Abstract
Acute erythroid leukemia (AEL) is a rare type of acute myeloid leukemia (AML) for which diagnostic criteria have been refined in the 2008 World Health Organization (WHO) classification of AML. The relationship of AEL to myelodysplastic syndromes (MDSs) and to AML with myelodysplasia-related changes (AML-MRC) is not clearly defined. We conducted a retrospective, multi-institutional study of patients with AEL and compared them with patients with MDS or AML-MRC with erythroid hyperplasia (≥ 50% erythroid cells). Among a total of 124 patients with AEL, 32% had a history of MDS or chronic cytopenia, 32% had therapy-related disease, and 35% had de novo disease. Sixty-four percent of patients had unfavorable AML risk-group karyotypes. FLT3 and RAS mutations were infrequent, occurring in 6% and 2%, respectively. The median overall survival (OS) of all AEL patients was 8 months, comparable with that of patients with MDS or AML-MRC with erythroid hyperplasia. The OS was related to cytogenetic risk group, but not blast count or morphologic dysplasia. Our findings suggest that AEL is in the continuum of MDS and AML with erythroid hyperplasia, where karyotype rather than an arbitrary blast cutoff represents the most important prognostic factor.
Introduction
Acute erythroid leukemia (AEL) is a rare form acute myeloid leukemia (AML), comprising less than 5% of cases of adult AML. The definition of AEL has undergone several revisions. Originally designated as M6 in the 1976 French-American-British (FAB) classification, its definition was refined in 1985 as an acute leukemia in which erythroid cells comprised at least 50% of all cells, and myeloblasts comprised at least 30% of the nonerythroid cells.1 In the 2001 World Health Organization (WHO) classification, the required blast count for all types of AML was lowered from 30% to 20%, which lowered the blast count defining AEL to 20% of the nonerythroid cells. In addition, a rare subcategory of AEL in which the neoplastic blasts were erythroid (so-called pure erythroid leukemia) was recognized.2 In the recent 2008 WHO classification of AML, the category of AML with myelodysplasia-related changes (AML-MRC) was proposed. This category includes all cases with blasts comprising 20% or more of all bone marrow cells and the presence of either morphologic evidence of significant multilineage dysplasia, specific myelodysplastic syndrome (MDS)–related cytogenetic abnormalities, or a history of MDS or a myelodysplastic/myeloproliferative neoplasm (MDS/MPN), irrespective of the presence of erythroid hyperplasia.3 According to this classification scheme, AEL cases with blasts comprising 20% or more of all bone marrow cells and fulfilling these criteria are classified as AML-MRC, whereas cases with blasts comprising less than 20% of all cells but 20% or more of the nonerythroid cells are classified as AEL.4 Moreover, if bone marrow blasts comprise less than 20% of nonerythroid cells, the case is then classified as MDS, not AEL. In effect, the distinction of AEL from MDS or AML-MRC with erythroid hyperplasia is based solely on the number of blasts, calculated as the proportion of nonerythroid cells in AEL but as the proportion of total bone marrow cells in MDS and AML-MRC. Given our limited understanding of the molecular genetic alterations in these diseases, this classification approach may arbitrarily divide biologically related myeloid neoplasms into different entities.
Because of the relative rarity of AEL, few large studies have examined its clinical and genetic features. Although AEL is now separated from AML-MRC, many publications have emphasized certain similarities between these disease categories, such as the high frequency of a preceding diagnosis of MDS or presence of multilineage dysplasia, as well as similarities in the observed cytogenetic alterations.5 AEL also has been shown to share similar karyotypic abnormalities with cases of AML with 20% to 30% blasts, previously categorized as refractory anemia with excess blasts in transformation (RAEB-t) in the FAB classification of MDS.6 Indeed, some leukemia experts have voiced concern that the lowered blast count of 20% of nonerythroid elements would lead to cases of MDS with excess blasts and transient erythroid hyperplasia being misdiagnosed as AEL.7 AEL has a relatively poor prognosis, with a median survival of 4 to 6 months. Cytogenetics appears to be a major factor in determining survival.8 Although allogeneic stem cell transplantation improves the outcome of AEL patients, most of these studies were based on AEL as defined by the FAB (≥ 30% blasts) system.9 No studies to date have reassessed AEL as currently defined in the 2008 WHO classification.
We studied the clinicopathologic and genetic features of 124 patients with AEL as defined using the criteria in the current WHO classification and examined their outcome in comparison with patients with MDS or AML-MRC associated with erythroid hyperplasia.
