Abstract
Twenty-four percent of sickle cell disease (SCD) patients have a stroke by the age of 45 years. Blood transfusions decrease stroke risk in patients deemed high risk by transcranial Doppler. However, transcranial Doppler has poor specificity, and transfusions are limited by alloimmunization and iron overload. Transfusion withdrawal may be associated with an increased rebound stroke risk. Extended blood typing decreases alloimmunization in SCD but is not universally adopted. Transfusions for thalassemia begun in early childhood are associated with lower rates of alloimmunization than are seen in SCD, suggesting immune tolerance. Optimal oxygen transport efficiency occurs at a relatively low hematocrit for SCD patients because of hyperviscosity. Consequently, exchange rather than simple transfusions are more effective in improving oxygen transport efficiency, but the former are technically more demanding and require more blood units. Although viscosity is of importance in the noncerebral manifestations of SCD, inflammation may play a larger role than viscosity in the development of large-vessel stroke. The future of SCD stroke management lies in the avoidance of transfusion. Hydroxyurea and anti-inflammatory measures may reduce the need for transfusion. Recent genome-wide association studies may provide methods for modulating fetal hemoglobin production enough to attenuate stroke risk and other complications of SCD.
Introduction
Sickle cell disease (SCD) is a protean disorder caused by elevations of intraerythrocyte and total blood viscosity. Hypoxia-induced gelation of hemoglobin S (HbS) deforms the erythrocyte and its membrane and causes massive cation loss as well as increased erythrocyte surface expression of adhesion molecule receptors. The concomitant lack of deformability and enhanced stickiness lead to obstructive adhesion of sickle cells to each other and to vascular endothelium.1 The result is ischemia reperfusion injury and endothelial cell damage with leakage of von Willebrand factor, platelet clumping, cytokine release, and attraction of granulocytes, macrophages, T cells, and invariant natural killer T (iNKT) cells to areas that become both infarcted and inflamed.2,3 The obstruction and inflammation in turn cause further hypoxia and acidosis and, consequently, further sickling, the so-called vicious cycle of SCD.
Of the many severe consequences of SCD, acute stroke and chronic cerebral ischemia are among the most disabling.4 The fragile cerebral vessels of young children are particularly vulnerable: crippling strokes may blight a child's life even before the age of 2 years. The terrible consequences of SCD-induced stroke have prompted multiple approaches to prevention of this pernicious complication.
Stroke in SCD was initially noted in 1923,5 13 years after the first description of the disease.6 A landmark angiographic case study in 1972 demonstrated the particular vulnerability of the internal carotid artery and circle of Willis7 with consequent formation of fragile collaterals. This can lead to Moyamoya syndrome, a characteristic finding on cerebral angiograms consisting of multiple small collateral vessels around the circle of Willis that give a “puff of smoke” appearance.
In this review, we offer a brief discussion of the natural history and prevention of stroke in SCD. We first discuss secondary prevention studies because they have led to landmark trials of primary prevention strategies. The review then highlights the potential pitfalls of nonspecific imaging modalities to screen SCD patients for stroke risk. The controversies surrounding the use of extended, phenotype-matched blood are explored together with the putative association of tranfusion initiation in early childhood to decreased alloimmunization. We then analyze the paradoxical relationships of hematocrit (Hct) and blood viscosity to the risks of stroke versus extra–central nervous system vascular obstruction in SCD. We conclude with recommendations for further research.
Natural history of stroke in SCD
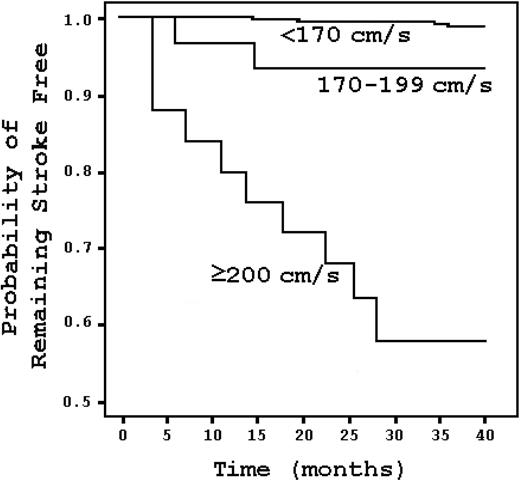
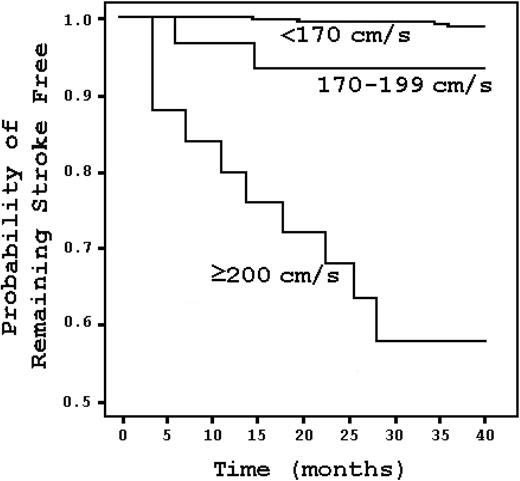
Hematologically normal black Americans have twice the stroke risk of white Americans.8 This suggests that a subset of blacks may have particularly vulnerable cerebral blood vessels. The risk is enormous in SCD. Approximately 11% of SCD patients have clinically apparent strokes before the age of 20.9 That risk increases to 24% by the age of 45.9 The risk of stroke is highest during the first decade, and it is most significant between ages 2 and 5, when it reaches 1.02% per year.9 The risk is lowest before the age of 2, probably because of the protective influence of fetal hemoglobin on sickling.9 A child with SCD has a stroke risk that is 333 times greater than that of a healthy child without SCD or heart disease.9,10 In children with “abnormal” transcranial Doppler (TCD) studies, the risk is more than 3000 times greater (Figure 1).11 The proportion of SCD patients with strokes before the age of 70 can be stratified by genotype, where the risk is highest for sickle hemoglobin (HbSS) and lowest for sickle β+-thalassemia.9
Kaplan-Meier survival analysis stratified by TCD result in SCD patients. Stroke risk increases with elevated TCD velocities but is most marked at 200 cm/s and higher. Adapted from Adams et al11 with permission.
