Abstract
Activation-induced cell death (AICD) plays an essential role in the contraction of activated T cells after eradication of pathogen. Fas (APO-1/CD95) is one of the key cell surface proteins that mediate AICD in CD4+ and CD8+ T cells. Despite its prime importance in cell death, regulation of Fas expression in T cells is poorly understood. Here we show that Cyclon, a newly identified cytokine-inducible protein, is induced in T cells on T-cell receptor ligation and important for immune homeostasis. Transgenic expression of Cyclon ameliorated autoimmune phenotype in mice lacking subunits of IL-2R. Transgenic expression of Cyclon markedly enhanced AICD through increased expression of Fas whose expression is essential for Cyclon action. Finally, we demonstrated that activated but not resting CD4+ T cells with targeted deletion of a Cyclon allele show reduced AICD and expression of Fas, indicating a critical role of Cyclon in Fas expression in activated T cells. We think that our data provide insight into expression regulation of Fas in T cells.
Introduction
Activation of acquired immunity induces rapid expansion of antigen-specific CD4+ and CD8+ T cells to eradicate the pathogens. After eradication of the pathogens, however, most activated T cells are removed.1,2 The elimination of activated T cells is largely mediated by apoptotic cell death.2 Activated T-cell autonomous death and activation-induced cell death (AICD) are the 2 mechanisms by which programmed death of activated T cells occurs.3-7 Activated T-cell autonomous death is mediated by the intrinsic death pathway,8-10 which is regulated by the B-cell lymphoma 2 (Bcl-2) family proteins. In contrast, the other mechanism of activated T-cell death, AICD, requires antigen restimulation of activated effector T cells and is triggered by the extrinsic death pathways regulated by signals of death receptors (DRs) of the tumor necrosis factor receptor (TNFR) family.1,11-13 Members of the DR subgroup include type I TNFR (TNFRI)/DR1, Fas/CD95/Apo1/DR2, DR3, TNF-related apoptosis-inducing ligand receptor 1 (TRAIL-R1)/DR4, TRAIL-R2/DR5/Apo2, and DR6. These receptors contain extracellular cysteine-rich domains and an intracellular death domain, which is necessary for transducing death signals.14-16 Binding of ligands to DRs triggers trimerization of the receptors, which is required for the recruitment of the adaptor protein Fas-associated death domain (FADD),16 and subsequently procaspase 8, also known as FLICE, or procaspase 10. The ensemble constitutes what is known as the death-inducing signaling complex, leading to auto-processing of initiator procaspases into active caspases, which activate downstream effector caspases.14,17,18 Other inhibitors of the pathway include the FLICE inhibitory protein (FLIP), which competes with procaspase 8 (FLICE) for binding to the DED of FADD.19-23
Fas ligand (FasL) and TNF are the key death ligands that mediate AICD in mature T cells.1,7,24-29 Importance of the Fas/FasL system in regulation of immune homeostasis has been established by genetic studies showing that lpr mice, which have a loss-of-function mutation in the Fas gene,30-32 and gld mice, which have a loss-of-function mutation in the Fasl gene,33-35 develop severe lymphadenopathy caused by defects in Fas-mediated cell death.36,37 In humans, defects in Fas and FasL cause similar abnormalities in the autoimmune lymphoproliferative syndrome type Ia and Ib, respectively.38-40
Expression levels of Fas or FasL markedly impact T-cell death. Regulation of FasL expression in primary T cells has been extensively examined.41-43 In contrast, surprisingly little is known about regulation of Fas expression in primary T cells. T-cell death-associated gene 51 (TDAG51) was shown to be required for T-cell receptor (TCR)–mediated expression of Fas in a T-cell hybridoma.44 However, it was later shown that TDAG51 is not essential for Fas regulation and apoptosis of primary mouse T cells in vivo.45 NF-κB was also implicated in Fas expression in a human T-cell line, Jurkat.46 However, TCR-mediated Fas up-regulation and AICD were normal in c-Rel/p50 doubly deficient primary T cells,47 suggesting that NF-κB is not essential for TCR-mediated Fas up-regulation in primary T cells.
Cytokines also play a role in the regulation of Fas-mediated activated T-cell death. Sensitivity to AICD is chiefly the result of the effect of interleukin-2 (IL-2) in inducing cell-cycle progression into late G1 to S phases, which confers susceptibility to death.1,7 It has been reported that the IL-2/IL-2 receptor (IL-2R) system is essential for AICD. IL-2R consists of IL-2Rα, IL-2Rβ, and the common cytokine-receptor γ-chain (γc).48-50 Mice deficient in IL-2, IL-2Rα, or IL-2Rβ gene develop massive enlargement of peripheral lymphoid organs and multiple autoimmune diseases, including hemolytic anemia and inflammatory bowel disease.51-54 It has also been reported that peripheral T cells in γc-deficient (γc−) mice are spontaneously activated.55 Defective AICD in mice lacking IL-2 signaling is at least partially responsible for the autoimmune phenotype of these animals.56 IL-2 was shown to increase transcription and surface expression of Fas and FasL and suppress transcription and expression of FLIP. However, it remains unknown how IL-2 receptor signals induce Fas up-regulation.
We have recently identified a novel nuclear protein, Cyclon, encoded by Ccdc86, which is induced on cytokine stimulation in the Ba/F3 cell line.57 Cyclon is a phosphorylated nuclear protein consisting of repetitive sequences in the amino-terminus and a coiled-coil domain in the carboxyl-terminus.57 Here, we examined in vivo function of Cyclon in T cells using Cyclon transgenic mice and Cyclon-deficient mice. In T cells, expression of Cyclon was also induced by TCR ligation. T cell–specific transgenic expression of Cyclon normalized the number of activated T cells, suppressed enlargement of peripheral lymphoid organs, and decreased serum IL-17 levels in IL-2Rα-deficient (IL-2Rα−) mice. Transgenic expression of Cyclon also normalized splenomegaly in the γc− background. Attenuation of these autoimmune phenotypes by Cyclon overexpression was correlated with increased Fas expression and enhanced AICD. Finally, AICD and Fas expression in activated but not in resting CD4+ T cells was substantially reduced in mice with targeted deletion of a Cyclon allele. These data collectively indicate a critical role of Cyclon in Fas expression and AICD in activated T cells. We think our data provide a clue to understanding of Fas expression regulation in primary T cells.
Methods
Mice
Generation of Cyclon-transgenic and Cyclon-deficient mice is described in supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article). IL-2Rα−, γc−, and Fas−lpr mice were purchased from The Jackson Laboratory. All animal experiments were approved by the Institutional Animal Care and Use Committee at New York University School of Medicine.
