Antigenic stimulation through the B-cell antigen receptor (BCR) is considered to promote the expansion of chronic lymphocytic leukemia (CLL) B cells. The spleen tyrosine kinase (Syk), a key component of BCR signaling, can be blocked by R406, a small-molecule Syk inhibitor, that displayed activity in CLL patients in a first clinical trial. In this study, we investigated the effects of BCR stimulation and R406 on CLL cell survival and migration. The prosurvival effects promoted by anti-IgM stimulation and nurselike cells were abrogated by R406. BCR triggering up-regulated adhesion molecules, and increased CLL cell migration toward the chemokines CXCL12 and CXCL13. BCR activation also enhanced CLL cell migration beneath marrow stromal cells. These responses were blocked by R406, which furthermore abrogated BCR-dependent secretion of T-cell chemokines (CCL3 and CCL4) by CLL cells. Finally, R406 inhibited constitutive and BCR-induced activation of Syk, extracellular signal-regulated kinases, and AKT, and blocked BCR-induced calcium mobilization. These findings suggest that BCR activation favors CLL cell homing, retention, and survival in tissue microenvironments. R406 effectively blocks these BCR-dependent responses in CLL cells, providing an explanation for the activity of R406 in patients with CLL.
Introduction
B-cell chronic lymphocytic leukemia (CLL) is a lymphoproliferative disorder with a highly variable clinical course, characterized by the clonal expansion of mature, antigen-stimulated CD5+/CD23+ B cells in blood, secondary lymphoid tissues, and the bone marrow.1 In CLL, most of the circulating cells are arrested in the G0/G1 phase of the cell cycle, and express high levels of antiapoptotic proteins.2 CLL therefore has been characterized as a process of defective apoptosis, rather than increased proliferation. In contrast, it is also known that a distinct fraction of CLL cells proliferates in vivo,3 which is thought to occur in tissue microenvironments, such as the lymphatic tissues and the marrow, where CLL cells can receive survival and growth signals from accessory cells that constitute the leukemia microenvironment. Despite their apparent longevity in vivo, CLL cells undergo spontaneous apoptosis in vitro, unless they are cocultured with accessory cells, collectively referred to as stromal cells. Nurselike cells (NLC),4,5 marrow stromal cells (MSC),6,–8 and follicular dendritic cells9 are examples of such stromal cells, and they protect CLL cells from spontaneous and drug-induced apoptosis in a contact-dependent fashion. Within the tissue compartments, microanatomical structures called pseudofollicles or proliferation centers, a hallmark finding in CLL pathology, are found. These proliferation centers are characterized by focal aggregates of activated, larger prolymphocytes and paraimmunoblasts, some of which express the proliferation marker Ki-67.10,–12 There is growing in vitro13,14 and in vivo15,16 evidence suggesting that chemokine receptors and adhesion molecules expressed on CLL cells are responsible for their trafficking between the blood and the tissues, and their homing and/or retention within these tissue microenvironments, guided by accessory cells that establish gradients of the respective ligands. Once CLL cells are in the appropriate microenvironment, B-cell antigen receptors may become engaged by microbial or autoantigens,1,17,18 which, along with other costimulatory signals, promote the expansion of the CLL clone.
Several lines of evidence suggest that antigen stimulation and B-cell receptor (BCR)–derived signals play a critical role in pathogenesis and prognosis of CLL (reviewed by Chiorazzi et al1 and Stevenson and Caligaris-Cappio19 ). First, the prognosis of CLL patients correlates with the amount of somatic mutations in the variable regions of the BCR.20,21 Second, CLL cells express a restricted set of BCR with immunoglobulin (Ig) heavy chain variable (V) gene sequences that are identical or stereotyped in subsets of patients,22,,–25 suggesting that these BCRs bind similar antigens that are relevant to the pathogenesis of CLL. Third, Ig-unmutated and/or ζ-associated protein-70 (ZAP-70)–positive patients respond preferentially to BCR stimulation26,27 and display gene expression profiles suggesting activation downstream of the BCR.28
BCR signaling is a complex process. The BCR is composed of an antigen-specific membrane Ig paired with Ig-α/Ig-β heterodimers (CD79α/CD79β). Engagement of BCRs by antigen induces phosphorylation of immunoreceptor tyrosine-based activation motifs in the cytoplasmatic tails of Ig-α and Ig-β,29 with subsequent recruitment of spleen tyrosine kinase (Syk) to BCR microclusters.30 Syk belongs to the Syk/ZAP-70 family of nonreceptor kinases, and is characterized by 2 N-terminal Src homolog 2 domains and a C-terminal kinase domain. Via the Src homolog 2 domains, Syk binds to activated immunoreceptor tyrosine-based activation motifs. Alternatively, Syk can also become activated by Src family kinases, or by autophosphorylation.31 Upon its phosphorylation, Syk propagates BCR-derived signals by activating downstream signaling pathways, including calcium mobilization and activation of AKT kinase, extracellular signal-related kinase 1/2 (ERK1/2, also called p44/42 mitogen-activated protein kinase), and myeloid cell leukemia-1.32,33 Syk-deficient mice display a severe defect of B lymphopoiesis, with a block at the pro-B to pre-B transition, consistent with a key role for Syk in pre-BCR signaling.34,35 Moreover, animal models demonstrated that Syk is critical for survival and maintenance of mature normal34 and malignant36 B cells. Besides their role in immune responses, BCR37 and Syk38,39 activation also modulates cell adhesion and chemotaxis of normal cells, such as B cells. In normal B cells, BCR engagement modulates the responsiveness to several chemokines,40 including CXC chemokine ligand (CXCL)12,37,41 suggesting that BCR and Syk participate in navigating and confining B cells within the lymphatic tissue microenvironment.
The aim of the present study was to investigate the role of BCR signaling in modulating migratory and survival responses in CLL cells. Furthermore, we tested the efficacy of a specific, adenosine triphosphate-competitive Syk inhibitor, R406,42,43 to abrogate the effects of BCR stimulation in CLL cells.
Methods
Cell purification
After informed consent, peripheral blood samples were obtained from patients fulfilling diagnostic and immunophenotypic criteria for B-cell CLL at the Leukemia Department of the M. D. Anderson Cancer Center. Patient consent for samples used in this study was obtained in accordance with the Declaration of Helsinki on protocols that were reviewed and approved by the Institutional Review Board at M. D. Anderson Cancer Center. The patients' characteristics are summarized in supplemental Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Peripheral blood mononuclear cells (PBMCs) were isolated via density gradient centrifugation over Ficoll-Paque (GE Healthcare) and used fresh or viably frozen in fetal bovine serum (SAFC Biosciences) plus 10% dimethylsulfoxide (DMSO; Sigma-Aldrich) for storage in liquid nitrogen.