Kaplan-Meier survival analysis stratified by TCD result in SCD patients. Stroke risk increases with elevated TCD velocities but is most marked at 200 cm/s and higher. Adapted from Adams et al11 with permission.
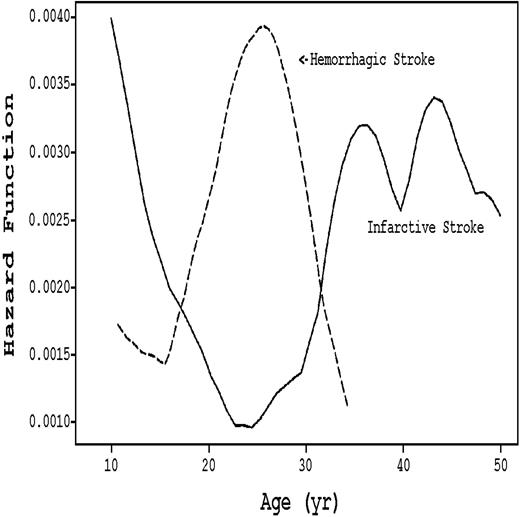
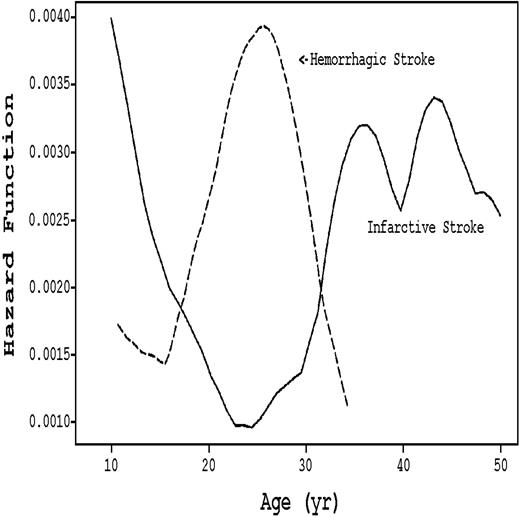
Stroke subtype varies by age in SCD patients, as seen in Figure 2. The incidence of the ischemic variant, which constitutes 54% of all cerebrovascular accidents (CVAs),9 is highest during the first decade and after age 30. During the 20s, ischemic CVA is replaced by hemorrhagic CVA.9 Although not characterized as age-dependent, 10% to 30% of SCD patients have silent strokes that exhibit radiologic findings consistent with diffuse white matter disease.12-15 These silent infarcts are associated with cognitive deficiencies.14
Hazard rates of infarctive versus hemorrhagic stroke stratified by age in SCD patients. (Solid line) Infarctive stroke. (Dashed line) Hemorrhagic stroke. Adapted from Ohene-Frempong et al9 with permission.
Hazard rates of infarctive versus hemorrhagic stroke stratified by age in SCD patients. (Solid line) Infarctive stroke. (Dashed line) Hemorrhagic stroke. Adapted from Ohene-Frempong et al9 with permission.
Secondary prevention
Blood transfusion16
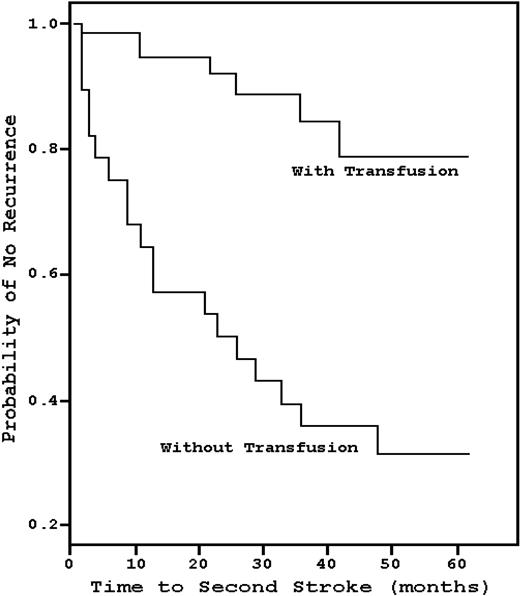
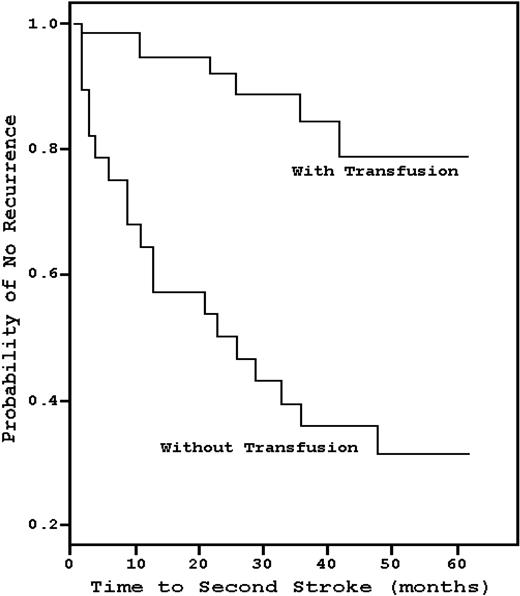
In 1978, Powars et al17 found that the recurrence rate of stroke in SCD patients was 66%, and the risk was greatest during the 2 to 3 years after the initial CVA (Figure 3). Conversely, Pegelow et al18 observed that, in 60 children with a prior stroke history who had been on chronic blood transfusions with a goal HbS less than or equal to 30%, only 8 patients developed a recurrent CVA over a mean of 38 months, a 13.3% recurrence rate that has been reproduced in other studies.19 However, analogous to the Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP II) trial,20 the data suggest that lifelong blood transfusions may be necessary, as there is a high risk of recurrence relatively soon after transfusion cessation. Wang et al21 prospectively discontinued transfusions in 10 patients who had been on such therapy for a median of 9.5 years; within 12 months, half developed an ischemic event. No study done before the termination of transfusions, such as angiography, magnetic resonance imaging, electroencephalogram, or neurologic examination, was predictive of recurrence.
