Abstract
Severe aplastic anemia (SAA) is a life-threatening bone marrow failure disorder that can be treated with bone marrow transplantation, immunosuppressive therapy, and high-dose cyclophosphamide. Here, we report long-term follow-up on 67 SAA patients (44 treatment-naive and 23 refractory) treated with high-dose cyclophosphamide. At 10 years, the overall actuarial survival was 88%, the response rate was 71% with the majority being complete, and the actuarial event-free survival was 58% in 44 treatment-naive SAA patients. Patients with refractory SAA fared less well after high-dose cyclophosphamide therapy; at 10 years, overall actuarial survival, response, and actuarial event-free survival rates were 62%, 48%, and 27%, respectively. High-dose cyclophosphamide is highly effective therapy for severe aplastic anemia. Large randomized controlled trials will be necessary to establish how results of high-dose cyclophosphamide compare with either bone marrow transplantation or standard immunosuppressive regimens, such as antithymocyte globulin and cyclosporine.
Introduction
Acquired severe aplastic anemia (SAA) is a rare, life-threatening hematopoietic stem cell disorder that manifests with pancytopenia and a hypocellular bone marrow.1,2 In most cases, bone marrow failure results from autoimmune destruction of hematopoietic stem cells. Fungal infections are the leading cause of death; however, hemorrhage, evolution to clonal disease (myelodysplastic syndromes [MDSs], leukemia, and paroxysmal nocturnal hemoglobinuria [PNH]), and transfusional iron overload are other causes of severe morbidity and mortality. SAA can affect all ages but is most common in children and young adults. Blood or bone marrow transplantation (BMT) from a human leukocyte antigen (HLA)–matched sibling donor can cure most patients with SAA, but fewer than 30% of patients have a suitable HLA-matched sibling. Moreover, the best results with allogeneic BMT are in children; adults, especially those older than age 40, do less well largely as a result of complications from graft-versus-host disease. Alternative donor transplantations (mismatched and unrelated donors) also have the potential to cure SAA, but such transplantations are usually reserved for second-line therapy because of their high rates of morbidity and mortality.
Immunosuppressive therapy is also a highly effective therapy for SAA and is generally front-line therapy for SAA patients who lack matched sibling donors or who are not good candidates for BMT. The most commonly administered immunosuppressive regimen is composed of antithymocyte globulin and cyclosporine A (ATG/CsA). ATG/CsA will induce a hematopoietic response in 60% to 70% of untreated SAA patients, and the probability of survival at 5 years ranges from 60% to 85%.3-6 However, up to 40% of patients eventually relapse, and an additional 10% to 40% develop a secondary clonal disease.3,4,7,8 Additional immunosuppressive drugs have been added to the ATG/CsA platform with hopes of decreasing relapse and secondary clonal disease, but so far no improvement in outcome has been observed.
High-dose cyclophosphamide is highly immunosuppressive and has been used to successfully treat SAA9-13 and other severe autoimmune diseases14-17 in several small trials. A pilot study of 10 patients treated with high-dose cyclophosphamide demonstrated that this approach has the potential to cure SAA; 6 of these patients had between 9 and 19 years of follow-up at the time of publication in 1996,9 and all 6 remain alive and disease free with normal blood counts out 21 to 31 years from initial therapy (R.A.B. and R.J.J., unpublished data, June 2008). However, another small study that combined high-dose cyclophosphamide with CsA for the treatment of SAA reported unacceptable rates of fungal infection and early mortality, limiting the enthusiasm for this approach.13 The purpose of this report is to document the response, survival, and long-term follow-up of a large cohort of SAA patients (44 treatment-naive and 23 refractory to one of more courses of immunosuppressive therapy) treated with high-dose cyclophosphamide at Johns Hopkins University since 1996.