Flow cytometry
The expression of surface molecules was analyzed by flow cytometry, using the following monoclonal antibodies (mAbs): CD5-fluorescein isothiocyanate (FITC), CD19-allophycocyanin, CD38-phycoerythrin (PE), CD40-FITC, CD44-FITC, CD49d-PE, CD54-PE, CD62L-FITC, CD80-FITC, CD86-FITC, CD183-PE (CXC chemokine receptor [CXCR]3), CD184-PE (CXCR4), CD185-PE (CXCR5), CD197-PE (CCR7), and the relevant isotype control mAbs, purchased from BD Biosciences or from R&D Systems (anti-CXCR5). A total of 106 mononuclear cells was incubated for 30 minutes at 4°C with saturating concentrations of mAbs, and the cells were washed twice and analyzed on a FACSCalibur (BD Biosciences).
NLC cocultures
For coculture with NLCs, PBMCs from patients with CLL were suspended in complete RPMI medium with 10% fetal bovine serum and penicillin-streptomycin-glutamine (HyClone) to a concentration of 107 cells/mL (total 2 mL) and incubated for 14 days in 24-well plates (Techno Plastic Products), as previously described.4
Stimulation of BCR on CLL cells: cell viability, expression of chemokine receptors and adhesion molecules, and CCL3 and CCL4 protein detection
To evaluate the effect of BCR stimulation on CLL cell viability, CLL cells (107/mL) were incubated in complete RPMI medium (control) or in complete medium supplemented with 10 μg/mL anti-IgM (polyclonal goat F(ab′)2 fragments to human IgM [MP Biomedicals] at 37°C in 5% CO2). After 24 and 48 hours, CLL cell viability was measured by analysis of mitochondrial transmembrane potential by 3,3 dihexyloxocarbocyanine iodine (DiOC6; Invitrogen-Molecular Probes) and cell membrane permeability to propidium iodide (PI; Molecular Probes), as described.13 Briefly, 200 μL cell suspension was collected at the indicated time points and transferred to fluorescence-activated cell sorter tubes containing 200 μL of 60 nM DiOC6 and 2 μg/mL PI in RPMI with 0.5% bovine serum albumin. Cells were then incubated at 37°C for 15 minutes and analyzed within 30 minutes by flow cytometry on a FACSCalibur (BD Biosciences). To evaluate the changes in expression of chemokine receptors and adhesion molecules, CLL cells were incubated with 10 μg/mL anti-IgM or control medium, and after 48 hours, cells were stained with saturating concentration of mAbs. Expression of adhesion molecules and chemokine receptors on CLL cells was determined by costaining with allophycocyanin-labeled anti-CD19 mAbs, and gating on the CD19+ cells. To determine the effect of BCR engagement and Syk inhibition on the induction of C-C chemokine ligand (CCL)3 and CCL4 protein secretion by CLL cells, CLL cells were stimulated for 24 hours with 10 μg/mL anti-IgM. Then supernatants were removed and assayed for CCL3 and CCL4 protein by quantitative enzyme-linked immunosorbent assay (R&D Systems), as described.44
The Syk inhibitor R406
R406 was provided by Rigel Pharmaceuticals. R788 (fostamatinib disodium), the clinically used oral formulation of R406, is a prodrug that is rapidly converted to R406 in vivo.42,45 Previous studies established that R406 is a relatively selective Syk inhibitor, although R406 also displayed (lower) activity against Flt3, Jak, and Lck.42 In vivo, R406 plasma levels are dose dependent; volunteers displayed plasma concentration of R406 between 1 μg/mL and more than 5 μg/mL after a single dose of R406. Weinblatt et al reported average steady-state R406 plasma concentrations of 1.5 μg/mL in a rheumatoid arthritis trial in patients treated with 150 mg of R877 by mouth twice daily.45 A 10 mM stock solution of R406, stored in aliquots at −80°C, was diluted in DMSO and added to the assay media to a final concentration of 5 μM or as specified. To determine the efficacy of R406 to antagonize BCR-derived survival signals, CLL samples (107 cells/mL) were preincubated in complete medium with R406 or carrier (1% DMSO). After 30 minutes of preincubation at 37°C, samples were incubated with or without 10 μg/mL anti-IgM for an additional 24 or 48 hours. Cell viability of CLL cells either in suspension or in coculture with NLC, expression of surface molecules, chemotactic response toward CXCL12 and CXCL13, pseudoemperipolesis, and phosphorylation of proteins downstream of the BCR were evaluated at indicated time points, and compared with controls incubated in complete medium, supplemented with carrier (1% DMSO).
Chemotaxis assay
The chemotaxis assay across polycarbonate Transwell inserts was performed as described.13 Briefly, 15 CLL samples (107 cells/mL) were incubated in complete RPMI medium (control) or in complete medium supplemented with 10 μg/mL anti-IgM at 37°C in 5% CO2. After 48 hours, CLL cells were suspended to a concentration of 107 cells/mL in RPMI 1640 with 0.5% bovine serum albumin, and a total of 100 μL, containing 106 cells, was added to the top chamber of a Transwell culture insert (Costar) with a diameter of 6.5 mm and a pore size of 5 μm. Filters then were transferred to wells containing medium with or without 200 ng/mL CXCL12 (Upstate Biotechnology) or 1 μg/mL CXCL13 (R&D Systems). The chambers were incubated for 3 hours at 37°C in 5% CO2. After this incubation, the cells in the lower chamber were suspended and divided into aliquots for counting with a FACSCalibur for 20 seconds at 60 μL/min in duplicates. A 1/20 dilution of input cells was counted under the same conditions.
Migration assay beneath marrow stromal cells (pseudoemperipolesis)
To evaluate the impact of BCR stimulation on migration of CLL cells beneath marrow stromal cells, we assayed for CLL cell pseudoemperipolesis, as described.13 Briefly, the murine stromal cell line M2-10B4 was seeded the day before the assay onto collagen-coated 12-well plates at a concentration of 1.8 × 105 cells/well in RPMI supplemented with 10% fetal calf serum and penicillin-streptomycin-glutamine. CLL cells from 6 patients were suspended at a concentration 107 cells/mL in medium and were incubated at 37°C in 5% CO2 in the following conditions: complete medium (control) and complete medium supplemented with 10 μg/mL anti-IgM, with or without 5 μM R406 for 30 minutes before addition of 10 μg/mL anti-IgM. After overnight incubation, CLL cells were counted and suspended to a concentration 107 cells/mL and added to the stromal cell layers. The plates were incubated at 37°C in 5% CO2. After 4 hours of incubation, cells that had not migrated into the stromal cell layer were removed by vigorously washing with RPMI medium 3 times. The complete removal of nonmigrated cells and the integrity of the stromal cell layer containing transmigrated cells were assessed by phase-contrast microscopy and documented photographically. The stromal cell layer containing transmigrated cells was detached by incubation for 1 minute with trypsin/EDTA (ethylenediaminetetraacetic acid) solution prewarmed to 37°C (GIBCO-BRL). Cells were then immediately suspended by adding 1 mL of ice-cold RPMI/10% fetal calf serum, washed, and suspended in 0.5 mL of cold medium for counting by flow cytometry for 20 seconds at 60 μL/min in duplicates. A lymphocyte gate was set according to the different relative size and granularity (forward scatter and side scatter) characteristics to exclude stromal cells from the counts. The number of migrated cells under each condition was expressed as percentage of the control.
