Abstract
A recent report demonstrated that free human T-cell leukemia virus 1 (HTLV-1) could infect plasmacytoid dendritic cells (pDCs). The major role of pDCs is to secrete massive levels of interferon-α (IFN-α) upon virus exposure; however, the induction of IFN-α by HTLV-1 remains unknown. We demonstrate here that cell-free HTLV-1 generated a pDC innate immune response by producing massive levels of IFN-α that were inhibited by anti–HTLV-1 antibodies. HTLV-1 induced costimulatory molecules and rapid expression of the apoptotic ligand tumor necrosis factor–related apoptosis-inducing ligand (TRAIL). Furthermore, HTLV-1 stimulated pDC-induced apoptosis of CD4+ T cells expressing DR5, transforming pDCs into IFN-producing killer pDCs. We also observed that an endosomal acidification inhibitor and a Toll-like receptor-7 (TLR7)–specific blocker drastically inhibited pDC response to HTLV-1. Three-dimensional microscopy analysis revealed that unstimulated pDCs were “dormant” IFN-producing killer pDCs with high levels of intracellular TRAIL that could be rapidly mobilized to the surface in response to TLR7 activation. Inhibition of viral degradation in endosomes by chloroquine maintained viral integrity, allowing virus detection by 3-dimensional microscopy. We demonstrate that pDCs respond to cell-free HTLV-1 by producing high levels of IFN-α and by mobilizing TRAIL on cell surface after TLR7 triggering. This is the first demonstration of an innate immune response induced by free HTLV-1.
Introduction
Human T-cell leukemia virus 1 (HTLV-1), the first characterized human retrovirus,1 has been identified as the causative agent for adult T-cell leukemia/lymphoma (ATLL)2,3 and HTLV-1–associated myelopathy/tropical spastic paraparesis,4 uveitis, and infective dermatitis in children.5 HTLV-1 virions infect CD4+ T cells, which represent the main target for HTLV-1 infection in peripheral blood. HTLV-1–associated diseases occur after long periods of virus latency.6 For years it has been thought that unlike other retroviruses, free virions were poorly infectious.7 However, Jones et al8 recently reported that freshly isolated myeloid dendritic cells (mDCs) and plasmacytoid dendritic cells (pDCs) are efficiently and productively infected by cell-free HTLV-1. Furthermore, infected mDCs and pDCs were able to transfer virions to autologous CD4+ T cells, clearly demonstrating that cell-free HTLV-1 can be infectious and target DCs.8
pDCs participate in innate and adaptive immunity,9,10 are located in blood and lymphoid organs,10,11 and produce up to 1000-fold more interferon-α (IFN-α) than other cell types in response to virus exposure.12 Three molecules have been characterized for HTLV-1 entry into cells, heparan sulfate proteoglycans13 and BDCA-4 (also called neuropilin-1)14 for the initial virus binding to target cells15 and glucose transporter 1 for the postattachment and the viral fusion.16,17 Interestingly, BDCA-4 is expressed by mDC and T cells18,19 but cells expressing the greatest level of BDCA-4 in blood are pDCs,20 strongly suggesting that HTLV-1 could interact with pDCs.
Nevertheless, HTLV-1–induced immune response by professional “sentinel” pDCs has not been reported. Viral activation of pDCs can be regulated by either of 2 Toll-like receptors (TLRs), TLR7 or TLR9,21 which are considered to be the receptors that human pDCs use for recognition of RNA/retroviruses22,23 and DNA,24 respectively. Virus-activated pDCs were recently reported to express the tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL).25 TRAIL, a TNF superfamily member,26 has been shown to induce apoptosis of cancer and infected cells expressing death receptor-4 or -5 (DR4, DR5).27-30 Virus-stimulated pDCs expressing TRAIL acquired cytotoxic activity, transforming them into a new subset of killer innate immune cells,25,31 which may play a central role in viral immunopathogenesis.