Isolation of cells and cell culture
CD4+ and CD8+ T cells were isolated from splenocytes by magnetic sorting (Miltenyi Biotec). Cells were cultured in RPMI complete medium.
Reverse-transcription polymerase chain reaction
Immunoblot analysis
Cell staining and flow cytometry
Cells were stained for 30 minutes at 4°C with fluorochrome-conjugated antibodies (Abs). Abs used for surface staining were fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)−, or PE-Cy7–conjugated CD4 (clone GK1.5), FITC- or Cychrome-conjugated CD8α (clone 53-6.7), PE-conjugated CD44 (clone IM7), PE-conjugated FasL (clone MFL3), and PE-conjugated Fas (clone Jo2; BD Biosciences). For T-reg staining, splenocytes were stained with FITC-labeled anti-CD4 inflow cytometry buffer, followed by staining with PE-conjugated anti-Foxp3 Ab in the Foxp3 staining kit (eBioscience) according to the manufacturer's protocol. Flow cytometric analysis was performed on FACSCalibur (BD Biosciences) and analyzed with FlowJo software (TreeStar).
IL-17 measurement
IL-17 in sera was analyzed by enzyme-linked immunosorbent assay with mouse IL-17 Quantikine assay kits (R&D Systems) according to the manufacturer's protocol.
AICD assay
We followed an authentic AICD protocol.61 Briefly, CD4+ and CD8+ T cells were purified from spleens by magnetic sorting and activated with anti-CD3 Ab (2 μg/mL) + anti-CD28 Ab (2 μg/mL) for 2 or 3 days. Live cells were purified by density centrifugation with Lymphocyte-M (Cedarlane) and restimulated with different concentrations of anti-CD3 Ab in the presence of IL-2 (5 ng/mL). Cells were incubated for 1 to 3 days, and viability was examined by propidium iodide (PI) staining and flow cytometry.
For analysis of Fas-mediated cell death, CD4+ and CD8+ T cells were purified from spleens by magnetic sorting and activated with anti-CD3 Ab (2 μg/mL) + anti-CD28 Ab (2 μg/mL) for 2 or 3 days. Live cells were purified by density centrifugation with Lymphocyte-M (Cederlane) and recultured for 1 day with the indicated concentrations of superFasL (Alexis) in RPMI complete media in the presence of IL-2 (5 ng/mL).
Statistical analysis
Statistical analysis was performed with the Prism software (GraphPad) using t test or 1-way analysis of variance.
Results
Cyclon mRNA expression is induced in both CD4+ and CD8+ T cells on activation
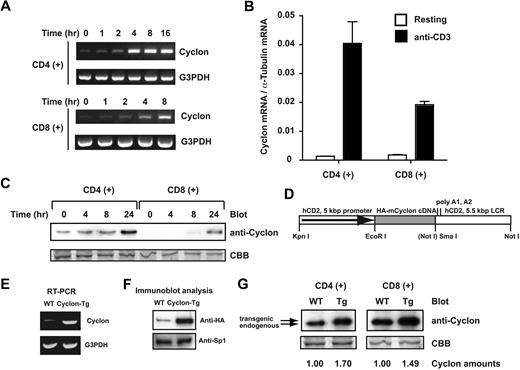
We found that Cyclon mRNA is induced in splenocytes by anti-CD3 stimulation (data not shown). To determine which subpopulations of T cells express Cyclon, purified splenic CD4+ and CD8+ T cells were stimulated with anti-CD3 and anti-CD28 Abs, and expression of Cyclon mRNA was examined (Figure 1A). TCR stimulation-dependent up-regulation of Cyclon was more evident in CD4+ T cells. Real-time PCR analysis showed that Cyclon mRNA was up-regulated by 30-fold 8 hours after stimulation in CD4+ T cells, whereas Cyclon mRNA was up-regulated by 13-fold in CD8+ T cells (Figure 1B). Immunoblot analysis with anti-Cyclon Ab57 revealed that Cyclon protein expression is induced more robustly in CD4+ than in CD8+ splenic T cells by anti-CD3 and anti-CD28 stimulation (Figure 1C). These results suggest a potential role of Cyclon in the regulation of function of activated T cells.
Activation-induced expression of Cyclon in primary T cells and generation of T-cell-specific Cyclon-Tg mice. (A) Induction of Cyclon mRNA in CD4+ and CD8+ splenic T cells by anti-CD3 and anti-CD28 Ab stimulation. Magnetically sorted cells were stimulated by plate-bound anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL). Total RNA was subjected to RT-PCR analysis. (B) Real-time RT-PCR analysis of expression of Cyclon mRNA in resting and activated CD4+ or CD8+ splenic T cells. Magnetically sorted cells were stimulated by plate-bound anti-CD3 Ab (2 μg/mL) for 8 hours. Expression of Cyclon mRNA was normalized against that of α-tubulin mRNA. (C) Induction of Cyclon protein expression in CD4+ and CD8+ T cells after anti-CD3 and anti-CD28 Ab stimulation. Cells were harvested at indicated time points after stimulation. Nuclear extracts were prepared and subjected to immunoblot analysis with anti-Cyclon Ab. (D) Schematic representation of the construct driving T cell–specific expression of HA-mCyclon. HA-mCyclon cDNA (shaded box) is being placed under the control of a human CD2 (hCD2) promoter and locus control region (LCR). The Not I site in parentheses was destroyed by blunting. (E) Expression of Cyclon mRNA in Cyclon-Tg splenocytes. mRNA expression was detected by RT-PCR analysis. (F) Expression of HA-mCyclon protein in Cyclon-Tg splenocytes. Nuclear extracts from splenocytes were subjected to immunoblot analysis with anti-HA Ab. (G) Expression of endogenous and transgenic Cyclon proteins in CD4+ and CD8+ T cells from WT and Cyclon-Tg mice. Cells were stimulated with anti-CD3 and anti-CD28 Abs for 24 hours. Nuclear extracts were prepared and subjected to immunoblot analysis with anti-Cyclon Ab. Relative amounts of Cyclon protein were shown. Please note that transgenic Cyclon has a larger molecular weight than endogenous Cyclon protein because of addition of the HA-tag.