Measurement of intracellular calcium mobilization
Calcium mobilization was measured by using the fluorogenic probe Fluo3-AM (Invitrogen).46 A total of 107 CLL PBMCs in complete RPMI 1640 was incubated with 4 μM Fluo3-AM (Invitrogen) and 0.02% Pluronic F-127 (Sigma-Aldrich) for 30 minutes at 37°C. Cells were then resuspended at 5 × 106 cells/mL and incubated for additional 10 minutes at 37°C to allow complete de-esterification of intracellular AM esters. Cells were then washed and resuspended at 5 × 106 cells/mL for further preincubation with or without 5 μM R406 for additional 30 minutes at 37°C. A total of 2 × 105 cells was then resuspended in 500 μL and warmed to 37°C for 5 minutes before acquisition. Anti-human IgM (10 μg/mL) was added to samples 20 seconds after acquisition of background fluorescence, and data acquisition continued up to 180 seconds on a BD FACSCalibur. The same procedure was performed for samples preincubated with or without R406. To confirm that the absence of anti-IgM–induced fluxes observed after treatment with R406 was not due to technical issues, these samples were subsequently treated with 2 μM ionomycin (Sigma-Aldrich), which elicited robust calcium fluxes in all cases. Data were analyzed using FlowJo software, version 8.5.2 (TreeStar). To calculate the percentage of cells that exhibited increased fluorescence after addition of anti-IgM, we established a background fluorescence threshold (T) for each sample at the fluorescence intensity of the 85th percentile of unstimulated cells.
Western blotting
For immunoblot assays, cells were lysed on ice for 30 minutes in lysis buffer containing 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 300 mM NaCl, 1.5 mM MgCl2, 0.5% sodium deoxycholate, 20 mM glycerophosphate, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 0.2 mM EDTA, 0.5 mM dithiothreitol, 1 mM sodium orthovanadate, and protease inhibitor. Cells were centrifuge at 14 000g for 15 minutes at 4°C, and supernatant was stored at −80°C until use. Protein content was determined using the detergent-compatible protein assay kit, according to manufacturer's instructions (Bio-Rad). Aliquots (25 μg) of total cell protein were boiled with Laemmli sample buffer and loaded onto 8% to 12% sodium dodecyl sulfate–polyacrylamide gels and transferred to nitrocellulose membranes (GE Osmonics Labstore). Membranes were blocked for 1 hour in PBS-Tween containing 5% nonfat dried milk and then incubated with primary antibodies either overnight or for 2 hours, followed by species-specific horseradish peroxidase-conjugated secondary antibody (diluted 1/5000) for 1 hour. The blots were visualized by enhanced chemiluminescence, according to manufacturer's instructions (Pierce Biotechnology), and normalized to the actin levels in each extract. Membranes were probed at 4°C with the following primary antibodies: p-ZAP-70Tyr319, p-SykTyr352, p-ERKThr202/Tyr204, p-AKTSer473, Syk, ERK, and AKT (Cell Signaling Technology). To quantify the results, we measured the protein levels using Odyssey Infrared Imaging system (LI-COR). Results were expressed as ratio of total protein and normalized to the corresponding control.
Data analysis and statistics
Results are shown as mean plus or minus standard error of the mean (SEM). For statistical comparison between groups, the Student paired t test was used. Analyses were performed using GraphPad Prism 4 software for Macintosh (GraphPad Software Inc). Flow cytometry data were analyzed using FlowJo software (TreeStar).
Results
BCR triggering increases CLL cell viability, and R406 abrogates BCR-derived survival signals
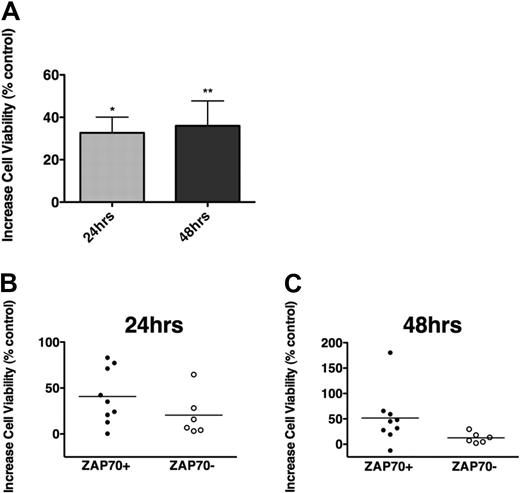
Cross-linking the BCR with anti-IgM triggers BCR activation; although not all CLL samples appear to respond, there was a preferential response to anti-IgM in nonmutated and ZAP-70+ cases.26,47 In our CLL samples, we noticed low (n = 3) and moderate increases (n = 11) in viability after 24 and 48 hours of stimulation with soluble anti-IgM, and induction of apoptosis only in one case (87.6% of viability at 48 hours), compared with controls. As depicted in Figure 1A, the mean increase in CLL cell viability after 24 hours was 32.7% plus or minus 7.2%, and 36.0% plus or minus 11.8% after 48 hours (mean ± SEM, n = 15). The induction of survival was higher in ZAP-70–positive (n = 9) than in ZAP-70–negative samples (n = 6), although the difference was not statistically significant (Figure 1B-C). A similar trend was noticed for unmutated and for CD38+ CLL cases (supplemental Figure 4).
BCR triggering protects CLL cells from spontaneous apoptosis. (A) The bars display increases in mean relative viability of CLL cells cultured in medium supplemented with 10 μg/mL anti-IgM, when assessed after 24 and 48 hours, and compared with CLL cells cultured in medium without anti-IgM. Displayed are the mean (± SEM) viabilities of 15 different CLL patient samples, and the asterisks indicate significant increases in viability with P < .05. (B-C) Increased viability of CLL cells after culture with anti-IgM in the subsets of ZAP-70–positive (●, n = 9) or ZAP-70–negative CLL samples (○, n = 6). After 24 (B) and 48 hours (C) of stimulation with anti-IgM, ZAP-70–positive CLL cells displayed higher levels of cell viability than ZAP-70–negative cells; however, these differences did not reach statistical significance.
BCR triggering protects CLL cells from spontaneous apoptosis. (A) The bars display increases in mean relative viability of CLL cells cultured in medium supplemented with 10 μg/mL anti-IgM, when assessed after 24 and 48 hours, and compared with CLL cells cultured in medium without anti-IgM. Displayed are the mean (± SEM) viabilities of 15 different CLL patient samples, and the asterisks indicate significant increases in viability with P < .05. (B-C) Increased viability of CLL cells after culture with anti-IgM in the subsets of ZAP-70–positive (●, n = 9) or ZAP-70–negative CLL samples (○, n = 6). After 24 (B) and 48 hours (C) of stimulation with anti-IgM, ZAP-70–positive CLL cells displayed higher levels of cell viability than ZAP-70–negative cells; however, these differences did not reach statistical significance.