Kaplan-Meier estimates of the probability of stroke recurrence with chronic blood transfusions compared with historical controls. Chronic blood transfusion significantly decreases the risk of stroke recurrence. Adapted from Pegelow et al18 with permission.
Kaplan-Meier estimates of the probability of stroke recurrence with chronic blood transfusions compared with historical controls. Chronic blood transfusion significantly decreases the risk of stroke recurrence. Adapted from Pegelow et al18 with permission.
Hydroxyurea
In view of concern about adverse effects of blood transfusions, hydroxyurea (HU) therapy has become an alternative option. Ware et al22 examined 16 children with stroke histories who were on chronic blood transfusions for a mean of 56 months and had their transfusions stopped because of alloimmunization, autoimmune hemolysis, iron overload, or noncompliance. HU therapy and intermittent phlebotomy to remove iron were offered to these patients, beginning 2 weeks after the last transfusion. After a mean of 22 months on HU therapy, the stroke rate was 19%. Although significantly lower compared with historical controls,17 the stroke rate was almost twice as high as that observed in patients receiving transfusions in the same institution (11%). Notably, the average time to stroke in those who had the complication was only 3 to 4 months.
Ware et al concluded that a switch to HU required overlap between transfusions and HU therapy. Therefore, the authors performed a follow-up study23 of 20 patients who were overlapped for a mean of 6 months and were followed for almost 3 years. Only 2 patients had a recurrence, suggesting that overlapped HU therapy provides efficacy equal to transfusion in the prevention of stroke recurrence in children. These findings led to the Stroke with Transfusions Changing to Hydroxyurea trial, an ongoing randomized phase 3 trial to compare chronic blood transfusions and iron chelation to HU and phlebotomy for secondary prevention of stroke and management of iron overload in children with SCD and previous stroke. The results of this trial are not yet reported.
Primary prevention
STOP I trial
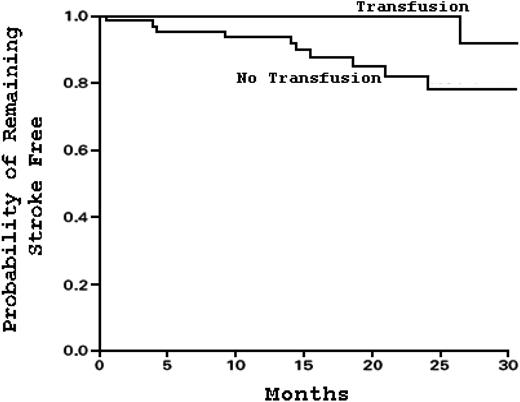
In 1997, Adams et al11 showed that children with “abnormal” TCD velocities, defined as greater than 200 cm/s in the large cerebral vessels, had an associated 40% stroke risk within 3 years (Figure 4). Given the efficacy of chronic blood transfusions for prevention of stroke recurrence,16 it was hypothesized that TCD could identify high-risk SCD patients and that chronic blood transfusions might decrease the risk of a first stroke. In the landmark study Stroke Prevention Trial in Sickle Cell Anemia (STOP I; NCT00000592),24 children 2 to 16 years of age with HbSS or sickle β0-thalassemia who had no prior stroke history but an “abnormal” TCD value were randomly assigned to either chronic blood transfusions every 3 to 4 weeks (goal HbS ≤ 30%) or standard care. The trial was terminated early when 12 strokes occurred, 11 of which were in the standard care group (Figure 5). The standard care group had a stroke incidence rate of 10% per year.
Kaplan-Meier estimates of the probability of remaining stroke-free in patients with SCD receiving chronic blood transfusions as opposed to standard care. Chronic blood transfusions decrease the risk of primary stroke compared with standard care. Adapted from Adams et al24 with permission.
Kaplan-Meier estimates of the probability of remaining stroke-free in patients with SCD receiving chronic blood transfusions as opposed to standard care. Chronic blood transfusions decrease the risk of primary stroke compared with standard care. Adapted from Adams et al24 with permission.