Methods
Patients
We searched the pathology archives at 4 institutions (Massachusetts General Hospital [MGH] between 2004 and 2008; University of Massachusetts Medical Center [UMASS] between 1999 and 2007; The University of Texas M. D. Anderson Cancer Center [MDACC] between 1992 and 2007; and University of Pittsburgh Medical Center [UP] between 2001 and 2007) for cases diagnosed as AEL. Cases fulfilling current WHO classification criteria for AEL of erythroid/myeloid subtype were included, whereas cases of AEL, pure erythroleukemia subtype, were excluded. Cases with 20% or more total blasts and 50% or more bone marrow erythroid elements were excluded from the AEL group. However, many of these cases fulfilled WHO 2008 classification criteria for AML-MRC (dysplasia in at least 50% of cells in at least 2 lineages, MDS-related cytogenetics, and/or a history of MDS) and were used as a comparison group (see next paragraph). The patients' records were reviewed for any history of antecedent chemotherapy, radiation therapy, hematologic neoplasm, or cytopenia(s). Based on the clinical presentation, the patients were assigned to 3 groups: AEL after MDS (MDS-AEL), comprising patients with a documented history of cytopenia(s) for at least 6 months preceding the diagnosis of AEL or a preceding diagnosis of MDS or MDS/MPN; therapy-related AEL (T-AEL), comprising patients who had an antecedent history of radiotherapy or chemotherapy or both; and de novo AEL (N-AEL), representing the patients who presented as acute onset disease with no history of MDS, chronic cytopenias, or therapy for an earlier neoplasm. Clinical follow-up information, including treatment modalities and overall survival (OS) from the date of AEL, was retrieved from the electronic medical records. All therapy with toxicity equivalent to standard “7 + 3” chemotherapy was classified as induction chemotherapy. This study was approved by the Institutional Review Boards of all participating institutions.
For comparison, 3 additional groups of patients were examined. The first group was that of 40 patients who had MDS with erythroid hyperplasia (≥ 50% erythroid cells) and blasts comprising 5% or more of nonerythroid cells collected from the pathology archives of UMASS. The clinicopathologic features of these MDS cases have been described previously.10 According to the current WHO classification, this MDS control group included 16 cases of refractory cytopenia with multilineage dysplasia, 13 cases of refractory anemia with excess blasts-1, 9 cases of therapy-related MDS, and 1 case each of refractory anemia with ring sideroblasts and MDS, unclassifiable. The International Prognostic Scoring System (IPSS) risk grouping of the 38 cases with available data was as follows: 6 low, 17 intermediate-1, and 15 intermediate-2 when the blasts were counted as a percentage of total cells, and 0 low, 10 intermediate-1, 17 intermediate-2, and 11 high when the blasts were counted as a percentage of the nonerythroid cells.10 The second group was that of 41 patients who had leukemias that fulfilled the 2001 WHO classification definition of AEL, but had features of AML-MRC according to the 2008 WHO classification (≥ 50% bone marrow erythroid elements with ≥ 20% blasts of total bone marrow cells, as well as dysplasia in ≥ 50% of cells from at least 2 hematopoietic lineages, an MDS-related cytogenetic abnormality, and/or a prior history of MDS).3,11 These cases were collected from the archives of the Department of Hematopathology at MDACC. Twenty-four of these patients had de novo disease, 9 developed AML-MRC after a diagnosis of MDS, and 8 had therapy-related neoplasms. Thirty-one of the MDACC AEL and AML-MRC cases had been included in a previous study evaluating treatment outcome in AML.12 The third group was that of 179 MDS patients lacking erythroid hyperplasia (< 50% erythroid cells) collected from the pathology archives of UMASS.
Pathology review
Bone marrow aspirate smears and core biopsy specimens were assessed by at least one observer at each institution (UP: C.G., MGH: R.P.H., UMASS: S.A.W. and G.T., MDACC: Z.Z., A.K., and S.A.W.) and the following pathologic parameters were scored: overall bone marrow cellularity based on the biopsy specimen; differential count (based on 500 cells, or all available cells) on bone marrow aspirate smears; dysplasia in the erythroid and myeloid lineages on aspirate smears; dysplasia of the megakaryocytic lineage on the biopsy specimen and aspirate smears; and presence of ring sideroblasts on a Perls-stained bone marrow aspirate smear (when available). Blast counts were calculated as a percentage of all nucleated cells and of the nonerythroid cells (excluding erythroid elements, but not other cell types), according to the WHO guidelines.11 For a diagnosis of dysplasia in any lineage, dysplastic changes had to be present in at least 10% of the cells in that lineage; however, for the purposes of including cases in the AML-MRC control group, dysplastic changes had to be present in at least 50% of the cells in at least 2 lineages. The criteria used for dysplasia by all the observers are stated in the 2008 WHO classification.13 A subset of the cases from each institution was re-reviewed by another observer at a different institution to evaluate concordance in the differential counts.