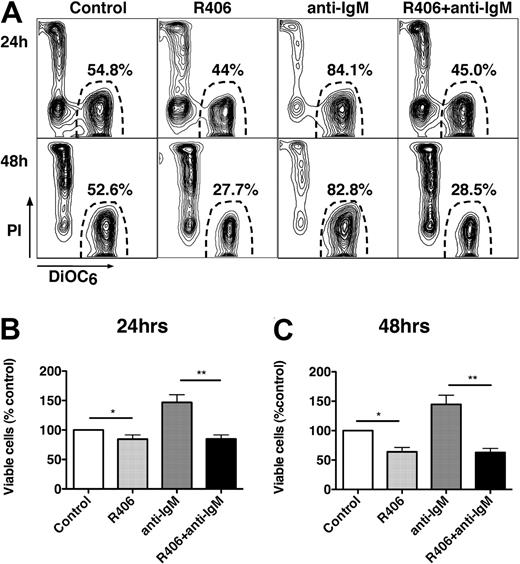
To determine whether R406 could inhibit BCR-derived prosurvival signals, CLL samples were cultured in complete medium (+1% DMSO), complete medium supplemented with anti-IgM, or medium supplemented with R406 with or without anti-IgM. Figure 2A displays the CLL cell viability of a representative case. As shown in Figure 2B and C, CLL cells stimulated with anti-IgM displayed an increased viability of 146.9% plus or minus 13.1% (24 hours) and 144.6% plus or minus 13.1% (48 hours) of respective controls (mean ± SEM, n = 11). Treatment with R406 plus anti-IgM abrogated the prosurvival effect of anti-IgM, leading to a reduced viability of 84.8% plus or minus 6.8% and 62.7% plus or minus 6.9% at 24 and 48 hours, respectively. A similar reduction in CLL cell viability was observed with R406 alone.
The Syk inhibitor R406 induces CLL cell apoptosis and abrogates BCR-derived survival signals. (A) Presented are contour plots that depict the relative green (DiOC6) and red (PI) fluorescence intensities of CLL cells from a representative patient on the horizontal and vertical axes, respectively. The viability was determined after 24 and 48 hours (as indicated on the left side) after incubation of CLL cells in medium alone (control), medium supplemented with 5 μM R406, medium with 10 μg/mL anti-IgM mAbs, or medium with anti-IgM mAbs and 5 μM R406, as indicated above the plots. The viable cell population is characterized by bright DiOC6 staining and PI exclusion, and is gated in the lower right corner of each contour plot. The percentage of viable cells is displayed above each of these gates. In this case, R406 reduced CLL cell viability from 54.8% to 44%. Anti-IgM stimulation increased CLL cell viability to 84.1%, which was completely abrogated by coincubation with anti-IgM plus R406, reducing CLL cell viability to 45% at 24 hours. Similar results were obtained at 48 hours (bottom row). (B-C) The bar diagrams represent the mean relative viabilities of CLL cells cultured in complete medium (control), or medium supplemented with 5 μM R406, 10 μg/mL anti-IgM, or the combination of 5 μM R406 and 10 μg/mL anti-IgM. Viabilities were normalized to the relative viability of control samples at the respective time points (100%), to account for differences in spontaneous apoptosis in samples from different patients. Displayed are the means (± SEM) from 11 different patient samples, assessed after 24 hours (B) and 48 hours (C). * and ** indicate P < .05.
The Syk inhibitor R406 induces CLL cell apoptosis and abrogates BCR-derived survival signals. (A) Presented are contour plots that depict the relative green (DiOC6) and red (PI) fluorescence intensities of CLL cells from a representative patient on the horizontal and vertical axes, respectively. The viability was determined after 24 and 48 hours (as indicated on the left side) after incubation of CLL cells in medium alone (control), medium supplemented with 5 μM R406, medium with 10 μg/mL anti-IgM mAbs, or medium with anti-IgM mAbs and 5 μM R406, as indicated above the plots. The viable cell population is characterized by bright DiOC6 staining and PI exclusion, and is gated in the lower right corner of each contour plot. The percentage of viable cells is displayed above each of these gates. In this case, R406 reduced CLL cell viability from 54.8% to 44%. Anti-IgM stimulation increased CLL cell viability to 84.1%, which was completely abrogated by coincubation with anti-IgM plus R406, reducing CLL cell viability to 45% at 24 hours. Similar results were obtained at 48 hours (bottom row). (B-C) The bar diagrams represent the mean relative viabilities of CLL cells cultured in complete medium (control), or medium supplemented with 5 μM R406, 10 μg/mL anti-IgM, or the combination of 5 μM R406 and 10 μg/mL anti-IgM. Viabilities were normalized to the relative viability of control samples at the respective time points (100%), to account for differences in spontaneous apoptosis in samples from different patients. Displayed are the means (± SEM) from 11 different patient samples, assessed after 24 hours (B) and 48 hours (C). * and ** indicate P < .05.
R406 induces CLL cell apoptosis in NLC cocultures
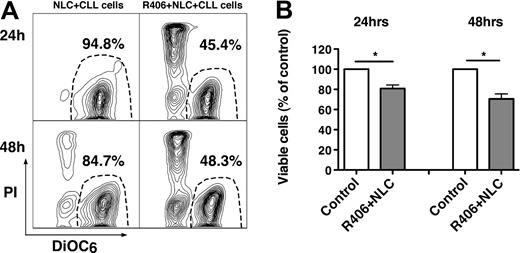
NLC protect CLL cells from spontaneous and drug-induced apoptosis through multiple pathways,4,48,49 which may include BCR activation.44 Therefore, we evaluated whether R406 could induce apoptosis in cocultures with NLC. Figure 3A displays CLL cell viability in a representative sample, cocultured with NLC in the presence or absence of R406. As depicted in Figure 3B, CLL cell viability in cocultures with NLC was significantly reduced by R406 to 80.8% plus or minus 3.5% at 24 hours, and to 70.6% plus or minus 5.0% at 48 hours (mean ± SEM, n = 24 for each time point, P < .05) of the respective controls, cultured with NLC in the absence of R406. We assume that this activity of R406 is largely due to a direct effect of R406 on the CLL cells, but we cannot exclude that R406 also affects NLC function and/or viability.
The Syk inhibitor R406 induces apoptosis of CLL cells in cocultures with NLC. (A) Displayed are contour plots that depict CLL cell viability, after staining with DiOC6 and PI (horizontal and vertical axes, respectively). The viable cell population is characterized by bright DiOC6 staining and PI exclusion, and is gated in the lower right corner of each contour plot. The percentage of viable cells is displayed above each of these gates. CLL cells were cocultured with NLC in presence or absence of the Syk inhibitor R406, as indicated above the plots. In this case, R406 reduced CLL cell viability in NLC cocultures from 94.8% to 45.4% at 24 hours, and from 84.7% to 48.3% at 48 hours. (B) The bars represent the mean relative viability of CLL cells cocultured with NLC (control) compared with CLL cells cocultured with NLC plus R406. Viabilities were normalized to the relative viability of control samples at the respective time points (100%), to account for differences in spontaneous apoptosis in samples from different patients. Viability was assessed after 24 and 48 hours. Displayed are the mean (± SEM) viabilities from 24 patient samples. *P < .01.
