In this issue of Blood, there are 2 reports on the increased risk of plasma cell disorders in the first-degree relatives of patients with multiple myeloma or MGUS.
Very little is known about familial/hereditary forms of multiple myeloma (MM) and monoclonal gammopathy of uncertain significance (MGUS).1-3 We recently described extensive familial phenotypic heterogeneity in 39 families with multiple cases of MM and MGUS wherein a subset showed a variety of hematologic and solid tumors that may well be integral to the familial clustering of MM.4 This subset consisted of cancer of the prostate, cancer of the pancreas, acute myelogenous leukemia, and myelofibrosis. It is important to realize that these heuristic findings are preliminary and will require many more MM families for a more complete assessment. In this issue of Blood, there is a well-conducted Swedish registry study reported by Landgren and colleagues that expands upon a growing data set of the familial associations of MGUS and MM.5
The rarity of familial MM has been a major problem in the understanding of MM's genetics and its heterogeneity. Physicians failing to take a comprehensive family history of MGUS, MM, and all cancers in the family further compound this problem. A case-finding approach has been used to address this limitation.2
In their literature review, Landgren et al noted the occurrence of case reports that have shown familial clusters of MGUS and MM2-4,6 in addition to other more rarely occurring syndromes that may be associated with MGUS.7 Recent population-based studies have identified an excess of MGUS among first-degree relatives of MM or lymphoplasmacytic (LPL/WM) patients.8 Landgren et al noted the absence of any large study quantifying risk for MGUS and related malignancies among first-degree relatives of individuals with MGUS. Therefore, they launched a study to characterize the pattern of familial disease.
A cohort of 4458 MGUS cases and 14 621 linkable first-degree relatives were compared to 17 505 matched controls with their linkable first degree relatives (n = 58 387) for plasma-cell and lymphoproliferative disorders.
The relatives of IgM MGUS patients had a 5.0-fold (1.1-23) increased risk of CLL, and risks for MM and LPL/WM were increased but did not achieve statistical significance. When they assessed risk by relation to the proband, age above or below 65 years at diagnosis of MGUS, or sex, results were quite similar. The risk of non-Hodgkin lymphoma (NHL) or Hodgkin lymphoma (HL) was not increased among MGUS relatives. In the study of first-degree relatives of MGUS patients, these investigators observed elevated risks of MGUS, MM, LPL/WM, and CLL, which they believe supports the role for germline susceptibility genes, in addition to the potential effect of shared environmental influences and/or their interaction with genetic susceptibility factors.
Landgren et al note that they are describing for the first time “… a critically and statistically significant increase in the prevalence of MGUS among first-degree relatives of patients with MGUS or MM compared with a reference population, using identical screening and diagnostic techniques.” They note that the first-degree family members of an MGUS or MM case show at least a 2-fold greater risk of MGUS when compared with population rates. For example, in considering absolute terms, the presence of MGUS when employed with the standard electrophoretic techniques, in first-degree relatives 80 years and older, is 13% compared with 7% in a reference population. This increase of MGUS in their investigation was found across both genders as well as in all ages of relatives 40 and older. Therefore, their findings provide valuable evidence of a familial aggregation of MGUS and MM, and certainly answers the concept that shared genes and their environment are crucial for development of MM or MGUS among relatives.
