Abstract
Autoimmune lymphoproliferative syndrome (ALPS) is a disorder of abnormal lymphocyte survival caused by dysregulation of the Fas apoptotic pathway. Clinical manifestations of ALPS include autoimmune cytopenias, organomegaly, and lymphadenopathy. These findings overlap with Evans syndrome (ES), defined by presence of at least 2 autoimmune cytopenias. We hypothesized a subset of patients with ES have ALPS and tested 45 children at 22 institutions, measuring peripheral blood double-negative T cells (DNTs) and Fas-mediated apoptosis. ALPS was diagnosed in 47% of patients tested. Markedly elevated DNTs (≥ 5%) were a strong predictor of ALPS (positive predictive value = 94%), whereas no patients with DNTs less than 2.5% had ALPS on apoptosis testing. Severity of cytopenias and elevated immunoglobulin levels also predicted ALPS. This is the largest published series describing children with ES and documents a high rate of ALPS among pediatric ES patients. These data suggest that children with ES should be screened for ALPS with DNTs.
Introduction
Autoimmune lymphoproliferative syndrome (ALPS) is a disorder of disrupted lymphocyte homeostasis caused by defective Fas-mediated apoptosis.1 Clinical manifestations include autoimmune cytopenias, organomegaly, lymphadenopathy, and increased risk of malignancy.2 Similar clinical features are observed in Evans syndrome (ES), a hematologic disorder defined by autoimmune destruction of at least 2 hematologic cell types after exclusion of other diagnoses.3 We hypothesized that a subset of patients diagnosed with ES may have ALPS and previously demonstrated in a small single institutional study that a significant percentage of patients diagnosed with ES have ALPS.4 To confirm our hypothesis, we conducted a multi-institutional observational study with primary aims to determine the prevalence of ALPS among patients diagnosed with ES and to evaluate double-negative T cells (DNTs) of phenotype CD3+CD4−CD8−TCR-α/β+ as a screening tool for ALPS in patients with ES.
Methods
We recruited patients diagnosed with ES or multiple unexplained autoimmune cytopenias at 22 institutions. Study inclusion and exclusion criteria are depicted in Table 1. Referring clinicians sent samples for centralized testing and conducted a standardized physical and laboratory assessment of lymphadenopathy, organomegaly, complete blood counts, hematologic autoantibodies, antinuclear antibodies (ANAs), serum immunoglobulin G (IgG), and antiphospholipid antibodies (APLAs). DNTs were measured in our clinical immunology laboratory, where the assay is performed routinely. We performed in vitro assessment of Fas-mediated apoptosis, the functional test used to confirm ALPS diagnosis. Methods for both of these assays are published and validated.4,8 All studies were performed using a Children's Hospital of Philadelphia Institutional Review Board–approved protocol with informed consent in accordance with the Declaration of Helsinki. Statistical analyses were performed using Prism 4 for Windows (GraphPad Software).
Results and discussion
A total of 73 patients were referred for evaluation. Of these, 11 did not meet diagnostic criteria for ES, and an additional 17 were excluded for incomplete clinical and/or laboratory data, leaving 45 patients for analysis (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Mean age at diagnosis was 7.1 years, with females (n = 12) comprising 24% of the cohort. Sex, age, and ethnicity were not associated with ALPS diagnosis (supplemental Figure 1B). No patients were related, and 6 patients had a first-degree relative with a history of autoimmune disease (3 with idiopathic thrombocytopenic purpura, 1 with autoimmune hemolytic anemia, 1 with common variable immunodeficiency, and 1 with celiac disease).
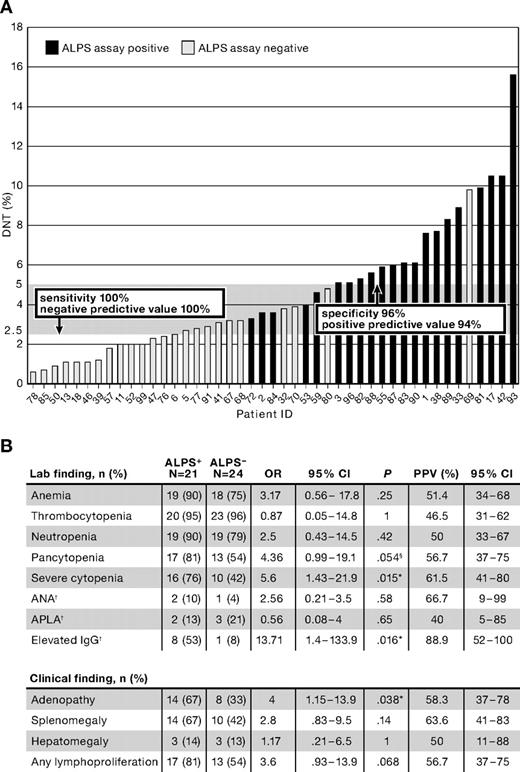
Twenty-one of 45 patients (47%) had elevated DNTs and defective Fas-mediated apoptosis consistent with a diagnosis of ALPS (Figure 1A). Clinical factors predictive of defective Fas-mediated apoptosis were (Figure 1B): severity of cytopenias, defined as requiring treatment with immunosuppressive medications at least twice a year (odds ratio [OR] = 5.6; 95% confidence interval [CI], 1.43-21.9; P = .015), presence of lymphadenopathy (OR = 4; 95% CI, 1.15-13.9; P = .038), and elevated IgG (OR = 13.7; 95% CI, 1.4-134; P = .016). Although clinically evident lymphoproliferation was a predictor of ALPS, 4 of 21 patients were found to have significant autoimmunity, elevated DNTs, and defective Fas-mediated apoptosis without clinically identifiable lymphoproliferation. Whether these patients have a forme fruste of ALPS or an ALPS-like syndrome is up for debate. Specific cytopenias and hematologic antibodies were not predictive of ALPS, nor were ANA, APLA, or hepatomegaly.