The Syk inhibitor R406 induces apoptosis of CLL cells in cocultures with NLC. (A) Displayed are contour plots that depict CLL cell viability, after staining with DiOC6 and PI (horizontal and vertical axes, respectively). The viable cell population is characterized by bright DiOC6 staining and PI exclusion, and is gated in the lower right corner of each contour plot. The percentage of viable cells is displayed above each of these gates. CLL cells were cocultured with NLC in presence or absence of the Syk inhibitor R406, as indicated above the plots. In this case, R406 reduced CLL cell viability in NLC cocultures from 94.8% to 45.4% at 24 hours, and from 84.7% to 48.3% at 48 hours. (B) The bars represent the mean relative viability of CLL cells cocultured with NLC (control) compared with CLL cells cocultured with NLC plus R406. Viabilities were normalized to the relative viability of control samples at the respective time points (100%), to account for differences in spontaneous apoptosis in samples from different patients. Viability was assessed after 24 and 48 hours. Displayed are the mean (± SEM) viabilities from 24 patient samples. *P < .01.
R406 blocks CCL3 and CCL4 secretion by CLL cells in response to BCR triggering
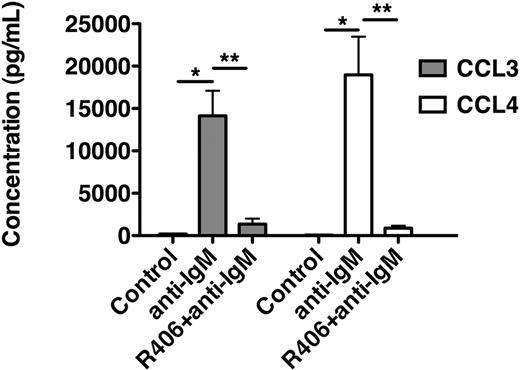
As displayed in Figure 4, anti-IgM stimulation of CLL cells increased CCL3 supernatant levels to 14 137 plus or minus 2953 pg/mL and CCL4 levels to 18 962 plus or minus 9000 pg/mL (mean ± SEM, n = 4). Preincubation with R406 significantly reduced the levels of these chemokines to 1359 plus or minus 650 pg/mL (CCL3) and to 882 plus or minus 290 pg/mL (CCL4), respectively (mean ± SEM, n = 4).
R406 blocks BCR-induced secretion of the chemokines CCL3 and CCL4 by CLL cells. This bar diagram displays CLL cell supernatant concentrations for CCL3 (■) and CCL4 (□) from CLL cells cultured in medium (control), medium supplemented with anti-IgM, or medium with anti-IgM plus R406. After 24 hours, supernatants were harvested and assayed by enzyme-linked immunosorbent assay. Whereas anti-IgM induced a robust secretion of CCL3 and CCL4, treatment with R406 almost completely abrogated the secretion of CCL3 and CCL4. Displayed are the mean (± SEM) supernatant concentrations from 4 different patient samples.
R406 blocks BCR-induced secretion of the chemokines CCL3 and CCL4 by CLL cells. This bar diagram displays CLL cell supernatant concentrations for CCL3 (■) and CCL4 (□) from CLL cells cultured in medium (control), medium supplemented with anti-IgM, or medium with anti-IgM plus R406. After 24 hours, supernatants were harvested and assayed by enzyme-linked immunosorbent assay. Whereas anti-IgM induced a robust secretion of CCL3 and CCL4, treatment with R406 almost completely abrogated the secretion of CCL3 and CCL4. Displayed are the mean (± SEM) supernatant concentrations from 4 different patient samples.
BCR activation modulates expression of adhesion molecules and chemokine receptors on CLL cells
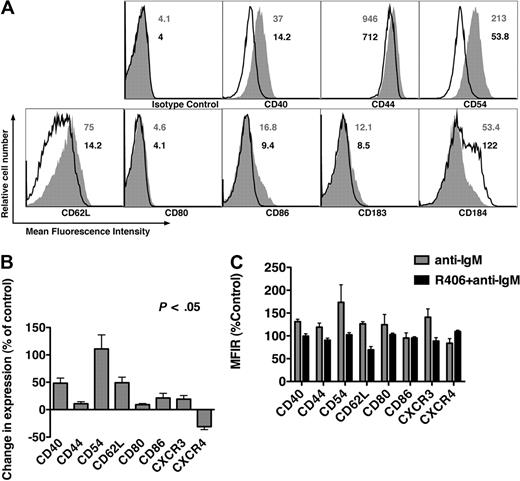
Figure 5A displays a representative case of CLL cells cultured in medium without (open histograms) or with anti-IgM (gray histograms). Comparing mean fluorescence intensities ratios of anti-IgM–stimulated CLL sample with control, we noticed significant increases in expression of CD40 (48.3% ± 9.3%), CD44 (10.7% ± 3.8%), CD54 (110.9% ± 25.6%), CD62L (49.1% ± 10.1%), CD80 (9.1% ± 5.0%), CD86 (21.3% ± 8.5%), and CD183 (CXCR3, 19.1% ± 6.6%), and a significant decrease in expression of CD184 (CXCR4, 31.1% ± 5.3%). No changes were observed for the other molecules tested (data not shown). These results are the mean (± SEM) relative changes after anti-IgM stimulation, compared with controls from 15 different CLL samples, as summarized in Figure 5B. CLL samples (n = 6) incubated with R406 plus anti-IgM did not display these changes in surface marker expression, as plotted in Figure 5C, indicating that these changes were dependent upon BCR signaling through Syk.
BCR triggering modulates surface expression of adhesion molecules and chemokine receptors, which is abrogated by R406. (A) Overlay histograms that depict relative fluorescence intensities of CLL cells from a representative patient, stained with fluorescence-labeled mAbs to the antigens displayed on the horizontal axes. CLL cells were cultured in medium (control) or in medium supplemented with anti-IgM. The solid gray histogram represents the staining of anti-IgM–treated CLL cells, whereas the black line represents the staining of control CLL cells. The numbers next to each of the histograms indicate the mean fluorescence intensity values for the anti-IgM–stimulated samples (in gray) or the controls (in black). Compared with the controls, anti-IgM induces up-regulation of CD40, CD44, CD54, and CD62L, and down-regulation of CXCR4. (B) Displayed are bar diagrams that depict the mean relative changes in surface molecule expression on CLL cells after stimulation with anti-IgM. Results were displayed as percentage of change in expression, relative to the control samples, based upon the mean fluorescence intensity ratios (MFIR) for each of the mAbs displayed on the horizontal axis. Each bar represents the mean (± SEM) from 15 different CLL patient samples, and each of the displayed changes was statistically significant, with P < .05. (C) R406 abrogates anti-IgM–induced changes in expression of surface molecules. The bars represent the mean (± SEM) relative expression of the antigens displayed on the horizontal axis, on CLL cells from 6 different patients. ▩ depicts the mean relative surface expression after 24 hours of stimulation with anti-IgM, whereas ■ represents the mean relative surface expression after 24 hours of stimulation with anti-IgM plus R406. The expression was normalized for each antigen to the relative antigen expression on control CLL cells from the respective patient, cultured in medium alone (100%).