Conventional cytogenetics
Conventional cytogenetic analysis was performed on G-banded metaphase cells prepared from unstimulated bone marrow aspirate cultures using standard techniques. Twenty metaphases were analyzed and the results reported using the International System for Human Cytogenetic Nomenclature. Based on the karyotype, cases were stratified according to the International Prognostic Scoring System (IPSS) of MDS14 and according to the United Kingdom Medical Research Council (UKMRC) criteria for AML.15 The specific cytogenetic abnormalities that constitute these 2 cytogenetic risk groups are shown in Table 1.
FLT3 and K- and N-RAS mutation analysis
A fluorescent-based multiplex polymerase chain reaction assay was used to detect internal tandem duplication and D835 point mutations of the FLT3 gene using DNA isolated from bone marrow aspirate or peripheral blood samples, as previously described.16 K-RAS and N-RAS mutations were tested using polymerase chain reaction followed by pyrosequencing as described previously.17
Statistical analysis
The Fisher exact test and χ2 test were applied to categoric variables. The Mann-Whitney test was used for numeric comparisons between groups. Patient survival was estimated by the Kaplan-Meier method from the date of AEL diagnosis until death from any cause or last patient follow-up date. Survival curves were compared by the log-rank test. Differences between 2 groups were considered significant if P values were less than .05 in a 2-tailed test. Multivariate analysis was performed by Cox proportional regression model to examine the relationship between survival time and patient characteristics. The significant factors were identified by Wald backward stepwise elimination.
Results
Clinical findings
A total of 124 patients had clinical and pathologic findings that fulfilled the current WHO criteria for AEL, including 88 males and 36 females with a median age of 64 years (range, 12-93 years). Thirteen patients originated from UP, 20 from UMASS, 16 from MGH, and 75 from MDACC. To evaluate concordance in differential counts, 40 of 75 MDACC cases originally reviewed by S.A.W. were rereviewed by R.P.H., 8 of 16 MGH cases originally reviewed by R.P.H. were rereviewed by S.A.W., and 5 of 20 UMASS cases originally reviewed by G.T. were rereviewed by R.P.H. The second reviewer was blinded to the counts of the original reviewer. The correlation coefficient of the rereviews was high (0.87 [P < .001] and 0.83 [P < .001], respectively, for erythroid and blast percentages). The mean difference in erythroid cell percentage between the original review and rereview was 1.9% (P = .04; paired t test). The mean difference in the blast count between observers at initial review and rereview was 0.2% (P = .68; paired t test).
The clinical features of the AEL cases are summarized in Table 2. Cytopenias were common: 54 (46%) of 117 patients were pancytopenic at the time of AEL diagnosis. Based on review of the patient records, these patients were further subclassified into 3 groups: 40 (32%) MDS-AEL, 40 (32%) therapy-related AEL (T-AEL), and 44 (35%) de novo AEL (N-AEL). The 40 patients subclassified as MDS-AEL included 11 patients with cytopenias of more than 6-month duration, 28 patients with a history of MDS, and 1 patient with a history of a MDS/MPN, unclassified. The 40 patients subclassified as T-AEL had a history of an earlier neoplasm that was treated with various chemotherapy regimens, irradiation, or both. The 44 patients with N-AEL did not have a history of an antecedent hematologic neoplasm, chronic cytopenias, or chemotherapy/radiation therapy. The clinical features of these subgroups are shown in Table 3.
Morphologic findings
The morphologic features of the AEL cases are summarized in Table 2. Most bone marrow core biopsy specimens were hypercellular and, by definition, bone marrow aspirate smears showed numerous erythroid elements comprising at least 50% of all cells on the smear. Morphologic dysplasia was commonly observed and was present in the erythroid lineage in almost all cases, and in megakaryocytes in most cases. The median blast count was 12% (range, 4%-19%) of all nucleated cells and 32% (range, 20%-58%) of all nonerythroid cells in the bone marrow.
Results of conventional cytogenetics and mutation analysis
The results of conventional cytogenetic analysis were available for 119 (96%) patients. Table 4 shows AEL cases in each cytogenetic risk group assignment using the IPSS and UKMRC schemes. A summary of the most frequent cytogenetic abnormalities is shown in Table 5. Deletions and monosomies of chromosomes 5 and 7 were common and 43% of patients fulfilled criteria for a monosomal karyotype as defined by Breems et al.18 Aside from the abnormalities shown in Table 5, other abnormalities included t(6;9) (3 cases, sole finding in 2 cases and associated with a complex karyotype in 1 case), 11q23 abnormality (1 case, sole abnormality), del(13q) (2 cases, sole abnormality in 1 case and associated with a complex karyotype in 1 case), isolated trisomy 21 (1 case), and isolated loss of the Y chromosome (1 case). Losses/deletions of chromosome 7 were closely associated with losses/deletions of chromosomes 5 (31/45 cases, 69%) and 20 (12/19 cases, 63%), but not with trisomy 8 (+8) (7/25 cases, 28%, P = .001 and P = .03 compared with loss/deletion of chromosome 5 and 20, respectively). Loss/deletion of chromosome 20 occurred together with +8 in only 1 patient. Differences in the UKMRC AML cytogenetic risk group distribution of the N-AEL, MDS-AEL, and T-AEL subgroups are shown in Table 3. There was a striking association of chromosome 7 abnormalities with a history of cytotoxic therapy: 26 (74%) of 35 T-AEL cases versus 5 (13%) of 38 MDS-AEL cases (P < .001) and 12 (28%) of 43 N-AEL cases (P < .001) had loss/deletion of chromosome 7.