Methods
Patient selection
Since January 1996, 44 patients with new-onset SAA and 23 patients with relapsed or refractory SAA referred to Johns Hopkins Hospital were enrolled in the protocol. Only patients with severe disease defined by the criteria of Camitta were eligible: bone marrow cellularity less than 25% with 2 of the following 3 laboratory abnormalities: absolute neutrophil count less than 0.5 × 109/L, platelet count less than 20 × 109/L, and an absolute reticulocyte count less than 60 000. Aplastic anemia was considered very severe (VSAA) if the patient met the criteria for SAA and had a neutrophil count less than 0.2 × 109/L. All patients gave informed consent for study participation approved by the Institutional Review Board of Johns Hopkins University in accordance with the Declaration of Helsinki.
Exclusion criteria included age older than 70 years, a serum creatinine of greater than 2.0 mg/dL, a cardiac ejection fraction of less than 45%, or any underlying malignancy unless it was thought to have been cured. Pregnant women, women of childbearing age who were unwilling to take oral contraceptives, and patients with Fanconi anemia or other congenital forms of aplastic anemia were also excluded. All eligible patients were offered treatment with high-dose cyclophosphamide. The patients were consecutively enrolled, and the only selection was for those younger than 30 years with an HLA-matched sibling donor, for whom we recommended allogeneic transplantation as initial therapy.
Treatment plan
The methods of administering high-dose cyclophosphamide and the posttreatment supportive care have been previously described.10 Briefly, cyclophosphamide at a dose of 50 mg/kg of ideal body weight daily was administered intravenously on 4 consecutive days. Intravenous 2-mercaptoethane sulfonate (10 mg/kg) for prophylaxis of hemorrhagic cystitis was administered 30 minutes before and 3, 6, and 8 hours after cyclophosphamide administration. On day 10 (6 days after the last dose of cyclophosphamide), all patients received granulocyte colony-stimulating factor (5 mg/kg daily) until the neutrophil count was 1.0 × 109 cells/L for 2 consecutive days.
All patients received prophylactic antibiotic support consisting of fluconazole (400 mg/day), norfloxacin (400 mg/day), and valacyclovir (500 mg twice daily) beginning on the day after the last dose of cyclophosphamide and continuing until the neutrophil count exceeded 0.5 × 109 cells/L. Prophylaxis against Pneumocystis carinii pneumonia with sulfamethoxazole (800 mg) and trimethoprim (160 mg) was given every Monday and Tuesday for 6 months after therapy. Packed red blood cell transfusions were administered to maintain a hematocrit greater than 0.25. Platelet transfusions were administered for bleeding or to maintain a platelet count greater than 10 × 109/L. Broad-spectrum antibacterials were started with first fever and adjusted according to cultures. Antifungal medications were added empirically for second fever.
Statistical analysis
Survival was defined as the time until death or last follow-up. Complete remission (CR) was defined as achieving normal peripheral blood counts for age and sex without the need for transfusions, hematopoietic growth factors, or immunosuppressive drugs. Partial remission (PR) was defined as achieving independence from blood transfusions without normalization of peripheral blood counts. The probabilities of survival and event-free survival (where death, relapse, MDS/AML, BMT, and PNH requiring treatment are defined as events) were estimated using the Kaplan-Meier method.18 The 95% confidence intervals (CIs) for the probability estimates were calculated using Greenwood formula. Deaths were censored using the longest follow-up time for time-to-remission analyses. Estimation of competing risk analysis was performed as previously described.19 All analyses were performed using Stata, Version 6.0 (Stata Corp).
Results
Patients and characteristics
From March 1996 through June 2008, 67 SAA patients were treated with high-dose cyclophosphamide: 44 patients were treatment-naive and 23 patients received at least one course of traditional immunosuppressive therapy consisting of either ATG/CsA (n = 21) or CsA alone (n = 2). Demographic and disease characteristics of the patients are shown in Table 1. In the treatment-naive group, 10 patients were being treated for an active infection at the time of cyclophosphamide administration. Two patients were being treated for presumed fungal infections and 8 were being treated for presumed bacterial infections. Of the patients with refractory SAA, 3 were being treated for an infection at the time of cyclophosphamide administration, 2 with presumed fungal infections and one with a bacterial infection.