BCR triggering modulates surface expression of adhesion molecules and chemokine receptors, which is abrogated by R406. (A) Overlay histograms that depict relative fluorescence intensities of CLL cells from a representative patient, stained with fluorescence-labeled mAbs to the antigens displayed on the horizontal axes. CLL cells were cultured in medium (control) or in medium supplemented with anti-IgM. The solid gray histogram represents the staining of anti-IgM–treated CLL cells, whereas the black line represents the staining of control CLL cells. The numbers next to each of the histograms indicate the mean fluorescence intensity values for the anti-IgM–stimulated samples (in gray) or the controls (in black). Compared with the controls, anti-IgM induces up-regulation of CD40, CD44, CD54, and CD62L, and down-regulation of CXCR4. (B) Displayed are bar diagrams that depict the mean relative changes in surface molecule expression on CLL cells after stimulation with anti-IgM. Results were displayed as percentage of change in expression, relative to the control samples, based upon the mean fluorescence intensity ratios (MFIR) for each of the mAbs displayed on the horizontal axis. Each bar represents the mean (± SEM) from 15 different CLL patient samples, and each of the displayed changes was statistically significant, with P < .05. (C) R406 abrogates anti-IgM–induced changes in expression of surface molecules. The bars represent the mean (± SEM) relative expression of the antigens displayed on the horizontal axis, on CLL cells from 6 different patients. ▩ depicts the mean relative surface expression after 24 hours of stimulation with anti-IgM, whereas ■ represents the mean relative surface expression after 24 hours of stimulation with anti-IgM plus R406. The expression was normalized for each antigen to the relative antigen expression on control CLL cells from the respective patient, cultured in medium alone (100%).
BCR triggering enhances CLL cell chemotaxis and pseudoemperipolesis in a Syk-dependent fashion
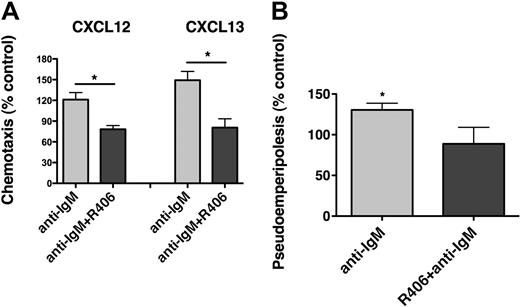
CLL cells display chemotaxis toward the chemokines CXCL12 and CXCL13 by activation of the respective chemokine receptors (CXCR4, CXCR5), expressed at high levels on CLL cells.13,14 To evaluate the effect of BCR triggering on chemotaxis, CLL cells from 15 different patients were assayed for chemotaxis toward CXCL12 (200 ng/mL) or CXCL13 (1 μg/mL) after incubation for 48 hours in medium (control) or in medium supplemented with 10 μg/mL anti-IgM. As shown in Figure 6A, anti-IgM significantly increased CLL cell chemotaxis toward CXCL12 to 135.4% plus or minus 15.2% of control, and toward CXCL13 to 170.5% plus or minus 31.5% of control (mean ± SEM, n = 15). Increases in chemotaxis of CLL cells after anti-IgM stimulation were higher in ZAP-70–positive compared with ZAP-70–negative samples (Figure 6B-C), although only for CXCL12 these differences were statistically significant. A similar trend was noticed for unmutated, but not for CD38+ CLL cases (supplemental Figure 5). To determine the importance of Syk in mediating the increased chemotaxis of CLL cells after BCR stimulation, CLL cells were incubated with anti-IgM with or without R406 before the chemotaxis assays. As displayed in Figure 7A, R406 decreased chemotaxis toward CXCL12 to 78.2% plus or minus 5.4% of untreated control, and toward CXCL13 to 80.6% plus or minus 12.8% of untreated control (mean ± SEM, n = 10). The migration of CLL cells beneath MSC (pseudoemperipolesis) is mediated by activation of CXCR4 through CXCL12, constitutively secreted by MSC. To evaluate whether BCR activation and its inhibition by R406 could modulate CLL cell pseudoemperipolesis, CLL cell pseudoemperipolesis was quantified after incubation in medium, medium supplemented with anti-IgM, or medium supplemented with anti-IgM plus R406. As shown in Figure 7B, stimulation with anti-IgM significantly increased CLL cell pseudoemperipolesis to 130.4% plus or minus 8.3% of control (mean ± SEM, n = 6). Incubation with anti-IgM plus R406 reduced CLL cell pseudoemperipolesis to 88.8% plus or minus 20.3% of untreated control.
BCR triggering increases chemotaxis of CLL cells toward CXCL12 and CXCL13. (A) CLL cells from 15 different patients were assayed for chemotaxis toward CXCL12 (▩) or CXCL13 (■) after incubation for 48 hours without (control) or with anti-IgM. Displayed is the mean relative chemotaxis (± SEM, n = 15) of anti-IgM–stimulated CLL cells, compared with unstimulated controls (100%). Anti-IgM treatment significantly increased CLL cell chemotaxis toward both CXCL12 and CXCL13, with P < .05, as indicated by the asterisks. (B-C) Displayed are scatter dot plots that represent the relative chemotaxis of 15 CLL samples after 48 hours of treatment with anti-IgM in ZAP-70–positive CLL samples (●, n = 9) and ZAP-70–negative CLL samples (○, n = 6) toward CXCL12 (B) or CXCL13 (C). Enhanced chemotaxis after anti-IgM treatment was more apparent in ZAP-70–positive samples; however, this difference between ZAP-70–positive and ZAP-70–negative CLL samples was significant only for CXCL12 (P < .05).
BCR triggering increases chemotaxis of CLL cells toward CXCL12 and CXCL13. (A) CLL cells from 15 different patients were assayed for chemotaxis toward CXCL12 (▩) or CXCL13 (■) after incubation for 48 hours without (control) or with anti-IgM. Displayed is the mean relative chemotaxis (± SEM, n = 15) of anti-IgM–stimulated CLL cells, compared with unstimulated controls (100%). Anti-IgM treatment significantly increased CLL cell chemotaxis toward both CXCL12 and CXCL13, with P < .05, as indicated by the asterisks. (B-C) Displayed are scatter dot plots that represent the relative chemotaxis of 15 CLL samples after 48 hours of treatment with anti-IgM in ZAP-70–positive CLL samples (●, n = 9) and ZAP-70–negative CLL samples (○, n = 6) toward CXCL12 (B) or CXCL13 (C). Enhanced chemotaxis after anti-IgM treatment was more apparent in ZAP-70–positive samples; however, this difference between ZAP-70–positive and ZAP-70–negative CLL samples was significant only for CXCL12 (P < .05).