Mutation analysis performed on the subset of patients with available material revealed a FLT3 internal tandem duplication mutation in 3 (6%) of 49 cases, including 2 T-AEL cases and 1 MDS-AEL case. A K-RAS mutation was identified in 1 (2%) of55 cases, in the T-AEL group. No N-RAS mutations were identified in any of the 55 cases examined.
Treatment and survival
Seventy-three (59%) of 124 patients received induction chemotherapy, followed by allogeneic bone marrow transplantation (BMT) in 21 (17%) patients. Thirty-three (27%) patients did not receive induction chemotherapy; of these, 2 were treated with azacitidine; 2, with thalidomide; 2, with lenalidomide; and 1, with decitabine, and the remaining patients received supportive care only. Treatment information was not available for 18 patients. In the N-AEL group, 15 (33%) patients were treated with BMT, 16 were treated with induction alone, and 10 did not receive induction therapy. In the MDS-AEL group, 3 (8%) received a bone marrow transplant, 22 were treated with induction alone, and 12 did not receive induction therapy. In the T-AEL group, 3 (10%) underwent BMT, 14 were treated with induction alone, and 11 did not receive induction therapy. Patients in the N-AEL group received a bone marrow transplant more often than patients in either the MDS-AEL (P = .006) or T-AEL groups (P = .02).
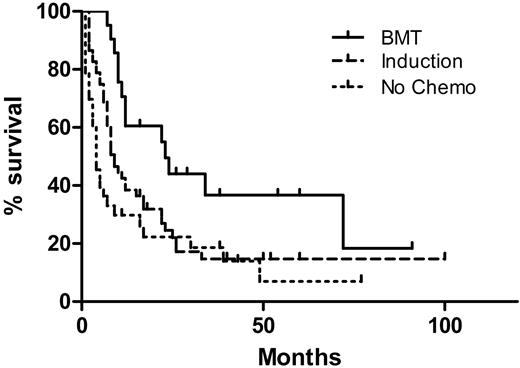
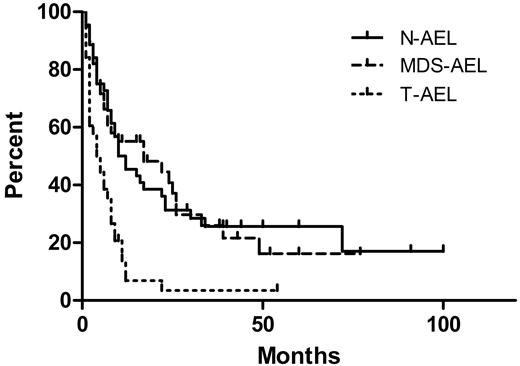
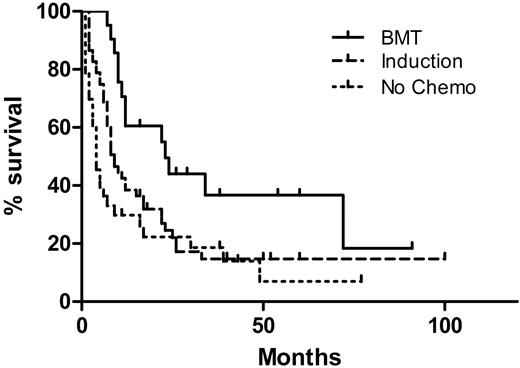
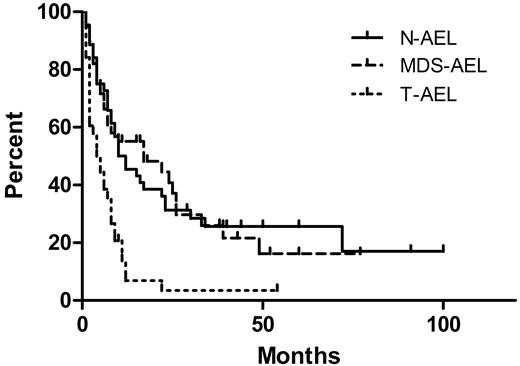
The median OS of all patients was 8 months, with a median follow-up time of 38 months for survivors and 8 months for all patients. The OS by treatment type is shown in Figure 1. Patients treated with induction and who received a bone marrow transplant had a median OS of 23 months, superior to patients treated with induction alone (9 months, P = .015), and patients receiving only supportive care or low-intensity therapy (4 months, P = .002). There was no significant difference in OS between patients receiving induction and those receiving only supportive or low-intensity therapy (P = .10). The OS by clinical presentation is shown in Figure 2. The median survival of patients with N-AEL (11 months) and MDS-AEL (17 months) was superior to patients with T-AEL (4.5 months; P < .001 for both). Excluding patients receiving a transplant, the median OS for patients with N-AEL (7.5 months) and MDS-AEL (17 months) was superior to T-AEL patients (5 months; P = .004 and P < .001, respectively). Within the MDS-AEL group, patients with chronic