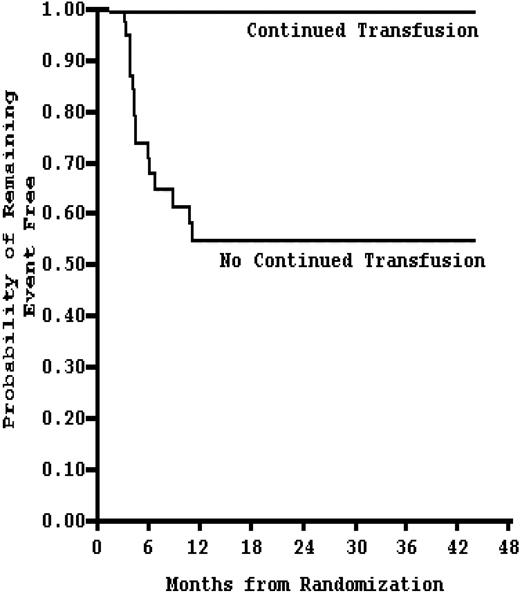
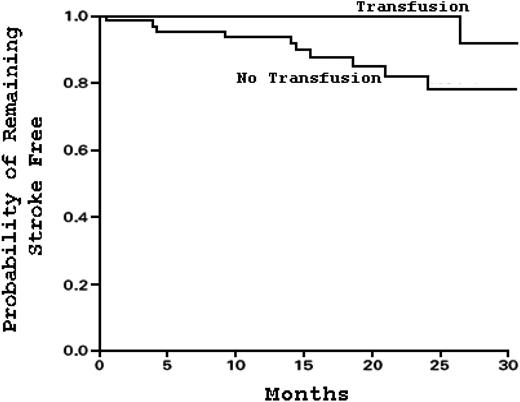
Kaplan-Meier estimates of the probability of remaining event-free (stroke or reversion to abnormal TCD velocity) in patients receiving continued blood transfusion (n = 41) compared with no continued transfusion (n = 38). Stopping chronic blood transfusion in SCD patients increases the risk of events within 12 months. Adapted from Adams and Brambilla20 with permission.
Kaplan-Meier estimates of the probability of remaining event-free (stroke or reversion to abnormal TCD velocity) in patients receiving continued blood transfusion (n = 41) compared with no continued transfusion (n = 38). Stopping chronic blood transfusion in SCD patients increases the risk of events within 12 months. Adapted from Adams and Brambilla20 with permission.
The consequent introduction of chronic transfusions for primary stroke prevention had an immediate impact on stroke rates in California. Only 2 years after the results of the STOP I trial were published, SCD stroke rates in the state of California dropped by a factor of 5.25
Transfusion complications
Even in the setting of optimal management, chronic blood transfusion management is complicated by alloimmunization and iron overload. Sixteen percent of transfused patients in the STOP I trial had treatment discontinued because of alloimmunization. Furthermore, ferritin levels rose by 1500% over 24 months in the transfusion group. This was probably because of the preferential use of simple transfusions, which accounted for 88% of all transfusions. Although exchange transfusions are iron neutral, they are technically more demanding and require more units of blood, which further increases the risk of alloimmunization. These limitations reduce enthusiasm for TCD screenings.26
Although iron chelation has been shown to reduce iron stores27-29 and reverse congestive cardiomyopathy resulting from iron overload,30 these results occur in only approximately half of the treated patients because compliance is quite poor resulting from discomfort, inconvenience, and cost.31-34 Indeed, mean serum ferritin levels are largely unchanged by the treatment. The use of an orally active iron chelator, such as deferasirox, may improve those poor results.
Transfusion: a Hobson's choice
Given the complication rate, if SCD patients are to undergo chronic blood transfusion, 2 critical questions must be answered: (1) Do elevated TCD values suffice to initiate chronic blood transfusions? (2) What are the consequences of discontinuing chronic transfusion?
In the STOP I trial, 15% of the children who had “abnormal” TCD results reverted to normal on follow-up imaging that occurred before randomization.24 It is unclear whether this variation in TCD results is intrinsic to the TCD machine, to operator variability, or to fluctuations in the hemodynamics of the cerebrovasculature of SCD patients. Nonetheless, in clinical practice, there is no established guideline for the appropriate length of time between confirmatory follow-up TCD evaluations. Although TCD has high sensitivity, ranging from 94% to 100%, its specificity is no better than the flip of a coin (51%).35 Consequently, an “abnormal” TCD value correctly predicts stroke only 36% of the time.11,35 TCD appears to serve as an excellent screening tool; but absent a confirmatory, high-specificity test, the thrust to institute chronic blood transfusions for primary stroke prevention in all SCD patients with “abnormal” TCD velocities remains controversial.
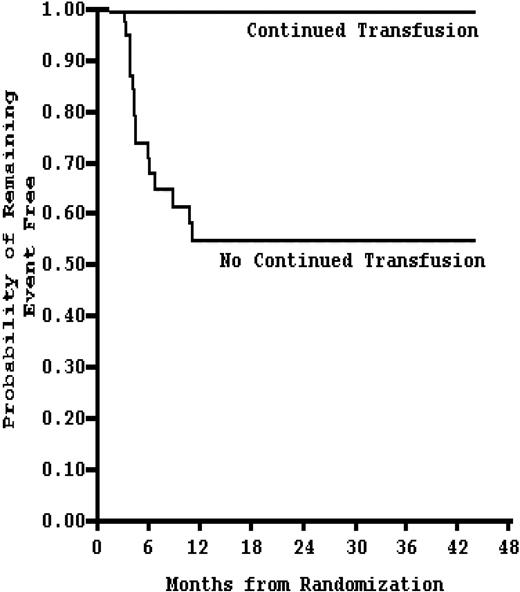
The STOP II trial20 (NCT00006182) evaluated the impact of cessation of chronic blood transfusions on stroke incidence. The endpoint, which was stroke or reversion to abnormal TCD with consequent need for transfusion, was achieved in 16 patients, all of whom were in the cessation group. Two of them had strokes. Unexpectedly, the endpoint was reached at a median time of only 3.2 months. Furthermore, in the STOP II trial, the probability of remaining stroke free at 30 months was only 50% in those who discontinued transfusions; whereas in the STOP I trial, the same probability was approximately 80% in the standard care group. This is particularly interesting given that the patients in the STOP II trial were older and did not include patients 2 to 5 years of age, the time frame with the highest risk of stroke development. Although speculative, the data suggest a rebound effect of increased stroke risk in the immediate period after cessation of transfusions, a rebound that may be suppressed by the use of HU therapy as noted by Ware et al.22,23
The HU alternative
In 1995, the Multicenter Study of Hydroxyurea in Sickle Cell Anemia trial36 (NCT00000586) showed that HU therapy increases total and fetal hemoglobin and decreases the incidence of acute chest syndrome and SCD pain crises. Although there was no difference in stroke incidence rates, this may have been the result of the age of the study population, all of whom were older than 18 years, an age range with a lower stroke risk among SCD patients. A follow-up study designed to examine the safety of HU in SCD children showed that HU therapy was well tolerated among patients 5 to 15 years of age with severe SCD.37 Furthermore, HU led to increased fetal hemoglobin. Given these findings and its relatively safe side effect profile, the role of HU for primary stroke prevention was examined 10 years later when a Belgian cohort study suggested that HU therapy is as efficacious as chronic blood transfusions.38 In this study of 34 patients with TCD velocities greater than 200 cm/s (and consequently at high risk of primary stroke risk by STOP I criteria), only 1 patient developed a stroke. Unlike the Multicenter Study of Hydroxyurea trial,36 the mean age of the patient population was 6 years, a high-risk population.