R406 antagonizes the increased chemotaxis and migration beneath marrow stromal cells (pseudoemperipolesis) of CLL cells after BCR triggering. (A) Displayed are bar diagrams that depict the mean relative chemotaxis of CLL cells after stimulation with anti-IgM (▩) or after stimulation with anti-IgM in the presence of R406 (■), compared with unstimulated CLL cells (controls, 100%). Displayed is the mean (± SEM, n = 10) chemotaxis toward CXCL12 and CXCL13, as indicated above the bars, for each of these conditions. R406 significantly inhibited the chemotaxis of CLL cells toward both CXCL12 and CXCL13 to levels that were even lower than the chemotaxis of control cells. The asterisks indicate a significant reduction of chemotaxis with P < .05. (B) Coculture of CLL cells with MSC results in the spontaneous migration of CLL cells beneath the MSC (pseudoemperipolesis). We investigated whether treatment with anti-IgM could increase migration of CLL cells beneath marrow stromal cells, and we also evaluated the effect of R406 on pseudoemperipolesis. As displayed, BCR triggering with anti-IgM significantly increased pseudoemperipolesis to 130.4% ± 8.3% of controls, and preincubation with R406 decreased the percentage of migrated cells to 88.8% ± 20.3% of control (mean ± SEM, n = 6). *P < .05.
R406 antagonizes the increased chemotaxis and migration beneath marrow stromal cells (pseudoemperipolesis) of CLL cells after BCR triggering. (A) Displayed are bar diagrams that depict the mean relative chemotaxis of CLL cells after stimulation with anti-IgM (▩) or after stimulation with anti-IgM in the presence of R406 (■), compared with unstimulated CLL cells (controls, 100%). Displayed is the mean (± SEM, n = 10) chemotaxis toward CXCL12 and CXCL13, as indicated above the bars, for each of these conditions. R406 significantly inhibited the chemotaxis of CLL cells toward both CXCL12 and CXCL13 to levels that were even lower than the chemotaxis of control cells. The asterisks indicate a significant reduction of chemotaxis with P < .05. (B) Coculture of CLL cells with MSC results in the spontaneous migration of CLL cells beneath the MSC (pseudoemperipolesis). We investigated whether treatment with anti-IgM could increase migration of CLL cells beneath marrow stromal cells, and we also evaluated the effect of R406 on pseudoemperipolesis. As displayed, BCR triggering with anti-IgM significantly increased pseudoemperipolesis to 130.4% ± 8.3% of controls, and preincubation with R406 decreased the percentage of migrated cells to 88.8% ± 20.3% of control (mean ± SEM, n = 6). *P < .05.
R406 blocks BCR downstream signaling in CLL cells
To determine the efficacy of R406 in blocking BCR downstream signaling, we analyzed phosphorylation of Syk, AKT, and ERK by Western blotting. As shown in Figure 8A, CLL cells display baseline activation of Syk, AKT, and ERK, which was enhanced after anti-IgM stimulation. Preincubation with R406 down-regulated the activation to levels that were lower than the baseline activation. Supplemental Figure 6 shows additional Western blot results. Because the mobilization of intracellular calcium is another important signaling response after BCR triggering, we evaluated whether R406 could block the intracellular calcium flux after anti-IgM stimulation. As shown in Figure 8B, treatment with R406 effectively blocked calcium mobilization in response to BCR stimulation.
R406 inhibits BCR signaling in CLL cells. (A) Displayed are immunoblots from CLL cells from 2 representative patients who were either unstimulated (control), or stimulated for 10 or 20 minutes with anti-IgM in the presence or absence of R406. These different conditions are displayed above the immunoblots. At the indicated time points, the cells were lysed and probed with mAbs to the antigens that are displayed on the left side. “P” indicates immunoblotting for the active, phosphorylated form, whereas “T” represents the nonphosphorylated, total amount of the respective proteins. We found that constitutive and anti-IgM–induced Syk activation, p44/42 mitogen-activated protein kinase (ERK), and AKT activation were inhibited by R406. Relative protein levels were determined by densitometry, normalized to the corresponding control, and are displayed below each of the phosphoprotein bands. (B) Calcium mobilization is another signaling response typically induced in B cells after BCR triggering. In this figure, CLL cells were labeled with the fluorescent calcium-indicator Fluo3-AM, and preincubated with or without R406. Then the CLL cells were stimulated with anti-IgM, and the fluorescence intensity was recorded over time. The lines represent the changes in fluorescence intensity (on the vertical axis) over time (horizontal axis) for control CLL cells (black lines) or cells preincubated with R406 (gray lines). Displayed is the anti-IgM–induced calcium mobilization in 4 different patients (labeled CLL#1-4), with a detectable calcium mobilization in the controls, and an abrogated calcium mobilization in R406-pretreated CLL cells.
R406 inhibits BCR signaling in CLL cells. (A) Displayed are immunoblots from CLL cells from 2 representative patients who were either unstimulated (control), or stimulated for 10 or 20 minutes with anti-IgM in the presence or absence of R406. These different conditions are displayed above the immunoblots. At the indicated time points, the cells were lysed and probed with mAbs to the antigens that are displayed on the left side. “P” indicates immunoblotting for the active, phosphorylated form, whereas “T” represents the nonphosphorylated, total amount of the respective proteins. We found that constitutive and anti-IgM–induced Syk activation, p44/42 mitogen-activated protein kinase (ERK), and AKT activation were inhibited by R406. Relative protein levels were determined by densitometry, normalized to the corresponding control, and are displayed below each of the phosphoprotein bands. (B) Calcium mobilization is another signaling response typically induced in B cells after BCR triggering. In this figure, CLL cells were labeled with the fluorescent calcium-indicator Fluo3-AM, and preincubated with or without R406. Then the CLL cells were stimulated with anti-IgM, and the fluorescence intensity was recorded over time. The lines represent the changes in fluorescence intensity (on the vertical axis) over time (horizontal axis) for control CLL cells (black lines) or cells preincubated with R406 (gray lines). Displayed is the anti-IgM–induced calcium mobilization in 4 different patients (labeled CLL#1-4), with a detectable calcium mobilization in the controls, and an abrogated calcium mobilization in R406-pretreated CLL cells.