cytopenia had a longer median OS (49 months) than the post-MDS patients (8 months; P = .026, log-rank test). Among all AEL patients, there was no difference in OS based on the presence of peripheral blood blasts or nucleated red blood cells. Patients with a single cytopenia had a superior median OS (22 months) compared with patients with 2 or 3 cytopenias (8 months; P = .049). There was no difference in OS between patients with morphologic evidence of dysplasia in 1, 2, or 3 lineages, nor was there a difference in OS between patients with and without ring sideroblasts.
Overall survival (OS) of 106 acute erythroid leukemic patients by treatment modalities. The 21 patients who underwent allogeneic bone marrow transplantation (BMT) had a superior OS (median, 23 months) compared with 52 patients who received induction chemotherapy without BMT (median, 9 months; P = .015) as well as 33 patients who did not receive high-intensity chemotherapy (median OS, 4 months; P = .002). There was no difference in OS between patients receiving induction versus those who did not receive high-intensity chemotherapy (P = .10).
Overall survival (OS) of 106 acute erythroid leukemic patients by treatment modalities. The 21 patients who underwent allogeneic bone marrow transplantation (BMT) had a superior OS (median, 23 months) compared with 52 patients who received induction chemotherapy without BMT (median, 9 months; P = .015) as well as 33 patients who did not receive high-intensity chemotherapy (median OS, 4 months; P = .002). There was no difference in OS between patients receiving induction versus those who did not receive high-intensity chemotherapy (P = .10).
Overall survival (OS) of acute erythroid leukemia (AEL) patients by clinical subgroups. The therapy-related AEL patients (T-AEL) had a median OS of 4.5 months, inferior to that of de novo AEL patients (N-AEL; median OS, 11 months; P < .001) and patients with AEL after an antecedent myelodysplastic syndrome or chronic cytopenia (MDS-AEL; median OS, 17 months; P = .001). There was no difference in OS between the N-AEL and MDS-AEL patients (P = .99).
Overall survival (OS) of acute erythroid leukemia (AEL) patients by clinical subgroups. The therapy-related AEL patients (T-AEL) had a median OS of 4.5 months, inferior to that of de novo AEL patients (N-AEL; median OS, 11 months; P < .001) and patients with AEL after an antecedent myelodysplastic syndrome or chronic cytopenia (MDS-AEL; median OS, 17 months; P = .001). There was no difference in OS between the N-AEL and MDS-AEL patients (P = .99).
