In this issue of Blood, Sutherland and colleagues describe an unusual in-frame deletion of exons 4-5 of the VWF gene associated with both dominantly inherited type 1 and with type 3 VWD.1
The mutation was initially identified in homozygous type 3 von Willebrand disease (VWD) patients, subsequently found in further compound heterozygous patients with type 3 VWD, and then sought in a previously studied cohort of type 1 VWD cases.2 It has thus been identified in 8 of 24 (33%) alleles of the type 3 VWD cohort of 12 apparently unrelated index cases (2 homozygotes and 4 compound heterozygotes) and additionally identified in 2 of 34 (6%) type 1 VWD index cases.
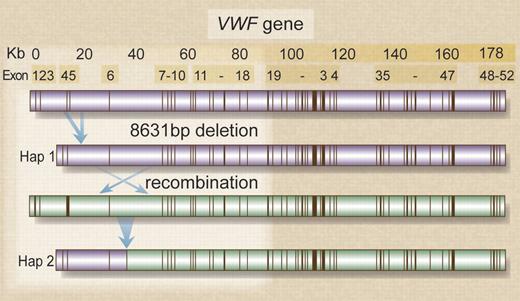
Alu-mediated VWF deletion followed by recombination in one family. The top portion of the figure shows the VWF gene intron-exon structure. Unequal homologous recombination between Alu Y repeats in introns 3 and 5 removes 8631bp and results in an in-frame deletion of exons 4-5. The deletion is located on an ancestral haplotype (Hap 1), present in all but one of the alleles investigated by Sutherland et al.1 A subsequent recombination event (panels 2 and 3) results in generation of haplotype 2 (Hap 2). The recombination location is likely to lie between intron 5 and exon 8. Professional illustration by Debra T. Dartez.
Alu-mediated VWF deletion followed by recombination in one family. The top portion of the figure shows the VWF gene intron-exon structure. Unequal homologous recombination between Alu Y repeats in introns 3 and 5 removes 8631bp and results in an in-frame deletion of exons 4-5. The deletion is located on an ancestral haplotype (Hap 1), present in all but one of the alleles investigated by Sutherland et al.1 A subsequent recombination event (panels 2 and 3) results in generation of haplotype 2 (Hap 2). The recombination location is likely to lie between intron 5 and exon 8. Professional illustration by Debra T. Dartez.
Most mutations identified in type 3 VWD are “null” mutations, resulting in a lack of expression due to non-sense, frameshift, splice site, and large deletion mutations. Large deletions typically result in a lack of von Willebrand factor (VWF) expression, and penetrance of bleeding symptoms in simple heterozygous individuals with such null mutations is very low. The deletion currently described is unusual in that a truncated VWF protein is produced, albeit in very low quantity, that has a dominant negative effect on expression of the second allele. The small group of heterozygous type 1 VWD patients with the mutation have mean VWF levels of around 25 IU/dL, and the mutation appears to result in bleeding in the majority of individuals investigated.
All but one family where the mutation was identified shared a common haplotype associated with the deletion comprising 25 SNP throughout VWF. A second related haplotype found in heterozygous form in only one type 3 family appeared to have arisen from a subsequent recombination 3′ to the deletion. The distribution of the deletion is not yet known; however, its presence in a number of presumed independent type 3 and type 1 VWD families suggests that it may be widespread in families of British origin, possibly including emigrant families. Analysis of the other 2 large type 1 VWD cohorts collected in Europe and Canada3,4 will help provide insight into the mutation's distribution. If it is sufficiently widespread, then the mutation should be sought first in any mutation screening strategy for types 1 and 3 VWD, prior to VWF DNA sequence analysis. A specific long polymerase chain reaction (PCR) described by the authors, which produces a deletion-specific 1084 bp amplicon along with a wild-type product of 1694 bp, will facilitate this analysis.
Examination of the sequence surrounding the deletion breakpoint implicated unequal homologous recombination between 2 Alu Y repeat elements in introns 3 and 5 as the mutation mechanism. There are approximately 100 000 Alu repeats in the human genome, and these have been repeatedly involved in the generation of deletion mutations. As the authors highlight, this is at least the third example of an Alu-mediated deletion in type 3 VWD and in early reports of VWF deletions, mutation mechanism was not investigated so the mechanism is likely underrecognized. Alu repeats may be involved in both homologous and non-homologous recombination, as a core 26 bp repeat sequence is reported to be recombogenic.5 A previously reported type 2 VWD patient (disease subtype not specified) had a large in-frame deletion of 31 kb (exons 26-34) associated with recombination between an Alu repeat in intron 25 and a short stretch of homologous sequence in intron 34.6 These examples suggest that many more large deletions, both in-frame and resulting in null alleles, will be identified as techniques for gene dosage (large deletion or duplication) analysis are developed. Many of these mutations are currently likely to be missed, where a heterozygous deletion is masked by the presence of an intact second allele. Initial analysis of the partial cloned sequence of VWF indicated that there were 14 Alu sequences identified,7 but there are likely to be many more than this in the entire gene. Close sequence similarity between repeat sequences can help promote unequal homologous recombination, resulting in deletion of the intervening sequence. In the current example, Alu Y sequences surrounding the breakpoint had over 80% sequence similarity.
The subsequent recombination event identified in one family by Sutherland et al is also of interest. SNP analysis suggests that the recombination site lies between intron 5 and exon 8. There may now be sufficient density of VWF SNP reported that the region involved could be identified and sequence features implicated in the recombination event could also be investigated.
Conflict-of-interest disclosure: A.C.G. received sponsorship from CSL-Behring for maintenance of ISTH-SSC on VWF website. ■