Recently in this journal, Kristinsson et al investigated genetic factors in the etiology of lymphoplasmacytic lymphoma/Waldenström's macroglobulinimia (LPL/WM).8 They identified 2144 LPL/WM patients (1539 WM [72%] and 605 LPL [28%]) who were diagnosed in Sweden, 8379 matched controls and their linkable first-degree relatives (n = 6177 for the patient group and n = 24 609 for the control group). The first-degree relatives of LPL/WM patients were found “… to have 20-fold (4.1-98.4), 3.0-fold (2.0-4.4), 3.4-fold (1.7-6.6) and 5.0-fold (1.3-18.9) increased risk of developing LPL/WM, non-Hodgkin lymphoma (NHL), chronic lymphocytic leukemia (CLL), and MGUS, respectively. However, there was no evidence of an increased risk of developing multiple myeloma or Hodgkin lymphoma.”8 They concluded that their findings of highly increased risk of developing “… LPL/WM, NHL, CLL, and MGUS support the operation of shared susceptibility genes that predispose to LPL/WM and other lymphoproliferative disorders.”8
Also in this issue, Vachon and colleagues found, perhaps for the first time, a clinically and statistically significant increase in the prevalence of MGUS by screening first-degree relatives of patients with MGUS or multiple myeloma when compared with a well-characterized reference population.9 First-degree family members of an MGUS- or MM-affected individual have at least a 2-fold greater risk of MGUS when compared with general population rates. These findings support a heritable genetic predisposition for the occurrence of MGUS.
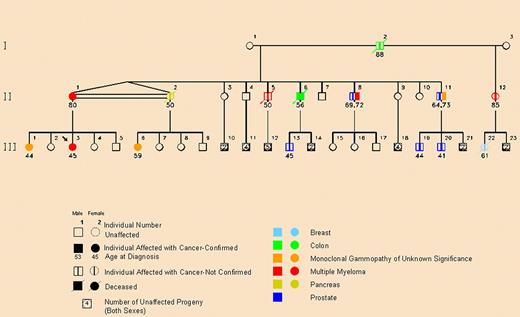
We have recently published a study on an African American family prone to myeloma with an association with prostate cancer (see figure).2 Noteworthy and consistent with some of our other observations is the fact that multiple cases of MGUS and prostate cancer as well as a smaller number of colon and pancreas cancers occurred in the direct lineage of MM-affected family members. In a subsequent New England Journal of Medicine issue, we responded to a group from Utah that showed evidence that prostate cancer may well be integral to the spectrum of familial MM.12,13
Pedigree showing family members with multiple myeloma or monoclonal gammopathy of undetermined significance (MGUS) in a pattern consistent with autosomal dominant transmission. The pedigree shows a decrease in the age of onset of prostate cancer, MGUS, and multiple myeloma from generation II to generation III. The arrow indicates the proband. The double horizontal line between family members II-1 and II-2 indicates that they are identical twins. Reprinted from Lynch et al2 with permission.
Pedigree showing family members with multiple myeloma or monoclonal gammopathy of undetermined significance (MGUS) in a pattern consistent with autosomal dominant transmission. The pedigree shows a decrease in the age of onset of prostate cancer, MGUS, and multiple myeloma from generation II to generation III. The arrow indicates the proband. The double horizontal line between family members II-1 and II-2 indicates that they are identical twins. Reprinted from Lynch et al2 with permission.
What does this mean clinically? First, MGUS is not rare. It affects more than 3% of the general population over the age of 50 and harbors a lifelong increased risk of multiple myeloma or related malignancy. However, what kind of related malignancy might be involved? In hereditary cancer research, it is always essential to examine cancer of all anatomic sites in a family because the pattern of cancer may aid significantly in a hereditary cancer syndrome diagnosis. Clearly, it will also indicate the need to screen and manage those cancers that constitute a particular hereditary syndrome. As mentioned, our research on multiple myeloma has disclosed differing solid tumors and hematologic malignancies, suggesting marked heterogeneity in the genetics of this disease.4 For example, we identified prostate cancer occurring in apparent excess in association with MGUS and multiple myeloma in an African American family.2
Evidence has emerged supporting the existence of a genetic predisposition that affects the incidence of monoclonal gammopathies,3,4,7,10 which is reinforced by findings in multigenerational families wherein multiple cases of MM and MGUS favored the possibility of a shared cancer-susceptibility locus.11 These studies of MM-prone families indicate that MGUS is perhaps the single best marker of a putative hereditary family, particularly when it segregates with cancer.2,4
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■