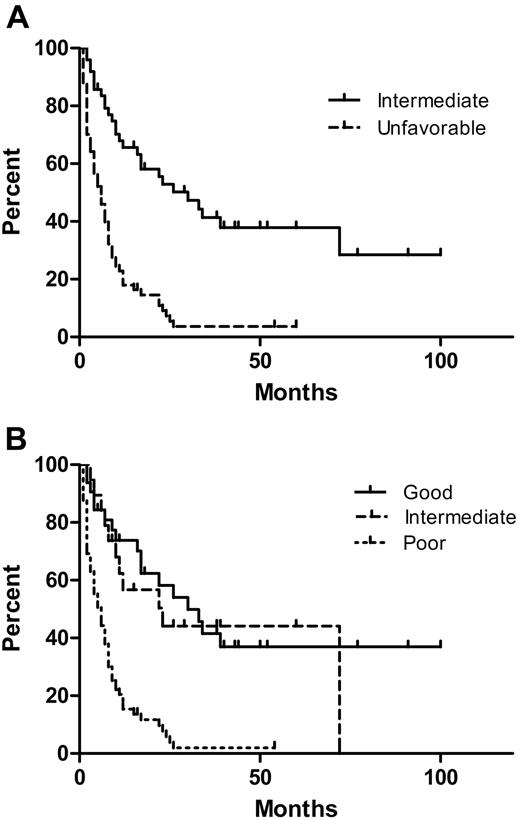
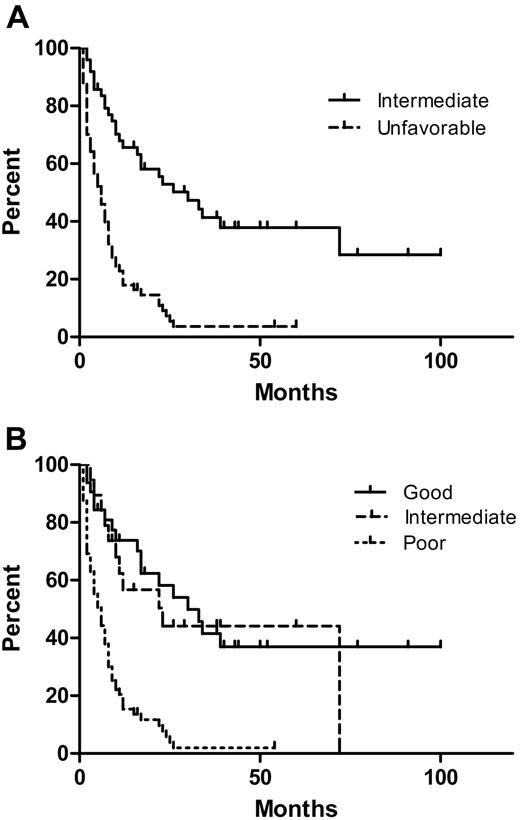
The OS of patients according to the UKMRC AML and the IPSS MDS cytogenetic risk groups are shown in Figure 3A and B. The median OS of patients with and without deletion/loss of chromosome 5 was 5.5 and 23 months, respectively (P < .001, log-rank test). The median OS of patients with and without deletion/loss of chromosome 7 was 7 months and 22 months, respectively (P = .001). Deletion/loss of chromosomes 5 and 7 occurred together in most cases. There was no significant difference in OS between patients with and without +8, nor was there difference in OS between patients with a normal karyotype compared with an abnormal karyotype in the AML intermediate cytogenetic risk group. The median OS of patients with or without a monosomal karyotype was 6.5 and 10 months, respectively (P = .02). However, UKMRC AML and IPSS cytogenetic risk groups were stronger predictors of OS than a monosomal karyotype. Within the AML intermediate cytogenetic risk group, there was no significant difference in the OS of patients who received a bone marrow transplant, were treated with induction chemotherapy alone, or did not receive induction chemotherapy (median survivals, 72, 26, and 30 months, respectively). However, AEL patients in the MRC AML unfavorable cytogenetic risk group benefited from more aggressive therapies: the 17-month median survival of those who received a BMT compared favorably with the 7-month median survival of those treated with induction only (P = .007) and the 2.5-month median survival of those not receiving induction therapy (P = .001). Induction therapy was also significantly correlated with a better OS compared with no induction therapy (P = .011) in this unfavorable cytogenetic risk group.
Overall survival (OS) of acute erythroid leukemia (AEL) patients by cytogenetic risk grouping. (A) The median OS of patients in the UKMRC AML intermediate and unfavorable groups was 30 and 6 months, respectively (P < .001). (B) The median OS of patients in the IPSS MDS good-, intermediate-, and poor-risk groups was 30, 23, and 6 months, respectively. The poor IPSS MDS risk group had a significantly inferior OS compared with the intermediate and good groups (both P < .001). There was no significant difference in OS between patients in the IPSS MDS good- and intermediate-risk groups (P = .68).
Overall survival (OS) of acute erythroid leukemia (AEL) patients by cytogenetic risk grouping. (A) The median OS of patients in the UKMRC AML intermediate and unfavorable groups was 30 and 6 months, respectively (P < .001). (B) The median OS of patients in the IPSS MDS good-, intermediate-, and poor-risk groups was 30, 23, and 6 months, respectively. The poor IPSS MDS risk group had a significantly inferior OS compared with the intermediate and good groups (both P < .001). There was no significant difference in OS between patients in the IPSS MDS good- and intermediate-risk groups (P = .68).