How might HU decrease stroke risk? Zimmerman et al39 demonstrated that HU therapy decreases TCD velocities and that such differences are greater when the baseline is higher; the latter presumably represents a higher risk for stroke. And, in a small case-control study,40 long-term HU therapy improved cerebral oxygen saturation, which was probably related to increased hemoglobin levels and lower total blood viscosity adjusted for Hct in the HU group. Lower TCD velocities may be associated with decreased turbulent flow and consequent endothelial damage around the stenosis, whereas improved oxygen saturation may raise the threshold for infarction by augmenting the oxygen reservoir. HU may also reduce the inflammatory component of vasculopathy41,42 by suppressing myelopoiesis. Finally, it is possible that HU therapy would be even more efficacious if patients were phlebotomized to reduce total blood viscosity still further.
Although HU seems to be a highly useful alternative with a relatively safe side effect profile,43 a randomized controlled trial to support its efficacy in preventing primary stroke has not been performed. The long-term effects of HU on the SCD hematopoietic system have not been completely evaluated. The fact that patients with polycythemia vera (PCV) treated with HU may have an increased risk of leukemia44,45 is probably not relevant because PCV is a myeloproliferative disease. No such risk has been detected in sickle cell anemia or secondary erythrocytoses.44,46
Thus far, only chronic blood transfusion has undergone the rigor of a randomized controlled trial to test its efficacy in primary stroke prevention. Although transfusions are efficacious, they carry significant risks, including alloimmunization and autoimmune hemolytic anemia, iron overload, and infection. Because TCD has a relatively low positive predictive value for stroke, the positive results of the STOP I trial must be weighed against the risks of transfusions.
Extended blood typing
The use of phenotypically matched blood for prevention of alloimmunization and autoimmunization and consequent hemolytic reactions is highly controversial. In the Cooperative Study of Sickle Cell Disease (CSSCD)47 (NCT00005277), the overall rate of alloimmunization in SCD patients was 18.6%, and this prevalence was directly related to the number of blood transfusions received. This rate is higher than that observed in patients with thalassemia,48-51 probably because in SCD the donor and recipient pools differ racially34 and because transfusions in thalassemia begin at an early age and are maintained. This contributes to immune paralysis. Consequently, the National Institutes of Health recommends that all SCD patients older than 6 months should have extensive antigenic phenotyping, defined as the following: ABO, Rh, Kell, Duffy, Kidd, Lewis, Lutheran, P, and MNS blood groups.52 However, these recommendations have not been universally adopted. In one study of 1182 North American laboratories, only 37% of community blood banks typed blood beyond ABO and Rh in SCD patients, and only one-fourth used the results to match donor red blood cells (RBCs) prophylactically.53 In contrast, 81% of academic centers54 performed full RBC phenotyping before transfusion, and 77% used that information to provide pretransfusion-matched RBCs, of which more than 90% were matched for the most commonly cited immunogenic antigens, C, E, and K.55-57 Nonetheless, only 25% of the academic centers surveyed provided phenotype data to their patients.54 Therefore, if a hypothetical patient were to be transfused at a hospital where extended blood typing is not regularly performed, all efforts to prevent alloimmunization in the previous years might be nullified.
What can we expect of extended phenotype matching? Castro et al58 determined that 29% of adult patients with SCD transfused with blood matched for only the ABO and D groups developed alloimmunization. Extrapolations suggest that phenotyping of C, c, E, e, and K antigens would reduce the alloimmunization rate by 50%, and an extra 25% reduction could be obtained if additional phenotyping was done beyond the C, E, and K antigens.58 However, it was also estimated that only 13.6% of the random white donor population could match for the C, E, and K antigens, and less than 1% of random white donors could match for other antigens in addition to C, E, and K (in this study, 90% of the donor pool was white). Clearly, efforts to extend the black donor pool will help patients with SCD.59
In the STOP I trial, matching for the C, E, and K antigens decreased the rate of alloimmunization by a factor of 6 per unit of exposure.60 Although extensive blood type matching can be challenging, blood transfusions containing antigens to which the donor is alloimmunized not only increases the risk of hemolytic reactions but also shortens the half-life of the infused RBCs61 ; consequently, additional transfusions are required to maintain a low HbS, which further increases the risk of iron overload, infection, and alloimmunization.