We show in this study that cell-free HTLV-1 virions generated pDC innate immune response. Indeed, HTLV-1–stimulated pDCs produced massive levels of IFN-α, which were inhibited by neutralizing HTLV-1 antibodies (HTLV-1 patients' serum or antienvelope antibody). HTLV-1 induced costimulatory molecules and rapid expression of TRAIL and down-regulated chemokine receptors. Furthermore, HTLV-1–stimulated pDCs induced apoptosis of CD4+ T cells expressing DR5 via their expression of membrane TRAIL, characterizing the IFN-producing killer plasmacytoid dendritic cells (IKpDCs). Thus, we investigated the mechanism by which HTLV-1 turned pDCs into IKpDCs. We observed that an endosomal acidification inhibitor (chloroquine) and TLR7-specific blocker (A151) drastically inhibited pDC response to HTLV-1. Three-dimensional (3D) microscopy analysis revealed that resting pDCs were “dormant” IKpDC with high levels of intracellular TRAIL that could be rapidly mobilized at the surface in response to TLR7 activation. Inhibition of viral degradation in endosomes by chloroquine maintained viral integrity, allowing virus detection in pDCs by 3D microscopy. In chloroquine-treated pDCs, intact particles were unable to stimulate the TLR7 pathway and consequently prevent TRAIL relocalization and IKpDC generation. Therefore, our study demonstrates that cell-free HTLV-1 virions induce pDC innate immune response, transforming them into functional IKpDCs.
Methods
Blood from HIV-1–seronegative blood bank donors was obtained from Etablissement Français du Sang (convention no. 07/CABANEL/106). Experimental procedures with human blood was reviewed and approved by the Necker Hospital Ethical Committees for human research and were done according to the European Union guidelines and the Declaration of Helsinki.
Isolation and culture of blood leukocytes
In vitro experiments were performed by the use of peripheral blood mononuclear cells (PBMCs) isolated by density centrifugation from peripheral blood lymphocyte separation medium (Cambrex). Cells were cultured in RPMI 1640 (Invitrogen) containing 10% fetal bovine serum (Hyclone). Fresh pDCs were isolated from healthy donor PBMCs by the use of the negative selection pDC untouched isolation kit (Miltenyi Biotec), allowing purification without any cell stimulation.
Stimulation and culture of pDCs
Purified pDCs were cultured alone, with influenza A virus, with supernatants from HTLV-1 virus–producing T-cell line (MT-2), or purified HTLV-1 from MT-2 supernatant. MT-2 cells were seeded at 106/mL overnight in culture medium, then supernatants were collected and ultra centrifuged for 2 hours at 100 000g. HTLV-1 viruses were resuspended in 100 μL of culture media and quantified by the use of the HTLV-1-p19 Elisa Kit (Gentaur). Several concentrations of p19-equivalent (0-1800 ng/mL) were used to stimulate pDCs in vitro. Blocking HTLV-1 envelope (gp-46) antibody (10 μg/mL; Zeptometrix) and sera-containing blocking HTLV-1 antibodies from HTLV patients (serum 1, serum 2) were used to block HTLV-I virions.
TLR blocking assays
pDCs were cultured with HTLV-I or CpG-A (Invivogen) in the presence of endosomal acidification inhibitor chloroquine diphosphate salt (0.1-1μM; MP Biomedical) or with TLR7 oligodinucleotide A151 blocker (T*T*A*G*G*G*T*T*A*G*G*G*T*T*A*G*G*G*T*T*A*G*G*G; Integrated DNA Technologies Inc). IFN-α production, TRAIL, CD40, CD83, CD86, CXCR4, and CCR5 expression by purified pDCs stimulated or not by HTLV-1 were quantified after chloroquine and A151 treatment overnight.