Clinical and laboratory features of children with ES. Forty-five patients with ES were evaluated for ALPS by in vitro Fas-mediated apoptosis assay and DNTs. Black columns represent patients with defective Fas-mediated apoptosis (consistent with ALPS); gray columns, patients with normal apoptosis assays (not consistent with ALPS; A). The ordinate depicts DNTs (%), and the gray shaded bar delineates DNTs between 2.5% (upper limit of normal) and 5% (marked elevation). All patients with DNTs below 2.5% had normal apoptosis testing. All patients with DNTs more than or equal to 5%, except for patient 69, had defective Fas-mediated apoptosis. Of note, this child was found to have an identifiable genetic mutation in FAS and may represent a false negative on apoptosis testing. Severe autoimmune cytopenias (requiring immunosuppressive treatment at least twice a year), lymphadenopathy, and IgG level were predictive of ALPS (B), whereas pancytopenia trended toward predicting ALPS in ES. Of note, 4 of 21 patients ultimately found to have ALPS had no clinical evidence of lymphoproliferation. †Limited clinical data were available for ANA (performed in 20 of 21 children with ALPS and 24 of 24 without ALPS), APLA (15 of 21 with ALPS and 14 of 24 without ALPS), and IgG (15 of 21 with ALPS and 13 of 24 without ALPS). §Trend toward statistical significance. *P value reaches significance. ALPS+ indicates patients with defective in vitro apoptosis; ALPS−, patients with normal in vitro apoptosis; and PPV, positive predictive value. P values were calculated using Fisher exact test.
Clinical and laboratory features of children with ES. Forty-five patients with ES were evaluated for ALPS by in vitro Fas-mediated apoptosis assay and DNTs. Black columns represent patients with defective Fas-mediated apoptosis (consistent with ALPS); gray columns, patients with normal apoptosis assays (not consistent with ALPS; A). The ordinate depicts DNTs (%), and the gray shaded bar delineates DNTs between 2.5% (upper limit of normal) and 5% (marked elevation). All patients with DNTs below 2.5% had normal apoptosis testing. All patients with DNTs more than or equal to 5%, except for patient 69, had defective Fas-mediated apoptosis. Of note, this child was found to have an identifiable genetic mutation in FAS and may represent a false negative on apoptosis testing. Severe autoimmune cytopenias (requiring immunosuppressive treatment at least twice a year), lymphadenopathy, and IgG level were predictive of ALPS (B), whereas pancytopenia trended toward predicting ALPS in ES. Of note, 4 of 21 patients ultimately found to have ALPS had no clinical evidence of lymphoproliferation. †Limited clinical data were available for ANA (performed in 20 of 21 children with ALPS and 24 of 24 without ALPS), APLA (15 of 21 with ALPS and 14 of 24 without ALPS), and IgG (15 of 21 with ALPS and 13 of 24 without ALPS). §Trend toward statistical significance. *P value reaches significance. ALPS+ indicates patients with defective in vitro apoptosis; ALPS−, patients with normal in vitro apoptosis; and PPV, positive predictive value. P values were calculated using Fisher exact test.
DNTs, an atypical T-cell population increased in ALPS patients, were assessed in all patients (Figure 1A). The upper limit of normal for peripheral blood DNTs at our laboratory is 2.5%, established by testing an extended panel of normal controls.4 In this cohort, 16 of 17 patients with DNTs more than or equal to 5% had defective Fas-mediated apoptosis. The patient with a normal apoptosis assay and elevated DNTs was subsequently found to have a FAS mutation, and his negative apoptosis assay may represent a false negative. All 13 patients with DNTs less than 2.5% had normal apoptosis assays. In our series, normal DNTs had 100% sensitivity and 100% negative predictive value for ALPS, whereas DNTs more than or equal to 5% had 96% specificity and 94% positive predictive value for a defective apoptosis assay. Of the patients with moderately elevated DNTs (2.5%-4.9%), 5 of 14 had defective apoptosis.