With respect to alloimmunization, is there an optimal time to begin chronic transfusions in SCD? This question may be somewhat irrelevant because transfusions are offered for episodic acute vasculopathy and vasoocclusion in SCD and generally not, as in thalassemia, for chronic anemia. The answer to the question is quite clear in thalassemia. Spanos et al showed that a Greek cohort of β-thalassemic patients who began regular chronic transfusions before the age of 3 years were less likely to develop alloantibodies.48 This conclusion was upheld within the cohort of patients with sickle-thalassemia who were undergoing periodic transfusions. Furthermore, a study62 comparing limited versus extended phenotype-matched blood found no difference in the rate of alloimmunization among thalassemic patients who began their first transfusion before 12 months of age. Beyond that age, however, the limited cohort group had a 20% absolute greater risk in developing alloimmunization. When both limited and extended patient cohorts were combined and analyzed, first transfusions begun before 12 months were almost 4 times less likely to be associated with alloantibodies. Although there are limited data for the SCD population, the CSSCD demonstrated that children who underwent their first blood transfusion before 10 years of age were 50% less likely to develop alloantibodies, even when corrected for total number of transfusions.47 This suggests that chronic transfusions begun in infancy or, perhaps, early childhood, are less likely to cause alloimmunization. By lowering the alloimmunization rate, the need for rare blood products is likely to fall, as currently, 33% of the requests to the American Rare Donor Program for RBC products are for alloimmunized patients with SCD.63
Functional limitations of the neonatal innate and adaptive immune systems may explain the lower rates of alloimmunization in early childhood.64 Studies show that neonatal monocytes have a decreased ability to produce proinflammatory cytokines65-67 and express lower levels of costimulatory molecules (CD40) that activate T cells.68 Furthermore, Takahata et al69 showed that cord blood contains many natural regulatory, CTLA-4–expressing T cells. These cells have an ability to suppress effector T cells after stimulation with mitogens and alloantigens. Importantly, the first year of infancy is a critical period of immune system development, as demonstrated by 2 studies showing that only children older than 1 year had T-cell responses comparable with adults after stimulation with alloantigens or vaccine antigens.70 These data suggest that initiation of transfusion during infancy may lead to lower rates of alloimmunization.
Are RBC alloantibodies and autoantibodies clinically significant? In a retrospective study of 78 pediatric SCD patients receiving a mean of 24 units of ABO- and D-matched blood, 23 (29%) and 6 (8%) developed alloantibodies and autoantibodies, respectively.71 In total, 7 patients (9%) developed delayed hemolytic or serologic transfusion reactions, and, among the patients with autoantibodies, 1 developed hyperhemolysis with the rest showing no evidence of hemolysis. The authors found that, except in the case of hyperhemolysis syndrome, which was not predictable in the study, all reactions were clinically neither serious nor life-threatening. In contrast, Castellino et al61 performed a review of 184 SCD patients receiving a median of 24 units of blood and found that 7.6% developed IgG autoantibodies. One-third of the patients demonstrated significant hemolysis (including 1 death).
The viscosity paradox
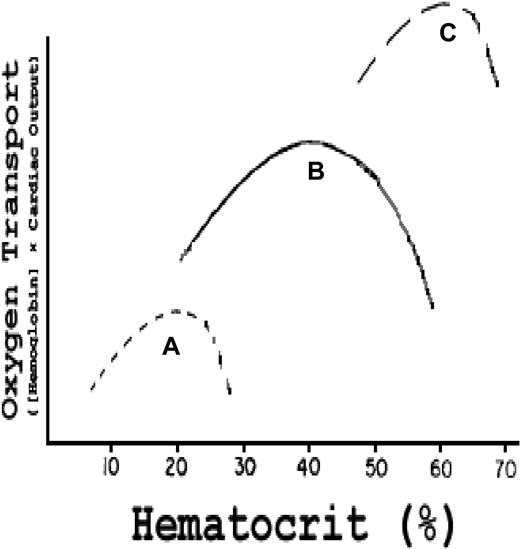
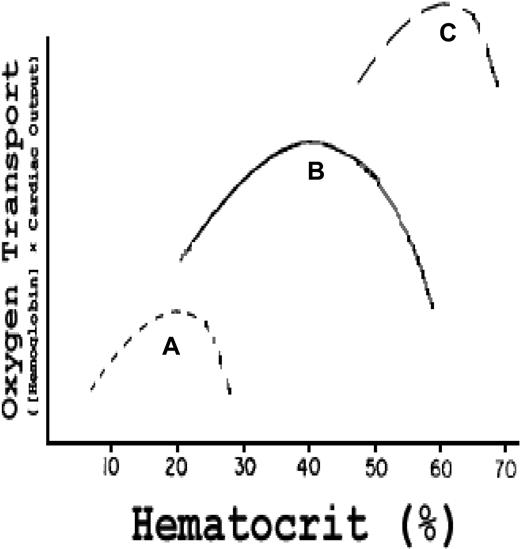
The role of blood viscosity is well established with respect to extra–central nervous system infarction crises in SCD9 and in primary and secondary polycythemia.72 The influence of Hct on oxygen transport was carefully analyzed by Richardson and Guyton,73 who demonstrated in an isovolumic canine model that the effect of Hct on oxygen transport describes a parabolic curve with a somewhat steeper negative slope above the peak Hct of 40% (Figure 6 curve B). That steeper negative slope is caused by a reduced cardiac output (CO) secondary to progressively increased peripheral resistance, the latter resulting from elevated blood viscosity.74-76 Canine RBCs, although smaller than human cells, have the same area-to-volume ratio77 and mean corpuscular hemoglobin concentration78 and, hence, similar deformability79,80 and internal viscosity. Therefore, hypothetical human red cell curves can be plotted on the same graph (Figure 6 curves A and C).
Effect of Hct on oxygen transport (hemoglobin concentration × CO) in vivo. Curve B (actual data): in normal dogs, maximum oxygen transport occurs at an Hct of 40%; curve C is a hypothetical curve observed in a human with established PCV; curve A is a hypothetical curve observed in a human with SCD. Modified from Richardson and Guyton73 with permission.
