The search is on for natural regulators of platelet reactivity. In this issue of Blood, Trifiro and colleagues confirm that the low frequency isoform of platelet GPVI (VIb) attenuates ligand-mediated signal transduction and dampens down platelet reactivity with collagen while not affecting GPVI receptor copy number.
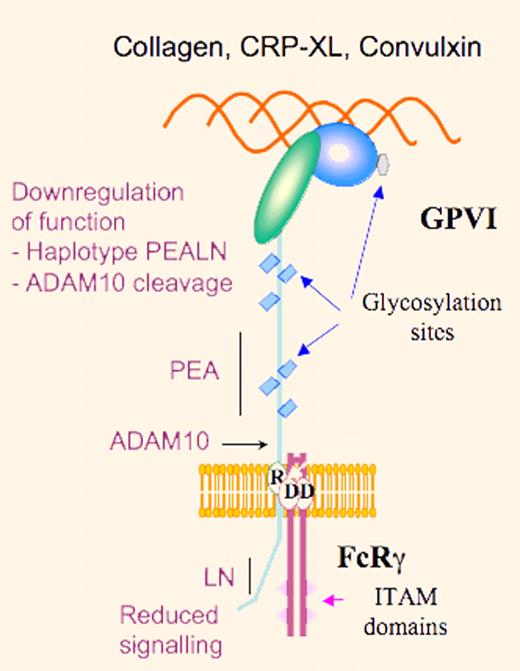
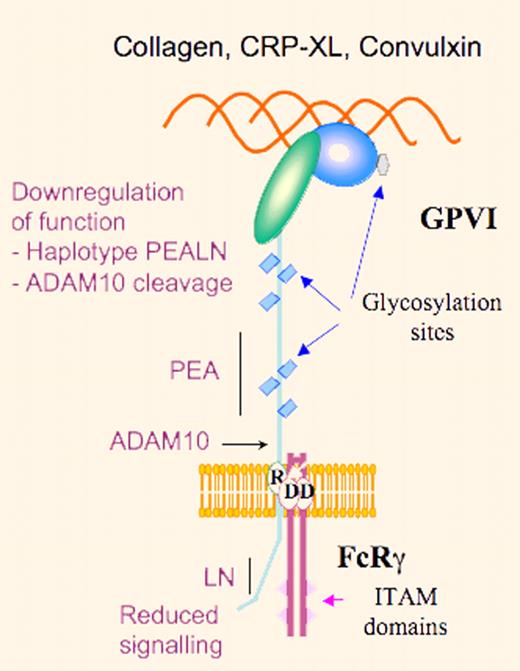
Platelet interaction with collagen is highly thrombogenic and is a significant factor in causing platelet accumulation at the site of atherosclerotic lesions. Platelets interact with collagen under shear through 2 receptors, the integrin α2β1 and glycoprotein VI (GPVI). Whereas these are independent receptors, the platelet response involves coordinated multistep activation. A major role is played by GPVI with signaling mediated by the noncovalently associated immunoreceptor tyrosine activation motif–containing FcRγ-chain. Significantly, platelet expression of both receptors is highly variable and their copy number may vary by up to 5-fold. This variability is in part under genetic control, and a number of candidate single nucleotide polymorphisms (SNPs) have been identified in both genes. The Kunicki laboratory has adopted a systematic approach to understanding the causes of changes in collagen receptor number between individuals; for example, they showed that single base changes in the 5′-regulatory region of the ITGA2 gene altered the binding of transcription factors SP1 and SP3 and modulated gene transcription.1 Subsequently, they used a mouse model to show how epigenetic factors, such as pre-mRNA splicing, were also involved in controlling α2β1 density.2 Attention also focused on GPVI when Croft et al described 2 common alleles and that a GP6 SNP was a risk factor for myocardial infarction.3 Other laboratories turned to new genome-sequencing technologies to identify platelet risk factors for cardiovascular disease. Ouwehand's group produced a high-resolution SNP map of GP6 and showed that the 2 common alleles (a and b) differed by 5 amino acid replacements, 3 in the stem (Ser199Pro, Lys217Glu, Thr229Ala) and 2 in the cytoplasmic domain (Gln297Leu and His302Asn).4 For these authors, the homozygous PEALN allele with an estimated frequency of 15.2% was associated with a somewhat reduced GPVI expression in platelets, a decreased tyrosine phosphorylation, and a lower functional response to the GPVI agonist cross-linked collagen-related peptide (CRP-XL). In widening the population profile for genomic studies, the newly formed Bloodomics consortium obtained a high-resolution SNP map of the GP6 locus, showing 12 additional rare GPVI isoforms and SNP variations between ethnic groups.5
Schematic representation of GPVI highlighting reduced platelet-collagen reactivity through down-regulation of GPVI signaling due to the PEALN haplotype or ADAM10-induced proteolysis.
Schematic representation of GPVI highlighting reduced platelet-collagen reactivity through down-regulation of GPVI signaling due to the PEALN haplotype or ADAM10-induced proteolysis.
In this context, Trifiro et al have re-examined the functional modifications in GPVI that accompany the change from the homozygous SKTQH to the PEALN allele.6 First, by Western blotting, they showed no differences between GP6 genotype and total platelet GPVI content among 132 normal subjects. Furthermore, there were no differences between the 2 haplotypes either as soluble products or as membrane-associated receptors in transfected DAMI cells with regard to their direct interaction with type I collagen, CRP, or the snake venom protein, convulxin (Cvx). However, both of the cytoplasmic domain substitutions in GPVIb had a significant effect on GPVI-dependent subcellular associations with signaling proteins and Cvx-induced signal transduction. More precisely, L317 increased binding affinity to calmodulin while N322 attenuates the rate and extent of Syk phosphorylation.
These observations once more emphasize the care needed in interpreting the significance of SNPs. The reasons for the 5-fold variation in GPVI expression in platelets remain to be explained and certainly other SNPs (perhaps in regulatory and promoter regions of GP6), epigenetic factors including variations in demethylation-based transcription7 as well as the known capacity of ADAM10 to cleave GPVI8 need to be taken into consideration. Also, what must be explained is the enigma that the decreased function of the GPVIb (PEALN) haplotype was first reported to be linked with an increased thrombotic risk. Notwithstanding, this study underlines the role of cytoplasmic domains of membrane receptors as regulators of signaling mechanisms.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■