Toxicity
Toxicity from high-dose cyclophosphamide was similar to that previously reported.10 All patients developed transient alopecia, most experienced nausea with or without vomiting that resolved within days after the last dose of cyclophosphamide, and most patients experienced at least one episode of febrile neutropenia. Hemorrhagic cystitis requiring continuous bladder irrigation occurred in 2 (4.5%) of the 44 treatment-naive patients and none of the 23 refractory patients. The median duration of hospitalization was 27.5 days (range, 1-330 days) for treatment-naive patients and 28 days (range, 1-86 days) for patients with refractory SAA. There have been 5 (11.4%) total deaths in the treatment-naive cohort (Table 2); 4 of these deaths occurred more than 5 months after the treatment and each occurred in patients with VSAA who failed to respond to high-dose cyclophosphamide. The causes of the death included infectious complications (n = 4) and complications from an unrelated bone marrow transplantation (n = 1).
There were 8 (18.2%) cases of severe fungal infections in the 44 treatment-naive patients, all within the first 3 months; 3 of these patients died, whereas the remaining 5 recovered. The cumulative incidence of developing such an infection, considering the one death that occurred at 2 months, was 21%. In contrast, 10 (43.5%) of the 23 refractory patients acquired a severe fungal infection after high-dose cyclophosphamide; 5 of these patients died and 5 recovered (Table 3). Nine of the 10 fungal infections occurred within the first 2 months, as did one death, corresponding to a 2-month cumulative incidence for fungal infection of 39%. However, one fungal infection occurred at 34 months, 2 months after treatment for a relapse, making the 34-month cumulative incidence 49%.
Hematopoietic recovery
For treatment-naive patients, the median time to a neutrophil count of 0.5 × 109/L was 60 days (range, 28-104 days), the median time to last platelet transfusion was 117 days (range, 24-640 days), and the median time to last red cell transfusion was 186 days (range, 30-1784 days). The patient who took 1784 days to become transfusion independent was an outlier; 6 other patients took longer than 1 year to become transfusion independent (range, 379-679 days). All other responding patients became transfusion independent within one year after treatment. For patients with refractory SAA, the median time to a neutrophil count of 0.5 × 109/L was 54 days (range, 35-119 days), the median time to last platelet transfusion was 103 days (range, 51-751 days), and the median time to last red cell transfusion was 210 days (range, 63-796 days). For the 24 patients who so far have achieved a CR, the median time to CR was 20 months (range, 4-70 months).
Response and survival of treatment-naive patients
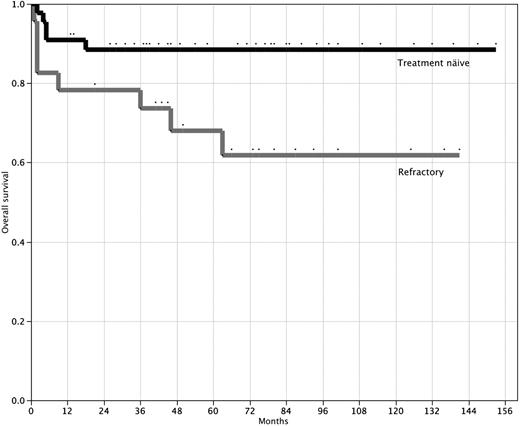
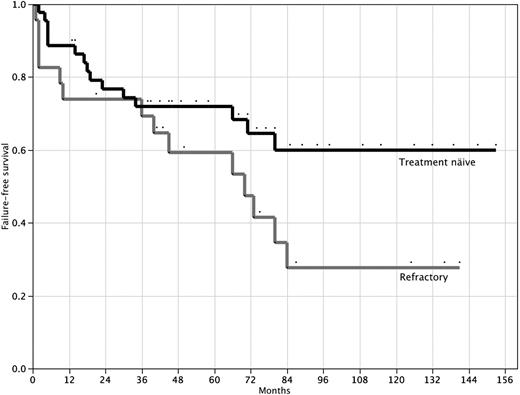