Effect of Hct on oxygen transport (hemoglobin concentration × CO) in vivo. Curve B (actual data): in normal dogs, maximum oxygen transport occurs at an Hct of 40%; curve C is a hypothetical curve observed in a human with established PCV; curve A is a hypothetical curve observed in a human with SCD. Modified from Richardson and Guyton73 with permission.
Figure 6 curve C represents a hypothetical patient with PCV and an elevated blood and, hence, stroke volume and CO. As derived from the studies of Cobb et al,72 the curve is shifted upward and to the right, but with a much steeper negative slope above the peak Hct because, at such very high viscosity and peripheral resistance, stroke volume and CO drop precipitously despite the high blood volume. The curve demonstrates that increments in blood volume can compensate in part for high viscosity.
Figure 6 curve A represents a hypothetical patient with SCD at the same relative blood volume as the normal “patient” in curve B. That curve is shifted downward and to the left, and the negative slope above the peak Hct is much steeper. This reflects the intrinsic elevated internal viscosity of sickle RBCs and their tendency to adhere to each other at low oxygen tensions producing higher total viscosity (and hence higher peripheral resistance) than normal RBCs at any given Hct.
The negative effect of rising Hct on oxygen transport is profound in SCD at least in its extra–central nervous system manifestations. Data derived from the CSSCD clearly established that high Hct (in the absence of α- or β-thalassemia trait) is a risk factor for vasoocclusive crisis, acute chest syndrome, and avascular necrosis, but, curiously, the reverse is true of stroke risk. In the latter case, anemia is a key risk factor.9 How can the effects of thalassemia be explained? Why does the central nervous system vasculature appear to obey different rules, and how do these rules affect transfusion policy?
The favorable effect of the concomitant thalassemia trait can be explained on a rheologic basis. Both α- and β-thalassemia have little, if any, effect on mean corpuscular hemoglobin concentration because a low hemoglobin content per cell is regularly associated with a reduced water and cation content, but they both induce a high area-to-volume ratio that improves the flow characteristics of red cells at high shear rates in large cerebral vessels and at low shear rates through endothelial cell channels. The high area-to-volume ratio also reduces cation losses and intracellular dehydration in deoxygenated SCD erythrocytes.81,82 One untoward result of the rheologic improvement is improved red cell life span and a concomitant rise in Hct, which may vitiate the benefit, particularly in the microvessels that are occluded and inflamed in extra–central nervous system crises.83
The paradoxical salutary effect of anemia on stroke risk may be more apparent than real. Stroke risk is highest in children between the ages of 2 and 5, ages at which aplastic crisis after viral infection is relatively frequent. Recovery from aplastic crisis simulates hemolytic anemia with reticulocytosis, thrombocytosis, and granulocytosis, all of which are probable risk factors for stroke in SCD. Adhesion molecule receptors are particularly enriched on reticulocytes.84 We suggest that all of these changes may endanger the already damaged cerebral vascular endothelial cells of SCD patients and provoke a complete occlusion. If these damaging factors were further complicated by an elevated Hct, stroke frequency would be even higher than it is.
The recent rheologic studies of Higgins et al85 may bear on the pathophysiology of the large-vessel damage that causes stroke in SCD. In an in vitro microfluidic model of the microcirculation, these investigators observed that, at an HbSS cell fraction greater than 65%, HbS polymerization alone is sufficient to halt blood flow without the contribution of coagulation proteins, platelets, or endothelium. However, their results also demonstrated that, at the flow velocities and pressures found in stenosed intracranial vessels, vaso-occlusion does not develop in this in vitro system, even in the setting of HbS polymerization. That finding emphasizes the importance of other factors, such as endothelial cell interactions and inflammation, in the development of large-vessel occlusion. The rheologic data reviewed here suggest that viscosity is more likely to play a direct role in the low shear rate conditions of the microvasculature, leading to vaso-occlusive disease, but may be indirect in the development of acute SCD stroke where the aforementioned inflammatory factors are probably involved.41,42 Indeed, postmortem samples of SCD patients with stenoses reveal proliferative intimal hyperplasia86-88 reminiscent of the inflammatory processes seen in atherosclerotic disease. Probably the return of anemia and its comcomitant cellular proliferation enhance the intimal damage and stroke risk observed soon after cessation of transfusion.
A very recent study by Wallace et al89 provides another potential stroke risk factor. SCD patients have very high levels of circulating iNKT cells. These cells readily exude toxic cytokines, such as Il-6, that attack endothelial cells and contribute to vasculopathy. The iNKT cell burden may explain why stroke risk is so very high in black SCD patients who are already at elevated risk on the basis of race alone.8
Although viscosity measured in vitro is enhanced by transfusion of normal red cells into an SCD patient unless the sickle red cell percentage is maintained below 30%, and preferably as low as 20%,90,91 the practical facts are that transfusion does improve the course of patients with stroke and extra–central nervous system manifestations of SCD. The task of clinical research is to accomplish equal clinical benefit without the use of red cell transfusion and its attendant complications.