Activation-induced expression of Cyclon in primary T cells and generation of T-cell-specific Cyclon-Tg mice. (A) Induction of Cyclon mRNA in CD4+ and CD8+ splenic T cells by anti-CD3 and anti-CD28 Ab stimulation. Magnetically sorted cells were stimulated by plate-bound anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL). Total RNA was subjected to RT-PCR analysis. (B) Real-time RT-PCR analysis of expression of Cyclon mRNA in resting and activated CD4+ or CD8+ splenic T cells. Magnetically sorted cells were stimulated by plate-bound anti-CD3 Ab (2 μg/mL) for 8 hours. Expression of Cyclon mRNA was normalized against that of α-tubulin mRNA. (C) Induction of Cyclon protein expression in CD4+ and CD8+ T cells after anti-CD3 and anti-CD28 Ab stimulation. Cells were harvested at indicated time points after stimulation. Nuclear extracts were prepared and subjected to immunoblot analysis with anti-Cyclon Ab. (D) Schematic representation of the construct driving T cell–specific expression of HA-mCyclon. HA-mCyclon cDNA (shaded box) is being placed under the control of a human CD2 (hCD2) promoter and locus control region (LCR). The Not I site in parentheses was destroyed by blunting. (E) Expression of Cyclon mRNA in Cyclon-Tg splenocytes. mRNA expression was detected by RT-PCR analysis. (F) Expression of HA-mCyclon protein in Cyclon-Tg splenocytes. Nuclear extracts from splenocytes were subjected to immunoblot analysis with anti-HA Ab. (G) Expression of endogenous and transgenic Cyclon proteins in CD4+ and CD8+ T cells from WT and Cyclon-Tg mice. Cells were stimulated with anti-CD3 and anti-CD28 Abs for 24 hours. Nuclear extracts were prepared and subjected to immunoblot analysis with anti-Cyclon Ab. Relative amounts of Cyclon protein were shown. Please note that transgenic Cyclon has a larger molecular weight than endogenous Cyclon protein because of addition of the HA-tag.
To analyze function of Cyclon in T cells in vivo, we generated transgenic mice overexpressing Cyclon in the T-cell lineages (Cyclon-Tg mice) under the control of the VA-hCD2 cassette (Figure 1D),62 which drives transgene expression in virtually all thymocytes as well as peripheral T cells.62,63 mRNA expression of the Cyclon transgene and its protein expression in splenocytes were confirmed by RT-PCR (Figure 1E) and by immunoblot analysis using anti-HA Ab (Figure 1F). In addition, immunoblot analysis with anti-Cyclon Ab revealed that expression levels of total Cyclon (endogenous and transgenic) were 1.70- and 1.49-fold higher in CD4+ and CD8+ T cells, respectively, from Cyclon-Tg mice than in those cells from WT mice (Figure 1G). These mice were backcrossed with C57BL/6 mice at least 6 times for further analysis. We obtained consistent results from different founder lines in the experiments described below.
Transgenic expression of Cyclon normalizes splenomegaly and lymphadenopathy in IL-2Rα− mice
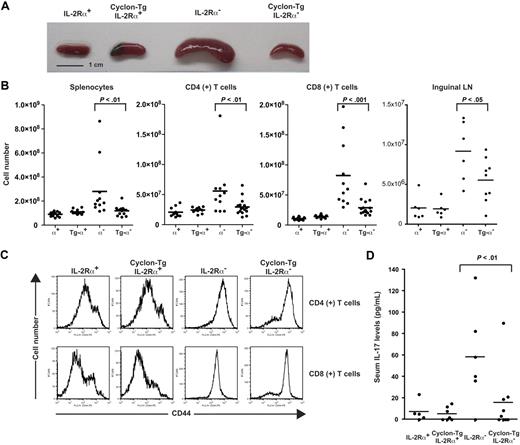
We found that T-cell development in the thymus and spleen was normal in Cyclon-Tg mice (data not shown). Because Cyclon is induced by stimulation with growth-promoting cytokines, including IL-2,57 we examined whether transgenic expression of Cyclon restores defective T-cell development and/or function in mice with defective IL-2 signaling. To determine whether forced expression of Cyclon rescues autoimmune phenotypes in mice with defective IL-2 signaling, we crossed Cyclon-Tg mice to the IL-2Rα− background. We observed splenomegaly in control 7-week-old IL-2Rα− mice as reported previously (Figure 2A). Interestingly, transgenic expression of Cyclon normalized the size of spleen in the IL-2Rα− background (Figure 2A). Numbers of total splenocytes, CD4+ T cells, and CD8+ T cells were significantly reduced by the transgenic expression of Cyclon in IL-2Rα− background (Figure 2B). Transgenic expression of Cyclon also reduced cell numbers of inguinal lymph nodes in IL-2Rα− mice (Figure 2B), suggesting that effects of transgenic expression of Cyclon are not specific on splenic T cells but general on peripheral T cells. We found that T cells from IL-2Rα− mice as well as those from Cyclon-Tg IL-2Rα− mice express high levels of CD44 (Figure 2C) and CD69 (data not shown), suggesting that transgenic expression of Cyclon does not normalize aberrant activation of peripheral T cells in IL-2Rα− mice.
Normalization of autoimmune phenotype of IL-2Rα− mice by transgenic expression of Cyclon. (A) Spleens from 7-week-old IL-2Rα+, Cyclon-Tg IL-2Rα+, IL-2Rα−, and Cyclon-Tg IL-2Rα− mice. (B) Cell numbers of splenocytes, CD4+, and CD8+ splenic T cells, and cells in inguinal lymph nodes from IL-2Rα+, Cyclon-Tg IL-2Rα+, IL-2Rα−, and Cyclon-Tg IL-2Rα− mice. (C) Expression of CD44 on CD4+ and CD8+ splenic T cells from IL-2Rα+, Cyclon-Tg IL-2Rα+, IL-2Rα−, and Cyclon-Tg IL-2Rα− mice. (D) Transgenic expression of Cyclon decreases IL-17 in serum of IL-2Rα− mice. Serum concentrations of IL-17 were analyzed in 7-week-old IL-2Rα+, Cyclon Tg IL-2Rα+, IL-2Rα−, and Cyclon TgIL-2Rα− mice by enzyme-linked immunosorbent assay.
Normalization of autoimmune phenotype of IL-2Rα− mice by transgenic expression of Cyclon. (A) Spleens from 7-week-old IL-2Rα+, Cyclon-Tg IL-2Rα+, IL-2Rα−, and Cyclon-Tg IL-2Rα− mice. (B) Cell numbers of splenocytes, CD4+, and CD8+ splenic T cells, and cells in inguinal lymph nodes from IL-2Rα+, Cyclon-Tg IL-2Rα+, IL-2Rα−, and Cyclon-Tg IL-2Rα− mice. (C) Expression of CD44 on CD4+ and CD8+ splenic T cells from IL-2Rα+, Cyclon-Tg IL-2Rα+, IL-2Rα−, and Cyclon-Tg IL-2Rα− mice. (D) Transgenic expression of Cyclon decreases IL-17 in serum of IL-2Rα− mice. Serum concentrations of IL-17 were analyzed in 7-week-old IL-2Rα+, Cyclon Tg IL-2Rα+, IL-2Rα−, and Cyclon TgIL-2Rα− mice by enzyme-linked immunosorbent assay.