The actuarial probability of survival at 10 years for the 44 treatment-naive patients is 88% (95% CI, 75%-95%) with a median follow-up of 58 months (range, 2-153 months; Figure 1). A hematologic response (transfusion independence) was achieved by 31 (70.5%) patients (19 of 24 with SAA and 12 of 20 with VSAA); 19 (43.2%) achieved a CR and 12 (27.3%) achieved PR. The median time to response was 5 months (interquartile range, 2-10 months). Actuarial failure-free survival is 58% (95% CI, 40%-74%) at 10 years (Figure 2). Late complications of disease/therapy are documented in Table 4. Two PR patients relapsed 66 and 80 months after treatment, respectively. One of these patients (VSAA) has been successfully re-treated with high-dose cyclophosphamide. Two patients, both nonresponders with SAA, have acquired an MDS at 20 and 70 months, respectively. Both of these patients are alive at 26 and 101 months, respectively: one patient, a 4-year-old, received a mismatched allogeneic BMT 24 months after high-dose cyclophosphamide; the other patient, a 58-year-old, developed refractory anemia with normal cytogenetics and is red cell– and platelet transfusion–dependent. Two patients (one in CR and one in PR) have more than 20% glycosylphosphatidylinositol anchor-deficient granulocytes by flow cytometry, but no patient has required therapy or developed symptoms of PNH (intravascular hemolysis, smooth muscle dystonias, or thrombosis).20 One patient, a 47-year-old man, developed a testicular cancer 6 years after treatment. He remains alive and disease-free after successful treatment with surgery and radiation. There were no other malignancies and no cases of avascular necrosis.
Overall survival after high-dose cyclophosphamide therapy. Overall survival for 44 treatment-naive patients (top line) and 23 patients refractory to prior immunosuppressive therapy (bottom line). P = .03 (log-rank test).
Overall survival after high-dose cyclophosphamide therapy. Overall survival for 44 treatment-naive patients (top line) and 23 patients refractory to prior immunosuppressive therapy (bottom line). P = .03 (log-rank test).
Failure-free survival after high-dose cyclophosphamide therapy. Failure-free survival for 44 treatment-naive patients (top line) and 23 patients refractory to prior immunosuppressive therapy (bottom line). P = .07 (log-rank test).
Failure-free survival after high-dose cyclophosphamide therapy. Failure-free survival for 44 treatment-naive patients (top line) and 23 patients refractory to prior immunosuppressive therapy (bottom line). P = .07 (log-rank test).
There were 6 patients with HLA-matched sibling donors who elected to receive high-dose cyclophosphamide as initial therapy. The median age of these patients was 40 years (range, 26-52 years), and all 6 remain alive with median follow-up of 44 months (range, 22-49 months). However, 2 patients, a 26-year-old and 52-year-old, did not respond to high-dose cyclophosphamide and received allogeneic transplantations 5 and 30 months, respectively, after initial therapy; both patients have engrafted and are disease-free without graft-versus-host disease. The remaining 4 patients with HLA-matched siblings remain in remission (3 in CR and 1 in PR) after high-dose cyclophosphamide.