Approximately 70% of patients with ALPS have identifiable genetic mutations in FAS, FASL, or CASP10.9 Seventeen of the 21 patients who met the laboratory diagnostic criteria for ALPS underwent genetic testing, and 9 of 17 (53%) had an identifiable mutation. The lower rate of detectable mutations in this study may reflect an underlying biologic difference in children who present with autoimmunity as a primary symptom rather than lymphoproliferation or the low prevalence of the disease and small numbers evaluated in prior studies.
An identified genetic mutation is a supportive criterion for ALPS and not part of the currently accepted standard definition.1,5,10 Polymorphisms in FAS are common, and a mutation must be proven functional to be useful for diagnosis.11 Accordingly, our Institutional Review Board only approved genetic testing on the patients with abnormal apoptosis. Our study classified ALPS by the accepted consensus definition first proposed by the National Institutes of Health in the 1990s. Several groups, including ours, have tried to identify new assays that predict ALPS (eg, soluble Fas ligand, vitamin B12, and interleukin-10), but these have only been successful in identifying ALPS type 1A patients (those with mutations in FAS) and are not strong predictors of other ALPS types.12 Currently, the only means of diagnosing ALPS type 3 patients is with the apoptosis assay. This group encompasses 20% to 30% of ALPS patients.
Patients with somatic mutation variant ALPS (1s) and Fas ligand variant (1b) have normal apoptosis assays. Fas ligand deficiency has been reported in 2 cases of ALPS.13 ALPS resulting from somatic DNT cells carrying a Fas mutation is thought to occur in 2% to 5% of patients and should be ruled out in patients with elevated DNT cells and appropriate clinical phenotype.14 Based on the rarity of these subtypes of ALPS, only 1 or 2 patients classified as “non-ALPS” in our series may have had ALPS. This small degree of possible misclassification would not change the findings of the study.
ALPS is presumed to be a rare condition with only a few hundred reported cases. Finding a high rate of ALPS in patients previously identified as having ES suggests that ALPS may be more prevalent than previously thought. Our detection of true prevalence of ALPS in patients diagnosed with ES may be skewed by referral bias. However, a clear diagnosis is crucial, as understanding the biology of these different entities may help with identifying targeted therapies, genetic counseling, and avoiding potentially harmful treatments in the future. In particular, treatment for ALPS may vary from that of ES, as clinical trials have identified mycophenolate mofetil and sirolimus to be effective medications for children with ALPS, whereas data for targeted therapies for ES are limited by its nature as a diagnosis of exclusion.15,16 Patients with ALPS who undergo splenectomy have an increased risk of developing postsplenectomy sepsis despite appropriate vaccination and antibiotic prophylaxis.1 If possible, splenectomy should be avoided in patients with ALPS. Trials have suggested that rituximab may be relatively contraindicated in ALPS patients as ALPS patients may develop long-term immunodeficiency after treatment.17 As ES is a diagnosis of exclusion, children with idiopathic autoimmune cytopenias should be tested for ALPS, especially before undergoing potentially harmful treatments, such as splenectomy or ritumixab. These results demonstrate that childhood ES may be a distinct entity from ES in adults, as the high prevalence of ALPS in children contrasts with a recent study of ES in adults that found no ALPS cases; notably, the study was not designed to detect prevalence of ALPS and only tested the few patients with atypical lymphoproliferation.18
In conclusion, we found that (1) a significant percentage of pediatric patients diagnosed with ES have ALPS, (2) severity in number and presentation of cytopenias predicts the diagnosis, and (3) some children without the classic lymphoproliferative signs are also at risk. Given the important differences in management between ALPS and ES, we recommend that all children with ES undergo ALPS screening with DNTs as part of their diagnostic evaluation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Richard Aplenc for review of statistical methods; Dr Eric Rappaport for genetic analyses; Drs Ellis Neufeld, Cindy Neunert, Susan Travis, Kathleen Sullivan, and John Choi for clinical expertise and advice; and Danniel Gaidula for assistance with preparing figures.
This work was supported by the United States Immunodeficiency Network (USIDNET, N01-A1-30070; D.T.T.), a Foerderer-Murray Award (D.T.T.), the Goldman Philanthropic Partnerships and the Rockefeller Brothers Fund (D.T.T.), the Larry and Helen Hoag Foundation Clinical Translational Research Career Development Award (D.T.T.), and the Sanford Chair and Weinberg Fund of the Children's Hospital of Philadelphia (S.A.G.).
Authorship
Contribution: A.E.S., C.S.M., S.A.G., and D.T.T. designed the research and drafted the manuscript; A.E.S., D.T.T., and C.S. performed research, analyzed and interpreted data, and performed statistical analysis; and all authors were involved in critical revision of the manuscript.
Conflict-of-interest disclosure: C.S.M. is a consultant for Bayer Healthcare, Baxter Healthcare, and Grifols US. The remaining authors declare no competing financial interests.
Correspondence: David T. Teachey, Divisions of Hematology and Oncology, Children's Hospital of Philadelphia, 3008 CTRB, 3501 Civic Center Blvd, Philadelphia, PA 19104; e-mail: teacheyd@email.chop.edu.