Transgenic expression of Cyclon reduces IL-17 levels in the sera in IL-2Rα− mice
It was recently shown that mice lacking IL-2 have increased T helper 17 (TH17) cells in the mesenteric lymph nodes and elevated serum levels of IL-17.64 WT and Cyclon-Tg mice had barely detectable levels of IL-17 (Figure 2D). Consistent with the previous report, we observed elevated levels of serum IL-17 in IL-2Rα− mice (Figure 2D). In contrast, Cyclon-Tg IL-2Rα− mice had much reduced levels of serum IL-17 (Figure 2D). These results suggest that transgenic expression of Cyclon also normalizes aberrant production of IL-17, one of the manifestations of autoimmune syndrome in IL-2Rα− mice.
Transgenic expression of Cyclon normalizes splenomegaly in γc− mice
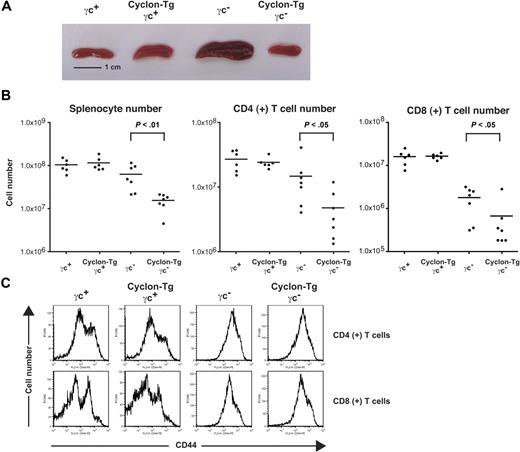
γc is the common subunit of cytokine receptors, including IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21.50 Loss of γc causes immunodeficiency characterized by profoundly diminished numbers of T and natural killer cells in human and mouse.50 Although γc− mice have markedly decreased numbers of thymocytes, accumulation of activated T cells in the periphery occurs as mice age.55 Accumulation of activated T cells can be caused at least partly by the lack of Foxp3+CD4+ regulatory T cells (T-regs) in γc− mice65 and by defects in AICD in γc− T cells.56 When we examined thymocyte development of γc− mice and Cyclon-Tg γc− mice, thymocyte development of γc− mice was not restored by transgenic expression of Cyclon (data not shown). As shown in Figure 3A, 8-week-old γc− mice had enlarged spleens compared with γc+ and Cyclon-Tg γc+ mice. As was observed in the Cyclon-Tg IL-2Rα− mice, Cyclon-Tg γc− mice had markedly smaller spleens than littermate γc− mice. Numbers of total splenocytes, CD4+ T cells, and CD8+ T cells were significantly reduced by the transgenic expression of Cyclon in γc− background (Figure 3B). Peripheral T cells in γc− mice and Cyclon-Tg γc− mice had activated phenotype shown by high expression levels of CD44 (Figure 3C) and CD69 (data not shown), suggesting that transgenic expression of Cyclon does not normalize aberrant activation of peripheral T cells in γc− mice. The size of T cells from γc− mice was much larger than that of T cells from γc+ mice (larger forward scatter values in flow cytometry, data not shown) because of their activated phenotype. This resulted in the increase in the size of spleens from γc− mice compared with γc+ mice (Figure 3A), although splenocyte numbers of γc− mice were slightly lower than those of γc+ mice (Figure 3B). These results collectively suggest that transgenic expression of Cyclon normalizes autoimmune-associated splenomegaly/lymphadenopathy and other autoimmune symptoms in mice lacking IL-2 signaling without normalizing activated phenotype of peripheral T cells.
Normalization of splenomegaly of γc− mice by transgenic expression of Cyclon. (A) Spleens from 8-week-old γc+, Cyclon-Tg γc+, γc−, and Cyclon-Tg γc− mice. (B) Cell numbers of splenocytes, CD4+ splenic T cells, and CD8+ splenic T cells from γc+, Cyclon-Tg γc+, γc−, and Cyclon-Tg γc− mice. (C) Expression of CD44 on CD4+ and CD8+ splenic T cells from γc+, Cyclon-Tg γc+, γc−, and Cyclon-Tg γc− mice.
Normalization of splenomegaly of γc− mice by transgenic expression of Cyclon. (A) Spleens from 8-week-old γc+, Cyclon-Tg γc+, γc−, and Cyclon-Tg γc− mice. (B) Cell numbers of splenocytes, CD4+ splenic T cells, and CD8+ splenic T cells from γc+, Cyclon-Tg γc+, γc−, and Cyclon-Tg γc− mice. (C) Expression of CD44 on CD4+ and CD8+ splenic T cells from γc+, Cyclon-Tg γc+, γc−, and Cyclon-Tg γc− mice.
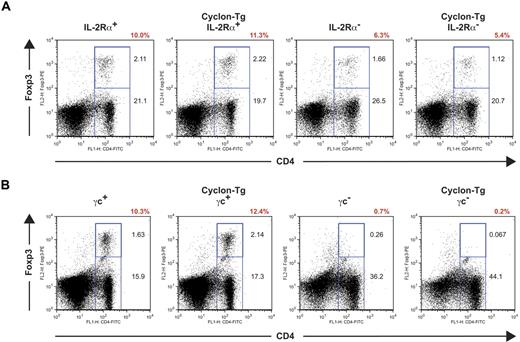
Transgenic expression of Cyclon does not restore development of T-regs
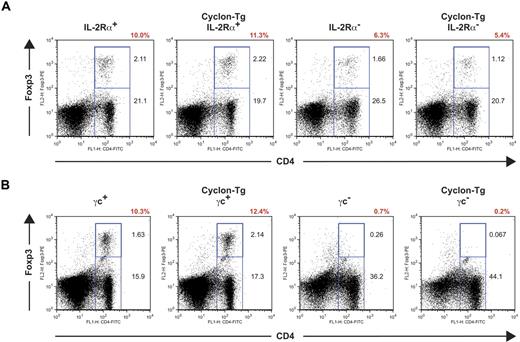
Autoimmunity in mice lacking IL-2 signaling can be caused at least partly by reduced numbers of T-regs in these mice65,66 and by defects in AICD. IL-2 signaling has been shown to be important for development and maintenance of Foxp3-expressing T-regs. Recent studies showed that IL-2Rα and IL-2Rβ are necessary for expansion of T-regs,65,66 whereas γc is essential for generation of T-regs in the thymus.65 To examine whether transgenic expression of Cyclon normalizes autoimmunity-associated splenomegaly through restoration of T-reg development in IL-2Rα− mice and γc− mice, we performed intracellular staining of Foxp3 in splenic CD4+ T cells. As shown in Figure 4A, IL-2Rα− mice had reduced percentages of T-regs. Transgenic expression of Cyclon failed to normalize numbers of T-regs in IL-2Rα− mice (Figure 4A). γc+ and Cyclon-Tg γc+ splenocytes contained sizable CD4+Foxp3+ T-reg populations (Figure 4B). In contrast, CD4+Foxp3+ cells were observed in neither γc− mice nor Cyclon-Tg γc− mice (Figure 4B), indicating that transgenic expression of Cyclon is unable to restore T-reg development in the absence of γc expression. These results indicate that Cyclon-mediated normalization of splenomegaly and lymphadenopathy in mice with defective IL-2 signaling is independent of function of T-regs.
