunization.20-22 This observation is consistent with our finding of a strong correlation between the IgG memory B-cell and IgG Ab responses at day 28 after immunization (r = 0.82, P = .004).
The most rapid memory B-cell response was detected among persons receiving their third immunization in the naive group, 56 days after the first dose, where a rise was seen as early as day 4. This may reflect faster activation of recently generated memory B cells either in extrafollicular foci or after reentry in GCs23,24 ; or activity of partially involuted GCs, which are present from the previous dose; or simply the size of the pool generated after a rapid 3-dose schedule. Indeed, a strong correlation was found between the IgG memory B-cell frequencies and the IgG Ab concentration at 28 days after the third dose (r = 0.88, P < .001). Perhaps, after months or years, the size of the initial pool wanes so that the B-cell response observed in the previously immunized group is not greater compared with the third immunization in the naive group. Alternatively, circulating Abs after doses 2 and 3 in the naive group and after booster immunization in the immune group may originate from long-lived plasma cells in the bone marrow.25
The kinetics and magnitude of the B-cell responses after primary and secondary immunization are not the same. Analysis of these responses is necessary to improve understanding of the nature of long-term humoral immunity after vaccination.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the volunteers who participated in the study and the team of Oxford Vaccine Group and Leiden University for their support, particularly Anja Jansen and Amy Slender for their technical assistance and Mary Warrell for advice on the rabies ELISA.
G.B.-R. was supported by the Swiss National Science Foundation. This work was supported by the Oxford Partnership Comprehensive Biomedical Research Center Program with funding from the Department of Health's National Institutes for Health Research Biomedical Research Centres funding scheme. This work was also supported by the Oxfordshire Health Services Research Committee. A.J.P. is a Jenner Institute Investigator.
The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health.
National Institutes of Health
Authorship
Contribution: G.B.-R. planned the study, conducted clinical trial work and laboratory work, analyzed the data, and wrote drafts of the paper; A.S.P. participated in planning the study and in the clinical trial work and reviewed drafts of the paper; C.M.J.-v.d.Z. developed some of the laboratory methodology, conducted laboratory work, and reviewed drafts of the paper; M.D.S. participated in planning the study and in the clinical trial work and reviewed drafts of the paper; and A.J.P. was the principal investigator, discussed and planned clinical and laboratory aspects of the study, and reviewed drafts of the paper.
Conflict-of-interest disclosure: A.J.P. acts as chief and principal investigator for clinical trials conducted on behalf of Oxford University, sponsored by vaccine manufacturers (Novartis Vaccines, GlaxoSmithKline, Sanofi-Pasteur, Sanofi-Pasteur MSD, and Wyeth Vaccines), but does not receive any personal payment from them. Industry-sourced honoraria for consultancy, lecturing, or writing and travel expenses and grants for educational activities are paid directly to an educational/administrative fund held by the Department of Paediatrics, University of Oxford. M.D.S. received assistance to attend scientific meetings from Wyeth Vaccines, GSK Pharmaceuticals, and Novartis Vaccines and has had travel and accommodation expenses paid by Novartis Vaccines while working in collaboration with Novartis Vaccines in Siena, Italy. The remaining authors declare no competing financial interests.
Correspondence: Geraldine Blanchard-Rohner, Centre for Clinical Vaccinology and Tropical Medicine, Churchill Hospital, Oxford, OX3 7LJ United Kingdom; e-mail: Geraldine.BlanchardRohner@paediatrics.ox.ac.uk.