Flow cytometry
After stimulation in culture, pDCs were incubated for 20 minutes at room temperature with fluorescein isothiocyanate–conjugated monoclonal antibody anti–human CD123 (MBL International), allophycocyanin (APC)–conjugated monoclonal anti–BDCA-2 antibody (Miltenyi Biotec), and APC-Cy7–conjugated anti-CD14 (BD Biosciences); VioBlue-conjugated anti-CD4 (Miltenyi Biotec); or with isotype-matched control antibodies (at 5 μg/mL each; BD Biosciences) in phosphate-buffered saline containing 2% mouse serum (Sigma-Aldrich). Cells were washed twice in ice-cold Dulbecco phosphate-buffered saline and fluorescence-activated cell sorting (FACS) analysis was performed on a FACSCanto 7-color flow cytometer by the use of FACSDiva software (BD Biosciences). CD4+CD123+BDCA-2+ CD14−–gated cells were then tested for the expression of surface markers by the use of phycoerythrin (PE)–labeled anti-TRAIL (eBioscience), peridinn chlorophyll protein–conjugated anti-CXCR4, PE-Cy5 anti-CD83 or PE-Cy5 anti-CD86, APC-Cy7–conjugated anti-CCR5, andAPC-Cy7 anti-CD40 (conjugated antibodies from BD Biosciences unless noted). FlowJo software (TreeStar Inc) was used to analyze flow cytometry data.
Cytotoxic activity assay
Fresh purified pDCs (effector) and CD4+ T cells (target) were cocultured. Purified pDCs were incubated with or without HTLV-1 for 6 hours, supernatants were removed, and cells were washed. In a 96-well plate, 5 × 103 target cells per well were incubated with pDCs at cell ratio effector/target 2:1. After 6 hours of coculture, cells were assayed for apoptosis by annexin-V/Topro-3 by flow cytometry. Annexin V/Topro-3 was measured on BDCA-2−CD3+CD4+ cells, which represent T cells.
Cytokine detection
Supernatants of cultured pDCs were tested for soluble IFN-α (Cell Sciences), or TNF-α (R&D Systems) by enzyme-linked immunosorbent assay according to the manufacturer's instructions.
Three-dimensional microscopy
pDCs were purified from healthy donor blood by the use of negative selection (Miltenyi Biotec) and were cultured overnight in the presence or absence of HTLV-1, HTLV-1 plus chloroquine, and HTLV-1 plus serum from HTLV-1–infected patients. pDCs were plated on poly-L-lysine (Sigma-Aldrich)–coated slides and then fixed in 4% paraformaldehyde, quenched with 0.1M glycine. Cells were incubated in permeabilizing buffer containing 1% saponin with mouse anti-TRAIL (clone RIK-2, eBioscience) and HTLV-1 patient's serum. Staining was revealed by goat anti–mouse IgG-Alexa488 (Jackson ImmunoResearch Laboratories) and goat anti–human IgG-Cy3 (Jackson ImmunoResearch Laboratories), respectively. Nucleus was stained by the use of DAPI (Molecular Probes). Mounted slides were scanned with a Nikon Eclipse 90i Upright microscope (Nikon Instruments Europe) by the use of a 100× Plan Apo VC piezo objective (NA 1.4) and Chroma bloc filters (ET-DAPI, ET-GFP, ET-Cy3) and were subsequently deconvoluted with a Meinel algorithm and 8 iterations and analyzed by the use of Metamorph (MDS Analytical Technologies). Specifications were as follows: Trail/DAPI/virus/Overlay/Confocal plane: representative 2D focal plan. XZ/YZ view of Confocal plane: XZ/YZ projection of the XY focal plan along the red cross axis. Overlay with bright: DIC. Projection overlays: 2D projections of the maximum intensity pixels along the Z axis. 3D: 3D reconstruction analysis of the total cell by the use of ImageJ64 software (National Institutes of Health) and Imaris 6.2 software (Bitplane; also used to generate videos).