Transgenic expression of Cyclon fails to restore defective T-reg development in IL-2R–deficient mice. (A) T-regs in spleens from IL-2Rα+, Cyclon Tg IL-2Rα+, IL-2Rα−, and Cyclon TgIL-2Rα− mice. (B) Lack of T-regs in Cyclon-Tg γc− mice. Splenocytes from 8-week-old mice were stained with mAbs to FITC-conjugated anti-CD4 Ab and PE-conjugated anti-Foxp3 Ab. Percentages of T-regs in CD4+ T cells are shown in red.
Transgenic expression of Cyclon fails to restore defective T-reg development in IL-2R–deficient mice. (A) T-regs in spleens from IL-2Rα+, Cyclon Tg IL-2Rα+, IL-2Rα−, and Cyclon TgIL-2Rα− mice. (B) Lack of T-regs in Cyclon-Tg γc− mice. Splenocytes from 8-week-old mice were stained with mAbs to FITC-conjugated anti-CD4 Ab and PE-conjugated anti-Foxp3 Ab. Percentages of T-regs in CD4+ T cells are shown in red.
Transgenic expression of Cyclon enhances AICD
Decreased T-cell numbers in peripheral lymphoid organs by transgenic expression of Cyclon in IL-2R-deficient mice could be caused by either reduced cell proliferation or increased cell death, or both. In this regard, proliferation of CD4+ and CD8+ T cells was indistinguishable between WT and Cyclon-Tg (supplemental Figure 1). In addition, no difference was observed in proliferation of T cells in IL-2R-deficient background (data not shown), suggesting that Cyclon does not affect cell proliferation. AICD is an important mechanism to limit lymphocyte expansion after eradication of pathogens. Both IL-2Rα− and γc− mice have defects in AICD.56 To determine whether transgenic expression of Cyclon affects AICD, we purified splenic CD4+ or CD8+ T cells from WT or Cyclon-Tg mice and examined cell death after secondary stimulation in the presence of IL-2. As shown in Figure 5A, Cyclon-Tg CD4+ or CD8+ T cells showed markedly increased AICD activity compared with WT CD4+ or CD8+ T cells. These data suggest that enhanced AICD by transgenic expression of Cyclon may be one of the mechanisms by which transgenic expression of Cyclon normalizes autoimmune phenotypes in mice deficient in IL-2 signaling.
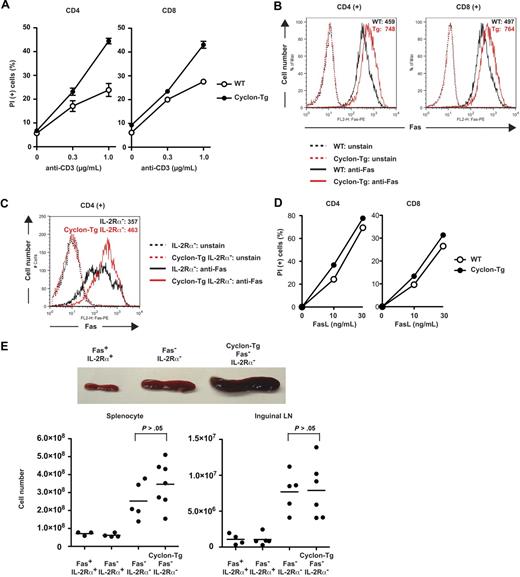
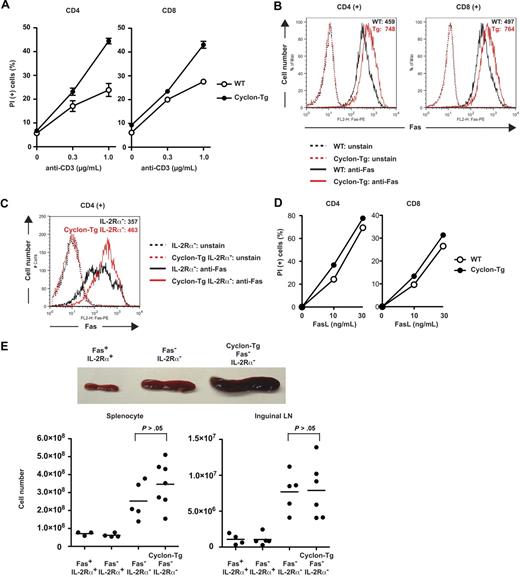
Cyclon positively regulates AICD through up-regulation of Fas expression. (A) Increased AICD of T cells by transgenic expression of Cyclon. CD4+ or CD8+ T cells sorted magnetically were activated with anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL) for 2 days. Live cells were purified by density centrifugation and restimulated with different concentrations of anti-CD3 in the presence of IL-2 (5 ng/mL). Cells were incubated for 2 days (left panel, CD4+ T cells) or 3 days (right panel, CD8+ T cells), and viability was examined by PI staining and flow cytometry. Indicated are representative data using WT and Cyclon-Tg littermates from 4 independent experiments. (B) Transgenic expression of Cyclon enhances Fas expression. CD4+ or CD8+ T cells were activated with anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL) for 2 days. Live cells were purified by density centrifugation and restimulated with anti-CD3 Ab (1 μg/mL) in the presence of IL-2 (5 ng/mL). Cells were incubated for 16 hours and stained with PE-conjugated anti-Fas Ab. (C) Transgenic expression of Cyclon enhances Fas expression on IL-2Rα− CD4+ T cells. CD4+ T cells sorted magnetically were activated with anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL) in the presence of IL-15 (100 ng/mL) for 2 days. Cells were stained with PE-conjugated anti-Fas Ab. (D) Increased cell death by FasL stimulation of T cells by transgenic expression of Cyclon. CD4+ or CD8+ T cells were activated with anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL) for 2 days. Live cells were purified by density centrifugation and restimulated with different concentrations of FasL in the presence of IL-2 (5 ng/mL). Cells were incubated for 24 hours, and viability was examined by PI staining and flow cytometry. Indicated are representative data using WT and Cyclon-Tg littermates from 4 independent experiments. (E) Cyclon transgenic expression fails to suppress splenomegaly and lymphadenopathy in Fas− IL-2Rα− mice. Spleens and numbers of splenocytes and inguinal lymph nodes from 7-week-old Fas+ IL-2Rα+, Fas− IL-2Rα−, and Cyclon-Tg Fas− IL-2Rα− mice are shown.