Discussion
In this study, we demonstrate that BCR signaling via Syk increased CLL cell viability, expression of adhesion and costimulatory molecules, and chemotaxis toward CXCL12 and CXCL13, 2 homeostatic chemokines that regulate B-cell trafficking to bone marrow and within lymphatic tissues. In addition, BCR triggering enhanced CLL cell migration beneath MSC, and induced calcium mobilization and activation of AKT, Syk, and ERK1/2. R406, an adenosine triphosphate-competitive, small-molecule Syk inhibitor, effectively blocked these survival and migration responses in CLL cells. Interestingly, R406 also induced CLL cell apoptosis in the absence of anti-IgM (Figure 2), which could be due to constitutive Syk activation. This is supported by our immunoblot findings of constitutive Syk activation, which was enhanced by anti-IgM stimulation in some cases, and (more consistently) suppressed by R406 (Figure 8 and supplemental Figure 6). These findings suggest either intrinsic Syk activation in CLL cells, and/or persistent Syk activation by external stimulation in suspension cultures even in the absence of anti-IgM.
R406 previously has been reported to inhibit proliferation of Syk-transformed pre-B cells,50 and to induce apoptosis in non-Hodgkin lymphoma (NHL)36,42,51 and CLL cells.52 R406 also displays clinical activity in patients with NHL, CLL,53 and rheumatoid arthritis.45 In CLL, this clinical response was characterized by an early, transient increase in peripheral lymphocyte counts, along with decreases in lymph node sizes, before induction of remissions in a significant proportion of CLL patients with relapsed disease.53 This clinical observation suggests that R406 induces the mobilization of CLL cells from tissue compartments to the blood, by antagonizing signals that confine CLL cells within tissues, before or along with the induction of CLL cell death. Our data provide an explanation for this remarkable clinical activity. First, the enhanced chemotaxis of CLL cells toward CXCL12 and CXCL13 after BCR stimulation was abrogated by R406. Consistent with this finding, R406-pretreated CLL cells displayed a reduced migration beneath marrow stromal cells (pseudoemperipolesis), an in vitro phenomenon that resembles migration and homing to the marrow microenvironment. Moreover, the BCR-dependent up-regulation of important adhesion and costimulatory molecules, such as CD54, CD62L, CD44, CD40, CD80, and CD86, was abrogated by R406. Collectively, these events could explain the transient lymphocytosis at the start of treatment with R406, due to the inhibition of chemokine- and adhesion molecule–related retention signals.
Side effects on treatment with R406 in patients with rheumatoid arthritis45 or B-cell lymphomas53 generally were mild; gastrointestinal side effects (predominantly diarrhea) and neutropenia were the most common toxicities, both of which were dose related. The most prominent abnormal hematologic finding was a reduction in neutrophil counts, which was rapidly reversible upon dose interruption, followed by dose reduction.45 Animal studies demonstrated additional immunomodulatory effects of R406, such as decreased thymus and spleen weights, hypocellularity of the bone marrow, and reduced lymphocyte counts, including T and B cells, which resolved during a 14-day treatment-free recovery period.54
BCR triggering and CLL coculture with NLC also induce the secretion of 2 T-cell chemokines, CCL3 and CCL4, by CLL cells, a recent finding44 that indicates that CLL cells are actively involved in creating a favorable microenvironment. CCL3/CCL4 secretion by CLL cells, along with other signals,55 could be a lead mechanism to explain the colocalization of T cells with CLL cells in pseudofollicles.11 We previously demonstrated that R406 abrogated CCL3 and CCL4 secretion by CLL cells in NLC cocultures,56 and now demonstrate that R406 also was remarkably effective in abrogating BCR-dependent induction of these chemokines. Because R406 inhibited CLL cell survival in NLC cocultures (Figure 3) and after BCR stimulation (Figure 2) and CCL3/CCL4 secretion after BCR triggering (Figure 4) and in NLC cocultures,44 we hypothesize that NLC cocultures activate CLL cells in a Syk-dependent fashion via the BCR. This is also supported by our recent data44 that NLC coculture induced expression of several other genes that become induced or activated in B cells upon BCR stimulation and that are (at least partially) dependent on Syk activation, including signal transducer and activator of transcription 1,56,57 EGR2, and EGR3,58 which are members of the early growth response (EGR) family of transcription factors, and the BCR regulatory molecule CD72 (see details in the Gene Expression Omnibus database under accession number GSE13811). A more formal and detailed investigation into the possibility of BCR activation in NLC cocultures is currently ongoing in our laboratory.
The effect of BCR triggering on CLL cell viability remains a controversial topic. Other investigators reported increased CLL cell viability33,59,60 or enhanced apoptosis61 after IgM triggering. Differences of antibodies used for BCR stimulation in respect to their specificity for IgM versus Ig or IgD, their affinity for the target(s), and polyclonal versus mAb interactions need to be considered for interpretation of these differences.
Collectively, our findings suggest that R406 disrupts the protective CLL microenvironment at multiple levels, that is, CLL cell survival, migration and homing, and paracrine circuits, such as CCL3/CCL4 secretion. Gobessi et al recently reported that R406 induces CLL cell apoptosis, and inhibits BCR-derived signals for CLL cell survival, AKT activation, and myeloid cell leukemia-1 up-regulation.52 As such, some of our findings confirm this recent study; the distinct, novel findings in this paper are related to CLL cell adhesion and costimulatory molecule expression, chemotaxis toward CXCL12 and CXCL13, pseudoemperipolesis, the viability data in NLC cocultures, and CCL3/CCL4 secretion. As such, our experiments take complex interactions between CLL cells and accessory cells into account, using models that resemble the CLL microenvironment in the marrow and lymphatic tissues.
In summary, our findings support a model in which BCR signaling in CLL cells enhances survival signals, cell adhesion, and chemotaxis. These effects are modulated by the BCR signaling capacity, and mediated through Syk. The effects on CLL cell chemotaxis and adhesion suggest that BCR signaling not only affects CLL cell survival, but also regulates trafficking and homing, and therefore may participate in the establishment and maintenance of the microarchitecture of the tissue microenvironments, such as the pseudofollicles. R406 effectively abrogates these BCR-derived responses at multiple levels. Its effect on chemotaxis and adhesion molecule expression provides a rational explanation for the fascinating clinical activity of R406 in CLL. Future studies of R406 (or second generation Syk inhibitors) will help us to determine whether these models are correct, and will be a major next step toward more treatments that target the microenvironment in patients with CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Ann Lowe and Yasumichi Hitoshi from Rigel Pharmaceuticals for valuable suggestions and for providing R406.
This work was supported by CLL Global Research Foundation grants (to J.A.B. and V.G.), a Sidney Kimmel Foundation for Cancer Research Scholar Award (to J.A.B.), and an American Society of Clinical Oncology Career Development Award (to J.A.B.).
Authorship
Contribution: M.P.Q. performed the experiments, analyzed the data, designed the figures, and wrote the paper with J.A.B.; K.B. performed the Western blots and reviewed the manuscript; A.V.K. and M.S. assisted with the experiments and reviewed the manuscript; M.J.K. and W.G.W. provided patients' samples and reviewed the manuscript; V.G. reviewed the manuscript; and J.A.B. designed the research, supervised the study, analyzed the data, and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan A. Burger, Department of Leukemia, Unit 428, University of Texas M. D. Anderson Cancer Center, PO Box 301402, Houston, TX 77230-1402; e-mail: jaburger@mdanderson.org.