Statistical analysis
Experiments were repeated at least 4 times. P values were determined by the use of a 2-tailed Student t test. Univariate distributions of flow cytometric data were performed by probability binning, in 300 bins by the use of FlowJo software (TreeStar).32
Results
IFN-α response to HTLV-1 stimulation
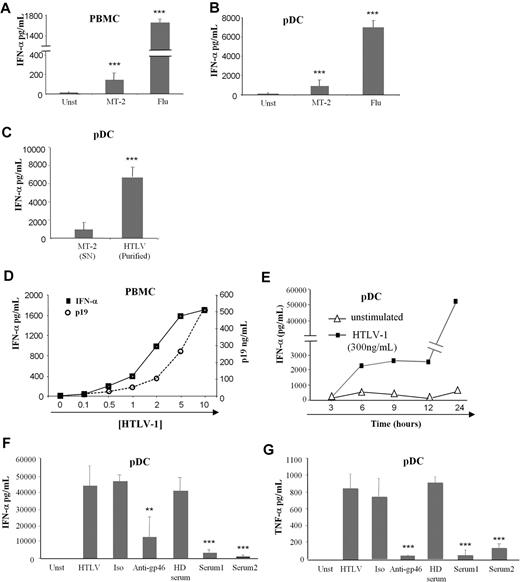
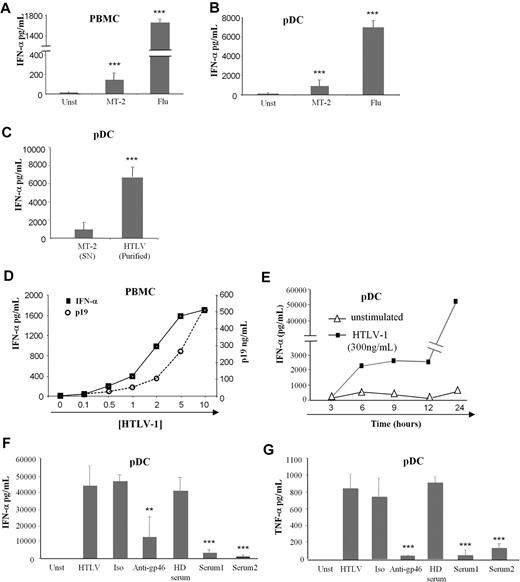
Because cell-free HTLV-1 could target immune cells and especially BDCA-4 (neuropilin-1) expressing pDCs,8 we studied the pDC response to this virus. The main characteristic of pDC viral stimulation is massive production of IFN-α that we followed by using an HTLV-1–infected CD4+ T-cell line (MT-2) as virus-producing cells. Low levels of IFN-α were secreted by HTLV-1–activated PBMCs and pDCs compared with influenza A virus stimulation (Figure 1A and B, respectively). Thus, we developed a purification method for HTLV-1 viruses by using ultracentrifugation of the MT-2 supernatants. Purified HTLV-1 virions (HTLV-1) induced greater levels of IFN-α by pDCs compared with MT-2 supernatants (Figure 1C). We also verified that ultracentrifuged supernatants from noninfected T-cell lines (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article) did not induce pDC IFN-α production (data not shown).
Cytokine production by HTLV-1–activated pDCs. IFN-α production by 106 PBMCs (A) and 105 purified pDCs (B) from healthy blood donors cultured alone in the presence of supernatants from HTLV-1 virus producing T-cell line (MT-2) or with influenza virus (Flu) as positive control. (C) Overnight IFN-α production by pDCs were assessed by the use of purified HTLV-1 virions (purified HTLV) from ultracentrifuged MT-2 supernatants (MT-2 SN). (D) IFN-α production by PBMCs in response to various HTLV-1 (p19-equivalent) concentrations (0-500 ng/mL). (E) Kinetic secretion of IFN-α by HTLV-1–stimulated pDCs (300 ng/mL of p19-equivalent) compared with unstimulated pDCs. HTLV-1 capacity to turn pDC into IFN-α (F) and TNF-α (G) producing pDCs was inhibited by sera containing blocking HTLV-1 antibodies from HTLV patients (Serum 1, Serum 2) or by blocking HTLV-1 envelope (gp46) antibody (10 μg/mL) but not using irrelevant antibody (10 μg/mL) or uninfected healthy donor serum (HD serum). Data shown are representative of at least 4 independent experiments. **P < .01; ***P < .001.