Cyclon positively regulates AICD through up-regulation of Fas expression. (A) Increased AICD of T cells by transgenic expression of Cyclon. CD4+ or CD8+ T cells sorted magnetically were activated with anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL) for 2 days. Live cells were purified by density centrifugation and restimulated with different concentrations of anti-CD3 in the presence of IL-2 (5 ng/mL). Cells were incubated for 2 days (left panel, CD4+ T cells) or 3 days (right panel, CD8+ T cells), and viability was examined by PI staining and flow cytometry. Indicated are representative data using WT and Cyclon-Tg littermates from 4 independent experiments. (B) Transgenic expression of Cyclon enhances Fas expression. CD4+ or CD8+ T cells were activated with anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL) for 2 days. Live cells were purified by density centrifugation and restimulated with anti-CD3 Ab (1 μg/mL) in the presence of IL-2 (5 ng/mL). Cells were incubated for 16 hours and stained with PE-conjugated anti-Fas Ab. (C) Transgenic expression of Cyclon enhances Fas expression on IL-2Rα− CD4+ T cells. CD4+ T cells sorted magnetically were activated with anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL) in the presence of IL-15 (100 ng/mL) for 2 days. Cells were stained with PE-conjugated anti-Fas Ab. (D) Increased cell death by FasL stimulation of T cells by transgenic expression of Cyclon. CD4+ or CD8+ T cells were activated with anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL) for 2 days. Live cells were purified by density centrifugation and restimulated with different concentrations of FasL in the presence of IL-2 (5 ng/mL). Cells were incubated for 24 hours, and viability was examined by PI staining and flow cytometry. Indicated are representative data using WT and Cyclon-Tg littermates from 4 independent experiments. (E) Cyclon transgenic expression fails to suppress splenomegaly and lymphadenopathy in Fas− IL-2Rα− mice. Spleens and numbers of splenocytes and inguinal lymph nodes from 7-week-old Fas+ IL-2Rα+, Fas− IL-2Rα−, and Cyclon-Tg Fas− IL-2Rα− mice are shown.
Cyclon positively regulates Fas expression, which is essential for reduction of splenomegaly by transgenic expression of Cyclon
It has been shown that the Fas/FasL system is critical for IL-2–dependent AICD.67,68 We examined whether enhancement of AICD by transgenic expression of Cyclon is the result of altered expression levels of Fas or FasL. We detected no changes in expression levels of FasL between WT and Cyclon-Tg T cells (supplemental Figure 2). In contrast, transgenic expression of Cyclon significantly enhanced expression levels of Fas both in activated CD4+ and CD8+ T cells (Figure 5B). Enhancement of Fas expression by transgenic expression of Cyclon was also observed on IL-2Rα− CD4+ T cells (Figure 5C). Fas expression levels on resting CD4+ T cells from Cyclon-Tg mice were not different from those on resting CD4+ cells from WT mice (supplemental Figure 3). In contrast, transgenic expression of Cyclon led to a mild increase in Fas expression on resting CD8+ T cells (supplemental Figure 3). Expression levels of other factors influencing cell death sensitivity, including FADD, FLIP-L, FLIP-S, caspase-8 precursor, and p18, were comparable in CD4+ and CD8+ T cells from WT and Cyclon-Tg mice (supplemental Figure 4A) and in CD4+ T cells from IL-2Rα− and Cyclon-Tg IL-2Rα− mice (supplemental Figure 4B).
To examine whether difference in expression levels of Fas affects sensitivity to Fas-mediated cell death, we analyzed effects of FasL on cell death of CD4+ and CD8+ T cells from WT and Cyclon-Tg mice. As shown in Figure 5D, Cyclon-Tg T cells were more susceptible to stimulation with FasL. In contrast, CD4+ and CD8+ T cells from WT and Cyclon-Tg mice showed comparable cell death responses to a nonspecific cell death inducer, puromycin (supplemental Figure 5), suggesting that enhanced cell death is specific to FasL stimulation. These data imply that increased expression of Fas by transgenic expression of Cyclon leads to enhanced Fas-mediated T-cell death.
To examine whether Fas expression is required for Cyclon-mediated normalization of splenomegaly/lymphadenopathy in IL-2Rα− mice, we compared sizes of spleens of Fas-deficient (Fas−, lpr) IL-2Rα− mice in the absence or presence of the Cyclon transgene. At 7 weeks of age, we observed that Fas− IL-2Rα− mice develop splenomegaly (Figure 5E). Transgenic expression of Cyclon did not normalize splenomegaly and enlargement of lymph nodes in Fas− IL-2Rα− mice (Figure 5E). In addition, splenomegaly of Fas− mice was not normalized by transgenic expression of Cyclon (supplemental Figure 6). These results indicated that Fas expression is required for Cyclon-mediated normalization of splenomegaly/lymphadenopathy in mice deficient in IL-2 signaling and hence suggested that Cyclon plays an important role in AICD through expression regulation of Fas.