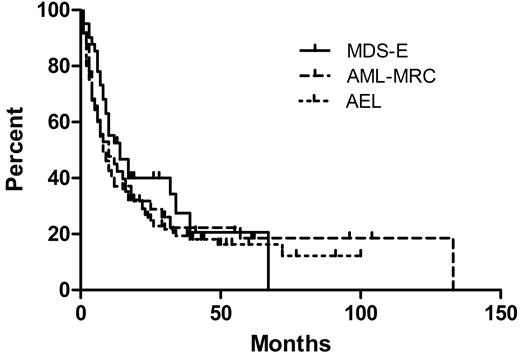
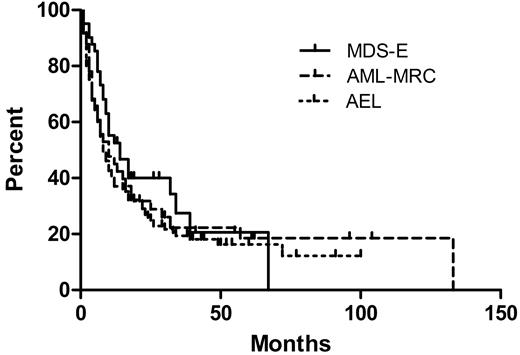
There was no correlation between bone marrow blast count (as a percentage of either all bone marrow cells or only nonerythroid cells) and survival among all the AEL cases (data not shown). When the groups of 40 MDS cases with erythroid hyperplasia and 41 AML-MRC cases with erythroid hyperplasia were compared with the 124 AEL cases, there was no difference in OS among any of the groups (Figure 4). Including all 3 groups (205 patients total), there was no significant difference in OS between strata when cases were stratified by blast counts of less than 5%, 5% to 9%, 10% to 14%, 15% to 19%, or 20% or more of all cells. There was also no difference in OS when the cases were stratified by blast counts of less than 10%, 10% to 19%, 20% to 29%, or 30% or more of nonerythroid cells (Table 6). However, similar to AEL, there was a significant difference in OS between the intermediate and unfavorable UKMRC AML cytogenetic risk groups among both the 35 AML-MRC cases with available cytogenetics (P = .001, log-rank test) and the 39 MDS with erythroid hyperplasia cases with available cytogenetics (P = .016, log-rank test). In contrast, survival analysis of 179 MDS cases lacking erythroid hyperplasia (< 50% erythroid elements) from UMASS showed a significant difference in OS based on blast count stratification (Table 6).
Comparison of 40 patients who had MDS with erythroid hyperplasia (MDS-E), 41 patients who had AML with myelodysplasia-related changes and erythroid hyperplasia (AML-MRC), and 124 patients with acute erythroid leukemia (AEL). The median OS values are 14, 8, and 10 months, respectively, which are not significantly different (P = .313).
Comparison of 40 patients who had MDS with erythroid hyperplasia (MDS-E), 41 patients who had AML with myelodysplasia-related changes and erythroid hyperplasia (AML-MRC), and 124 patients with acute erythroid leukemia (AEL). The median OS values are 14, 8, and 10 months, respectively, which are not significantly different (P = .313).
Multivariate analysis by Cox proportional regression was performed analyzing OS and the following factors: age, sex, clinical subgrouping, treatment type, bone marrow cellularity, bone marrow erythroid percentage, bone marrow blast count, hemoglobin level, white blood count, absolute neutrophil count, platelet level, and AML cytogenetic risk group. Only the AML cytogenetic risk group, treatment type (allogeneic BMT vs no BMT), and hemoglobin level were significantly associated with OS (Table 7).
Discussion
We analyzed the clinicopathologic features of the largest series of AEL patients reported to date in which both conventional cytogenetics and outcome data were available, for the purpose of reassessing AEL in light of the recent refinements in criteria proposed by the 2008 WHO classification. Our findings highlight a close relationship of AEL to AML-MRC and to MDS with erythroid hyperplasia. We found that AEL shares similar cytogenetic features to those of AML-MRC. If the blast counts were disregarded, 63% of the AEL cases in our series would fulfill WHO criteria for AML-MRC based on cytogenetic findings, and an additional 24% would qualify for AML-MRC based on preceding MDS and/or multilineage dysplasia. However, unlike most other types of AML (including AML-MRC), mutations that provide a proliferative advantage such as FLT3 and RAS (so-called class I mutations) are rare in AEL.19,20 In aggregate, these data suggest that AEL, AML-MRC, and MDS with erythroid hyperplasia are likely biologically related diseases that appear to be arbitrarily separated into different entities in the current WHO classification scheme.
The prominent, often trilineage dysplasia, frequent ring sideroblasts, cytopenias, and lack of class I mutations suggest a relationship of AEL to MDS. However, in contrast to MDS21-23 survival of AEL patients in our series was not related to bone marrow or peripheral blood blast counts or to the number of dysplastic lineages. The number of cytopenias in AEL patients did significantly correlate with survival, as patients with a single cytopenia had a better survival (P = .049) than patients with 2 or 3 cytopenias. When we extended these observations to the subset of MDS patients with erythroid hyperplasia and to patients with AML-MRC and erythroid hyperplasia, we found that these patients had a similar survival to AEL patients and, like AEL patients, OS was not affected by bone marrow blast count.