Future outlook
Transfusion-based approaches
The belief that chronic transfusions begun in infancy lead to lower alloimmunization rates is appealing but has not been tested in SCD. Furthermore, there are no guidelines for TCD screening of children just under the age of 2, the critical time frame for stroke prevention strategies. Such guidelines need to be developed. The alternative (to give chronic transfusions to all SCD children when they turn 2 in a manner akin to transfusion-dependent children with thalassemia) is clearly distasteful because most SCD patients are not at risk of stroke and would incur unnecessary adverse side effects as a result of such a prophylactic policy. Whether genome-wide association studies might clearly identify children who would benefit from chronic transfusions at 2 years of age is a devout hope of some but unlikely to be productive because the risk factors detected in most genome-wide association studies are much too low to be useful.92,93
Two important trials will shed light on transfusion-induced dilemmas. The phase 3 Stroke with Transfusions Changing to Hydroxyurea trial, promulgated by Ware et al22,23 (NCT00122980), compares transfusion and chelation to HU and phlebotomy for the prevention of stroke recurrence and iron overload. Unfortunately, it excludes children aged 2 to 5, the period with the highest stroke risk in SCD. To this trial has been added the recently initiated TCD with Transfusions Changing to Hydroxyurea Trial. The Silent Cerebral Infarct Multicenter Transfusion trial (NCT00072761) will compare chronic transfusions to observation for the prevention of silent strokes in children with SCD. Although not a stated aim of the study, it will be valuable to assess the effect of simple versus exchange transfusions on the primary outcome. Simple transfusions increase viscosity, which probably plays a role in the development of small-vessel ischemia that is thought to generate silent infarcts.
Non–transfusion-based approaches
Although we have focused on red cell transfusions in this review, the future lies in transfusion reduction. Effective management of SCD and avoidance of RBC transfusions require a better approach to fetal hemoglobin stimulation in sickle erythroblasts than has been achieved thus far with HU.
Recent genome-wide association studies suggest that single nucleotide polymorphisms in the BCL11A gene modulate fetal hemoglobin levels,94,95 and a study by Lettre et al96 additionally demonstrated that hemoglobin F–associated single nucleotide polymorphisms are correlated with pain crisis rates in SCD patients. BCL11A may be a unique suppressor of γ chain synthesis.97,98 Conceivably, modulation of BCL11A and the proteins associated with it may provide a therapeutic approach to SCD, including stroke prevention.
Statins are anti-inflammatory97,99 drugs that reduce activated, cholesterol-laden macrophages within the blood vessel intima. Moreover, studies show that statins prevent damage to blood vessels independently of their cholesterol-lowering effect.100 In a SCD mouse model—mice are hypocholesterolemic animals—Solovey et al101 demonstrated that lovastatin inhibits endothelial cell expression of tissue factor. A phase 1/2 study called Simvastatin Therapy in Sickle Cell Disease (NCT00508027) using simvastatin in patients with SCD is under way and will evaluate the effect of simvastatin on biomarkers of vasoreactivity, endothelial adhesion, and inflammation.
Surely, other approaches to control inflammation in SCD, such as engagement of adenosine receptors on iNKT cells,102-106 will also prove to be productive. Interestingly, a recent study shows that statins inhibit CD1d-mediated antigen presentation,99 a human leukocyte antigen–like molecule used by antigen-presenting cells to present lipid antigens to invariant NKT cells.107
In small studies,108,109 bone marrow transplantation (BMT) has proven to cure SCD and might be expected to stop progression of cerebral disease. However, strokes have been noted after BMT,110 and standard BMT is limited by a requirement for human leukocyte antigen–matched sibling donors,108,111 and it requires acutely toxic myeloablative therapy. Furthermore, BMT can be complicated by graft-versus-host disease.109,112 The results of the Sickle Cell Unrelated Transplant Study (NCT00745420), which will evaluate the safety and efficacy of either BMT or umbilical cord transplantations from unrelated donors in children with severe SCD who receive a reduced-intensity conditioning regimen before transplantation,113 may provide critical information about whether such reduced-intensity BMT is a feasible and safe alternative.
In conclusion, flagrant ischemic or hemorrhagic stroke is a terrible consequence of SCD, affecting one-fourth of patients by the age of 45 years. Unfortunately, the incidence of stroke peaks between 2 and 5 years of age, blighting childhood. Chronic but silent cerebral infarcts also accumulate in these patients and may well inhibit intellectual performance.114 Stroke prevention should be the highest priority of a national SCD program.
In an effort to prevent stroke, SCD programs have adopted TCD to select patients for chronic RBC transfusions. Such transfusions do prevent many strokes, but the TCD is highly nonspecific, and transfusion is fraught with risks of iron overload, blood group antigen sensitization, and unknown future infections. New chelating agents and improved matching alleviate some of these complications, but chronic transfusions remain a very unwelcome option. Furthermore, transfusion is not a simple solution because blood viscosity is high in SCD even after sickle cells are diluted by transfusion. Indeed, Hct is inversely proportional to red cell flow at any sickle cell fraction more than 20%.
HU treatment, perhaps accompanied by phlebotomy in some cases, may represent a better option than transfusion for stroke prevention. A trial is under way to make that determination. HU may be effective because it increases RBC fetal hemoglobin, but the drug may also inhibit phagocyte production and thereby reduce the inflammatory as well as the obstructive result of sickle cell adherence to endothelial cells. Nonphagocytic inflammatory cells, such as iNKT cells, have recently been implicated in SCD.89 Whether they are impacted by HU is currently unknown. In any case, stroke-causing large cerebral vessel disease is probably the result of vessel wall inflammation in addition to sickle cell adhesion. Statins and other approaches to inflammatory blood vessel disease may be highly relevant in the search for prevention of this forbidding complication, but the most promising long-term solution to this and other manifestations of sickle cell anemia lies in the control of fetal hemoglobin gene expression. Fortunately, progress is finally being made in this critical area.
Acknowledgments
The authors thank Drs Luigi Notarangelo, John Higgins, and H. Franklin Bunn for their critical review of the manuscript and Drs Elliot Vichinsky, Steven Sloan, Ellis Reinherz, Orah Platt, Ronald McCaffrey, and Judith Lin for their suggestions and encouragement.
Authorship
Contribution: L.A.V. designed the review; and L.A.V. and D.G.N. analyzed the literature, wrote and edited the manuscript, and modified the figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David G. Nathan, Dana 1644, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: david_nathan@dfci.harvard.edu.