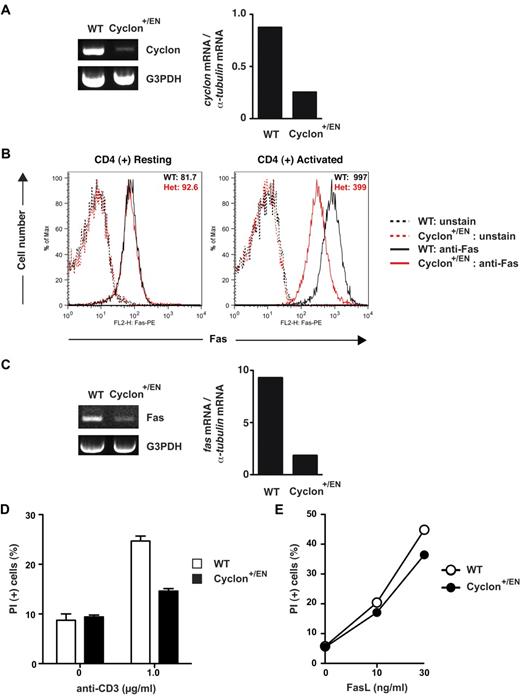
Decreased expression of Fas and AICD in CD4+ T cells with reduced Cyclon expression
To further examine the roles of Cyclon in Fas expression, we generated mutant mice in which enhanced green fluorescent protein and the neor gene were inserted into the exon 1 of a Cyclon allele to disrupt expression of Cyclon (Cyclon+/EN mice; supplemental Figure 7). Mice homozygous for this mutant allele were not born, suggesting that complete loss of Cyclon expression leads to embryonic/perinatal lethality (supplemental Figure 8). Development of T cells in Cyclon+/EN mice was normal (data not shown). Numbers of total splenocytes, splenic CD4+ and CD8+ T cells, expression of CD44, and T-cell proliferation induced by anti-CD3 and anti-CD28 Abs were normal in Cyclon+/EN mice (supplemental Figure 9). Expression levels of Cyclon mRNA were markedly (∼ 3-fold) reduced in activated CD4+ T cells from Cyclon+/EN mice compared with control WT CD4+ T cells (Figure 6A). We observed no difference in Fas expression levels in resting CD4+ T cells from WT and Cyclon+/EN mice (Figure 6B left panel). In contrast, activated Cyclon+/EN CD4+ T cells showed significant reduction of cell surface expression (∼ 2.5-fold) and mRNA expression of Fas (∼ 5-fold; Figure 6B right panel, C). Surface expression of Fas can be regulated by multiple steps, including translation, sorting to the plasma membrane, internalization, and degradation. Therefore, it is possible that some of these mechanisms may contribute to difference in reduction between surface Fas protein and Fas mRNA. We did not detect change in Fas expression levels in resting and activated CD8+ T cells from Cyclon+/EN mice (supplemental Figure 10A). Because Cyclon expression levels were similarly decreased in activated CD8+ T cells (supplemental Figure 10B), Fas expression may be differentially regulated in CD4+ and CD8+ T cells. Expression levels of other factors influencing cell death sensitivity, including FADD, FLIP-L, FLIP-S, caspase-8 precursor, and p18, were comparable in CD4+ T cells from WT and Cyclon+/EN mice (supplemental Figure 11). Anti-CD3-mediated AICD of CD4+ T cells from Cyclon+/EN mice was markedly lower than that of T cells from WT mice (Figure 6D). Furthermore, CD4+ T cells from Cyclon+/EN mice showed significantly reduced cell death in response to different concentrations of FasL (Figure 6E). CD4+ T cells from WT and Cyclon-Tg mice had comparable cell death responses to a nonspecific cell death inducer, puromycin (supplemental Figure 5), indicating that enhanced cell death is specific to FasL stimulation. These data clearly indicate that Cyclon plays a critical role in up-regulation of Fas expression and AICD in CD4+ T cells on activation.
Decreased expression of Fas and AICD in CD4+ T cells by reduced Cyclon expression. (A) Expression levels of Cyclon mRNA in activated CD4+ T cells from WT and Cyclon+/EN mice. (B) Surface expression of Fas on activated CD4+ T cells from WT and Cyclon+/EN mice. CD4+ T cells sorted magnetically were activated with anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL) for 3 days and cultured in the presence of IL-2 for 1 day, and then stained with PE-conjugated anti-Fas Ab. (C) Expression of Fas mRNA in activated CD4+ T cells from WT and Cyclon+/EN mice. (D) Decreased AICD of CD4+ T cells from Cyclon+/EN mice. CD4+ T cells sorted magnetically were activated with anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL) for 3 days. Live cells were purified by density centrifugation and restimulated with different concentrations of anti-CD3 in the presence of IL-2 (5 ng/mL). Cells were incubated for 2 days, and viability was examined by PI staining and flow cytometry. (E) Decreased FasL-induced cell death of CD4+ T cells from Cyclon+/EN mice. CD4+ T cells were activated with anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL) for 3 days. Live cells were purified by density centrifugation and restimulated with different concentrations of FasL in the presence of IL-2 (5 ng/mL). Cells were incubated for 24 hours, and viability was examined by PI staining and flow cytometry.
Decreased expression of Fas and AICD in CD4+ T cells by reduced Cyclon expression. (A) Expression levels of Cyclon mRNA in activated CD4+ T cells from WT and Cyclon+/EN mice. (B) Surface expression of Fas on activated CD4+ T cells from WT and Cyclon+/EN mice. CD4+ T cells sorted magnetically were activated with anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL) for 3 days and cultured in the presence of IL-2 for 1 day, and then stained with PE-conjugated anti-Fas Ab. (C) Expression of Fas mRNA in activated CD4+ T cells from WT and Cyclon+/EN mice. (D) Decreased AICD of CD4+ T cells from Cyclon+/EN mice. CD4+ T cells sorted magnetically were activated with anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL) for 3 days. Live cells were purified by density centrifugation and restimulated with different concentrations of anti-CD3 in the presence of IL-2 (5 ng/mL). Cells were incubated for 2 days, and viability was examined by PI staining and flow cytometry. (E) Decreased FasL-induced cell death of CD4+ T cells from Cyclon+/EN mice. CD4+ T cells were activated with anti-CD3 Ab (2 μg/mL) and anti-CD28 Ab (2 μg/mL) for 3 days. Live cells were purified by density centrifugation and restimulated with different concentrations of FasL in the presence of IL-2 (5 ng/mL). Cells were incubated for 24 hours, and viability was examined by PI staining and flow cytometry.
Discussion
In this study, we showed that T-cell activation induces expression of Cyclon, a novel cytokine-inducible nuclear protein (Figure 1). Initially, Cyclon was identified as an immediately early gene induced by growth-promoting cytokines, including IL-3 and IL-2.57 Because TCR-mediated expression of Cyclon mRNA is promptly induced, we think that TCR stimulation directly induces Cyclon expression but not indirectly, such as by IL-2 produced by activated T cells. In other words, there may be different mechanisms of Cyclon induction by different stimuli, such as growth-promoting cytokine (eg, IL-3, IL-2) and TCR stimulation. At this stage, we do not know the significance of IL-2− or IL-3−mediated induction of Cyclon in T cells and other cell lineages.