Cytogenetic risk group (AML intermediate vs unfavorable and IPSS risk group good or intermediate vs poor) was strongly correlated with OS in AEL, as well as in MDS and AML-MRC with erythroid hyperplasia. According to the IPSS scheme, we found no significant difference in OS between AEL patients with good- and intermediate-risk cytogenetics; these 2 cytogenetic risk groups also fail to stratify MDS patients with refractory cytopenia with multilineage dysplasia.24 Of note, a prior study of 26 patients with AEL also found that OS had no significant correlation with bone marrow blast count or dysplasia, but did correlate with cytogenetic subgrouping.8 However, this prior study was based on the FAB classification and thus was limited to AEL cases with blasts comprising 30% or more of nonerythroid elements. This previous study also found a higher frequency of chromosome 5 deletion/loss (62% of their cases) compared with chromosome 7 deletion/loss (42% of their cases) and raised this as a distinction from therapy-related AML, in which deletion/loss of chromosome 7 is more frequent.8 In our series, the frequency of chromosome 5 deletion/loss was similar to the frequency of chromosome 7 deletion/loss in AEL. It is noteworthy that T-AEL patients had a much higher incidence of unfavorable cytogenetics (particularly deletion/loss of chromosome 7). However, in the multivariate analysis including cytogenetic risk group, cytotoxic therapy was not an independent risk factor. AEL patients with a history of an antecedent hematologic neoplasm also had similar survival to patients presenting de novo. Similar to MDS (but unlike most other types of AML), patients with AEL do not appear to benefit from induction chemotherapy unless followed by bone marrow transplantation.22 Among patients with AEL lacking high-risk cytogenetics, we found no survival benefit of high-intensity therapy with or without BMT over those receiving supportive care and low-intensity therapies.
The underlying biology of AEL, AML-MRC, and MDS with marked erythroid hyperplasia is not well understood. One concern is that prior erythropoietin (EPO) treatment may have caused transient erythroid hyperplasia leading to an erroneous diagnosis of AEL, especially in patients with a preexisting history of MDS or anemia (MDS-AEL cases). However, in one study, MDS patients treated with EPO had relatively reduced rather than increased bone marrow erythroid elements, presumably as a result of EPO promoting functional erythroid differentiation.25 Furthermore, MDS cases with marked erythroid hyperplasia were no more likely than other MDS cases to be treated with EPO and had similar endogenous EPO levels to MDS cases lacking relative erythroid hyperplasia.10 EPO was not administered to any of the patients in our series with de novo AEL and thus could not be responsible for the relative erythroid hyperplasia present in that subgroup. Moreover, our finding that the survival in AEL was similar to MDS with erythroid hyperplasia and was related to neither the absolute blast count nor the blast count as a percentage of nonerythroid elements argues against any significant effect of EPO. Thus, intrinsic cytogenetic and molecular alterations rather than exogenous EPO administration appear to underlie the marked proliferation of erythroid elements that characterize AEL and other myeloid neoplasms with excess blasts and marked erythroid hyperplasia.
In summary, AEL presents in a heterogeneous manner, either after MDS or chronic cytopenia, as a therapy-related neoplasm, or de novo. Disease aggressiveness is strongly associated with cytogenetic findings, but not with blast count, a finding replicated in MDS and AML-MRC cases with erythroid hyperplasia. The extent of morphologic dysplasia, history of MDS, or history of cytotoxic therapy had no independent effect on OS. Our findings suggest that MDS with erythroid hyperplasia, AEL, and AML-MRC with erythroid hyperplasia may form a continuum in which subdivision based on traditional morphologic features such as blast count or dysplastic lineages may not be relevant. In this sense, this group of diseases may be analogous to therapy-related myeloid neoplasms, in which clinical outcome is similar irrespective of classification based on blast count and dysplasia.26 Although AEL cases with high-risk cytogenetics may be best considered as a morphologic subgroup of AML-MRC, the optimal biologic subgrouping of AEL patients lacking high-risk cytogenetics is uncertain; these patients do not appear to benefit from high-intensity therapy and this AEL cytogenetic subgroup may be more akin to MDS with erythroid hyperplasia. Further study is needed to determine the most effective therapy for patients with AEL who are not eligible for allogeneic bone marrow transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This publication was made possible by grant no. UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
Authorship
Contribution: R.P.H. and Z.Z. performed research, analyzed data, and wrote the paper; C.G., G.T., and A.K. collected cases and analyzed data; R.L., L.V.A., and L.J.M. analyzed data and assisted with writing the paper; H.M.K. assisted with writing the paper; and S.A.W. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert P. Hasserjian, Department of Pathology, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114; e-mail: rhasserjian@partners.org.
References
Author notes
*R.P.H. and Z.Z. contributed equally to this study.