Response and survival of refractory patients
The actuarial probability of survival for the 23 patients with refractory SAA is 61.8% (95% CI, 40%-80%) at 10 years with a median follow-up of 63 months (range, 1-141 months; Figure 1). A hematologic response was achieved by 11 (47.8%) patients. The median time to response was 11 months (interquartile range, 3-17 months). Failure-free survival for this group of patients is 27.7% (95% CI, 12%-53%) at 10 years (Figure 2). Late complications of disease/therapy are documented in Table 5. One patient achieving a CR relapsed at 70 months and is in a second CR more than 2 years after re-treatment with high-dose cyclophosphamide. A nonresponder was diagnosed with MDS 40 months after treatment with high-dose cyclophosphamide and ultimately died of complications of an unrelated allogeneic BMT. Two patients, one of whom had 23% PNH granulocytes before treatment with high-dose cyclophosphamide, developed symptomatic PNH; 1 patient did not achieve remission after high-dose cyclophosphamide and died of an intracerebral hemorrhage 63 months after therapy, and the other was in a PR for more than 6 years after high-dose cyclophosphamide before acquiring symptomatic PNH. This patient now has intermittent episodes of intravascular hemolysis, requires occasional red blood cell transfusions, and is contemplating treatment with eculizumab.
Discussion
High-dose cyclophosphamide is a highly immunosuppressive agent that, in addition to its successful use as a treatment for aplastic anemia9-11 and other severe refractory autoimmune diseases,14,16,21,22 has proven efficacy in allogeneic BMT as both a conditioning regimen23 and graft-versus-host disease prophylaxis.24 Cyclophosphamide's unique metabolism is responsible for its immunosuppressive yet stem cell-sparing properties. The major mechanism of cyclophosphamide inactivation is cellular aldehyde dehydrogenase (ALDH), primarily the ALDH1 family of isoenzymes.1 ALDH1, also known as retinaldehyde dehydrogenease, is highly expressed in hematopoietic stem cells and other cells requiring retinoic acid because of their high proliferative potential.1 Thus, such cells are resistant to cyclophosphamide, whereas lymphocytes that express relatively little ALDH1 remain sensitive to its cytotoxic effects.
Long-term follow-up of this series of SAA patients treated with high-dose cyclophosphamide confirms the efficacy of this treatment. At 10 years, the overall actuarial survival is 88%, the response rate is 71%, and the actuarial event-free survival is 58% in 44 treatment-naive SAA patients. There were no secondary clonal disorders or deaths in responding patients. One patient took more than 4 years to achieve complete transfusion independence, raising the possibility that this patient achieved a “spontaneous” remission. Nevertheless, these data are similar to the results of allogeneic BMT and ATG/CsA for treating SAA. After ATG and cyclosporine therapy, Rosenfeld et al reported a 55% overall survival at 7 years; in patients with VSAA, 12 of 48 patients died within 3 months after treatment compared with 3 of 74 patients with presenting neutrophil counts of greater than 0.2 × 109.4 We also found a higher mortality risk in patients with VSAA (Table 2), with 4 of 5 deaths occurring in the 20 patients with VSAA. The overall survival for the 24 treatment-naive patients without VSAA in our series was 96%, with the only death occurring at 18 months after high-dose cyclophosphamide. Moreover, only one of 44 treatment-naive patients in our series died in the first 3 months after treatment. Thus, the myelosuppressive properties of high-dose cyclophosphamide did not appear to influence early mortality.
The risk for relapse or secondary clonal disorders in treatment-naive SAA patients is low after high-dose cyclophosphamide. In our initial pilot study of high-dose cyclophosphamide, 7 of 10 patients responded to therapy and no one relapsed or acquired a secondary clonal disease.9 In the current series, only 2 of 31 responders (6.5%) relapsed; 1 of these patients has achieved a continuous second remission after a second course of therapy. MDS/leukemia is the most feared secondary clonal disease to evolve from aplastic anemia, with monosomy 7 the most common chromosomal abnormality to arise in these patients.4,8,25,26 In the treatment-naive group, 2 patients have developed MDS: one with normal cytogenetics and one with monosomy 7. An additional 2 patients in the treatment-naive group also have developed PNH cells, but neither has developed clinical PNH. Although relapse and secondary clonal disease are rare after allogeneic BMT, relapses occur in up to 40% of patients treated with ATG/CsA and MDS occurs in 3% to 15% of these patients.3,6,27-29 A potential advantage of high-dose cyclophosphamide over ATG/CsA is that the majority of responses are complete and that response is not dependent on continued administration of immunosuppressive drugs; more than 40% of all the treatment-naive patients achieved a CR, and none has relapsed or developed secondary clonal disease.