Next, we examined roles of Cyclon in the regulation of T-cell function in vivo. Transgenic expression of Cyclon specifically in the T-cell lineages suppressed development of splenomegaly, lymphadenopathy, and aberrant production of IL-17 in mice lacking IL-2 signaling (Figures 2,3). Because IL-17 has been linked to autoimmunity,69 reduction of IL-17 expression by transgenic expression of Cyclon may also contribute to normalization of autoimmune phenotype of IL-2Rα− mice. Autoimmunity in mice with defective IL-2 signaling is considered to be caused at least partly by the defects in T-regs65,66 and AICD.56 We showed that transgenic expression of Cyclon neither restores development of T-reg in mice (Figure 4) nor corrects aberrant activation of peripheral T cells in mice deficient in IL-2 signaling (Figure 2C,3C). These results are consistent with the interpretation that Cyclon-mediated normalization of autoimmune symptoms in these mice is independent of function of T-regs. In contrast, transgenic expression of Cyclon enhanced AICD both in CD4+ and CD8+ T cells (Figure 5A), whereas CD4+ T cells from Cyclon+/EN mice showed reduced AICD (Figure 6D), indicating that enhancement of AICD is one of the mechanisms of Cyclon action.
The Fas-dependent death pathway is well characterized, and its importance in immune homeostasis is firmly established.37 IL-2 was shown to increase transcription and surface expression of FasL and suppress transcription and protein expression of FLIP, an inhibitor of apoptosis. We showed that transgenic expression of Cyclon significantly enhances up-regulation of Fas on activated CD4+ and CD8+ T cells in WT and IL-2Rα− background (Figure 5B-C). Furthermore, Fas up-regulation and AICD were attenuated in activated CD4+ T cells from Cyclon+/EN mice (Figure 6B-D). In contrast, expression of other proteins involved in the Fas pathway was not affected (supplemental Figures 2,4,11). Furthermore, expression levels of Fas were directly correlated with sensitivity to FasL-mediated cell death (Figures 5D,6E). In addition, our genetic analysis showed that Cyclon-mediated normalization of splenomegaly and lymphadenopathy of IL-2Rα− mice requires expression of Fas (Figure 5D), collectively suggesting that Cyclon plays a critical role in AICD presumably through regulation of Fas expression after TCR stimulation.
Apoptosis is regulated by the extrinsic stimuli, such as signals through the FasL/Fas pathway as well as the intrinsic pathway involving Bcl-2 family members.1,2 It was recently shown that T-cell death after acute infection is mainly regulated by the intrinsic pathway in which Bim plays a critical role, whereas the Fas pathway regulates T-cell death in chronic infection and autoimmunity.3-5 Consistent with this notion, there was no difference in contraction of T cells between WT and Cyclon-Tg mice after injection of staphylococcal enterotoxin B (data not shown), further confirming that Cyclon regulates Fas expression but not expression or activities of regulators of the intrinsic pathway.
Expression of Fas on CD4+ and CD8+ T cells was differentially affected by an increase or decrease in expression levels of Cyclon. In CD4+ T cells, the effects of an increase or decrease in Cyclon expression on Fas expression were observed in activated but not resting cells (Figures 5–6, supplemental Figure 3). These results suggest an essential role of Cyclon in Fas up-regulation on activation in CD4+ T cells. Because Cyclon expression was low in resting T cells and induced by T-cell activation, it may be reasonable to assume that Cyclon is involved in enhancement of Fas expression on T-cell activation. On the other hand, transgenic expression of Cyclon increased expression of Fas in activated as well as resting CD8+ T cells (Figure 5, supplemental Figure 3), suggesting that forced expression of Cyclon is sufficient for up-regulation of Fas in CD8+ T cells. In contrast, reduction of Cyclon expression in CD8+ T cells did not affect Fas expression levels (supplemental Figure 10). These results suggest that enhancement of Fas expression in CD8+ T cells requires less Cyclon protein than in CD4+ T cells. Analysis of conditional deletion of Cyclon in T cells would be an interesting future issue to clarify roles of Cyclon in Fas expression in CD8+ T cells.
Understanding of regulation mechanisms of Fas expression in T cells is important for development of novel therapeutic approaches for autoimmune diseases and lymphoma/leukemia. However, surprisingly little is known about regulation mechanisms of Fas expression in primary T cells. It has been shown that TDAG51 is important for Fas induction in a protein kinase C−dependent manner in a T-cell hybridoma.44 Transcription factors, including p53,41 c-myc single-strand binding protein,70 Sp3,71 NF-κB,46,47 and AP-1,72 have been suggested to be involved in Fas expression regulation in various cell lines. However, involvement of these proteins in regulation of Fas expression in primary T cells has not been clearly demonstrated. In this regard, it was shown that neither TDAG51 nor NF-κB is essential for Fas expression regulation and apoptosis of primary mouse T cells in vivo.45,47 Information on critical elements in promoter/enhancer of the Fas gene in primary T cells is also very limited. Our preliminary data showed that the 6-kbp fragment upstream of the start codon of mouse Fas gene is not sufficient to support expression of a reporter gene in primary mouse CD4+ T cells (S.S.F. and H.F., unpublished observation, February 2008), suggesting that elements outside of this fragment might be important for expression of the Fas gene in primary T cells. Our present study revealed a nuclear modifier of Fas expression in primary T cells in vivo for the first time. Cyclon is a phosphorylated nuclear protein with a coiled-coil domain at the C-terminus.57 Coiled coils can be involved in signal-inducing events, molecular recognition, mechanical stability of cells, and movement processes.73 Therefore, it would be reasonable to assume that Cyclon interacts with Cyclon itself or other proteins via its coiled-coil domain to regulate expression levels of Fas. Considering the fact that Cyclon is localized in the nucleus57 and Fas mRNA levels were decreased by the reduction of Cyclon expression (Figure 6C), candidate Cyclon-interacting proteins would be transcription factors involved in the regulation of expression levels of Fas. We think that our data provide an important clue to elucidation of Fas expression regulation in T cells and that future investigation of mechanisms of Cyclon action will be an important step for understanding of molecular mechanisms of Fas expression regulation. Furthermore, our data suggest that pharmacologic regulation of Cyclon expression levels may be a potential clinical intervention of Fas expression levels in the treatment of autoimmune diseases and leukemia/lymphoma.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dan R. Littman for his kind and generous help, the Transgenic Core Facility of New York University Cancer Institute for generation of transgenic mice, Lina Kozhara and Derya Unutmaz for flow cytometric sorting, and Adriana Arita and Quazi Al-Tariq for technical assistance.
This work was supported by National Institutes of Health grant R01 AI059315 (H.F.).
National Institutes of Health
Authorship
Contribution: S.S.F. designed and performed research and wrote the paper; A.H. and K.K. performed research; T.E. contributed to generation of Cyclon-deficient mice; and H.F. directed, designed, and performed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hodaka Fujii, Combined Program on Microbiology and Immunology, Research Institute for Microbial Diseases, Osaka University, 3-1 Yamadaoka, Suita-shi, Osaka 565-0871, Japan; e-mail: hodaka@biken.osaka-u.ac.jp.