Patients with refractory severe aplastic anemia fared less well after high-dose cyclophosphamide therapy; at 10 years, overall actuarial survival, response, and actuarial event-free survival rates were 62%, 48%, and 27%, respectively. The toxicity of high-dose cyclophosphamide was also higher in this group with an early mortality rate of 17% and a 44% risk for invasive fungal infections in this group. These inferior results are probably multifactorial; these patients are more heavily transfused, are more immunosuppressed, and have had longer disease durations. Nevertheless, the 26% event-free survival at 10 years demonstrates that, similar to unrelated or mismatched allogeneic BMT30 or repeated courses of immunosuppressive therapy,31,32 high-dose cyclophosphamide can salvage patients with refractory SAA.
The use of high-dose cyclophosphamide as primary therapy in SAA has generated considerable controversy. In particular, a small randomized trial comparing high-dose cyclophosphamide and ATG/CsA found a very high rate of invasive fungal infections on the high-dose cyclophosphamide arm.13 It is possible that the inclusion of CsA on the cyclophosphamide arm, the absence of prophylactic granulocyte colony-stimulating factor and antifungal coverage, and/or the small sample size (only 15 patients were accrued to high-dose cyclophosphamide) may have accounted for the high rate of fungal infections in that study. In our series, 8 treatment-naive patients acquired an invasive fungal infection after treatment; 3 of these patients died, but 5 had a complete recovery and remain in remission. The incidence of fungal infections and the associated mortality in our series is actually less than that reported after treatment with ATG/CsA.4 The recent approval of several less toxic and more effective antifungal agents (eg, voriconizole, caspofungin, and posiconazole) probably reduces the treatment-related morbidity and mortality for patients with prolonged periods of deep aplasia even further. It is conceivable that patient selection through our referral base or through insurance clearance may have contributed to our high overall survival and event-free survival; however, all eligible patients were offered treatment with high-dose cyclophosphamide. The best predictor of survival in aplastic anemia is severity of disease, and the percentage of patients with VSAA in our treatment-naive cohort is comparable with that of other published series of immunosuppression for SAA.3,4,33 Furthermore, children with SAA tend to have better outcomes after immunosuppressive therapy than adults. The median age of our treatment-naive cohort was 32 years, older than most published series of immunosuppression.4,6,33,34
In conclusion, high-dose cyclophosphamide is an effective and potentially curative therapy for patients with treatment-naive SAA. Early deaths secondary to invasive fungal infection were no higher after high-dose cyclophosphamide in our series than what has been reported after ATG/CsA. Relapse and secondary clonal disease may occur in a minority of patients, but approximately 60% of patients achieve durable hematopoietic recovery and do not require further immunosuppressive agents. High-dose cyclophosphamide is less effective for patients with refractory SAA, but durable responses occur in approximately one-fourth of these patients. Because SAA is a rare disease, a large multi-institutional study comparing high-dose cyclophosphamide versus traditional immunosuppressive therapy will be necessary to determine the best approach for managing SAA patients who are not ideal candidates for allogeneic BMT.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This study was supported by the National Institutes of Health (grant P01CA70970).
National Institutes of Health
Authorship
Contribution: R.A.B. and R.J.J. designed and performed research, analyzed data, and wrote the paper; A.R.C. analyzed data and contributed to patient care and accrual; D.D. collected and analyzed data; S.N.G. analyzed data; and E.J.F., C.A.H., L.L., B.D.S., W.H.M., and R.F.A. collected and analyzed data and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert A. Brodsky, Division of Hematology, Department of Medicine, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross Bldg, Rm 1025, Baltimore, MD 21205; e-mail: brodsro@jhmi.edu.