Cytokine production by HTLV-1–activated pDCs. IFN-α production by 106 PBMCs (A) and 105 purified pDCs (B) from healthy blood donors cultured alone in the presence of supernatants from HTLV-1 virus producing T-cell line (MT-2) or with influenza virus (Flu) as positive control. (C) Overnight IFN-α production by pDCs were assessed by the use of purified HTLV-1 virions (purified HTLV) from ultracentrifuged MT-2 supernatants (MT-2 SN). (D) IFN-α production by PBMCs in response to various HTLV-1 (p19-equivalent) concentrations (0-500 ng/mL). (E) Kinetic secretion of IFN-α by HTLV-1–stimulated pDCs (300 ng/mL of p19-equivalent) compared with unstimulated pDCs. HTLV-1 capacity to turn pDC into IFN-α (F) and TNF-α (G) producing pDCs was inhibited by sera containing blocking HTLV-1 antibodies from HTLV patients (Serum 1, Serum 2) or by blocking HTLV-1 envelope (gp46) antibody (10 μg/mL) but not using irrelevant antibody (10 μg/mL) or uninfected healthy donor serum (HD serum). Data shown are representative of at least 4 independent experiments. **P < .01; ***P < .001.
We then quantified purified virions by using HTLV-1 p19 protein titration (Figure 1D). IFN-α production by PBMCs was strictly dependent on the quantity of HTLV-1 (p19 equivalent; Figure 1D). The optimal HTLV-1 concentration (300 ng/mL) was used in subsequent experiments. To better characterize IFN-α secretion by pDC, we performed time-course experiments. IFN-α secretion by HTLV-1–stimulated pDCs was quantified at 3-hour intervals. HTLV-1 induced rapid, as soon as 6 hours, IFN-α secretion (Figure 1E).
Furthermore, IFN-α secretion by pDCs was strictly mediated by HTLV-1 particles. Purified pDCs were cultured with HTLV-1 in the presence or absence of anti-gp46 or serum from HTLV-1–infected patients (containing HTLV-1 neutralizing antibodies). Both anti-gp46 and patients' serum significantly reduced IFN-α secretion by pDC (Figure 1F). Moreover, HTLV-1 also induced TNF-α production by pDCs that could be blocked by HTLV-1 neutralizing antibodies (Figure 1G). Serum from uninfected healthy blood donors and irrelevant antibody were used as negative controls and did not have any effect on IFN-α and TNF-α secretion by activated pDC. Taken together, these results demonstrated that cell-free purified HTLV-1 induced a virus-dependent cytokine-mediated response by innate immune cells.
Phenotypic and functional characterization of HTLV-1–stimulated pDCs
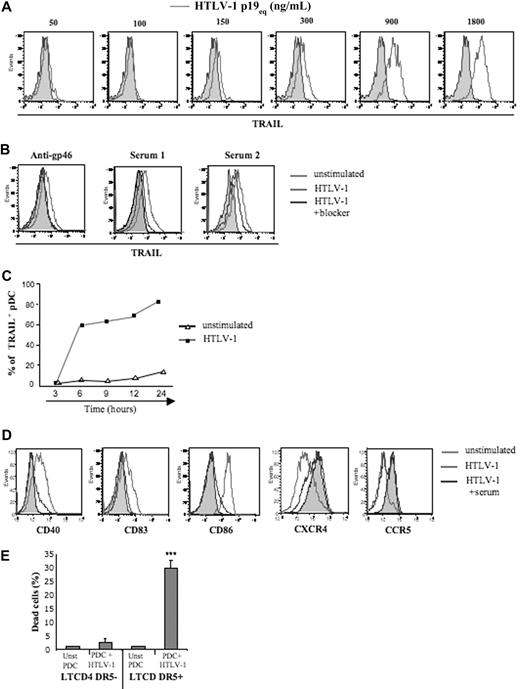
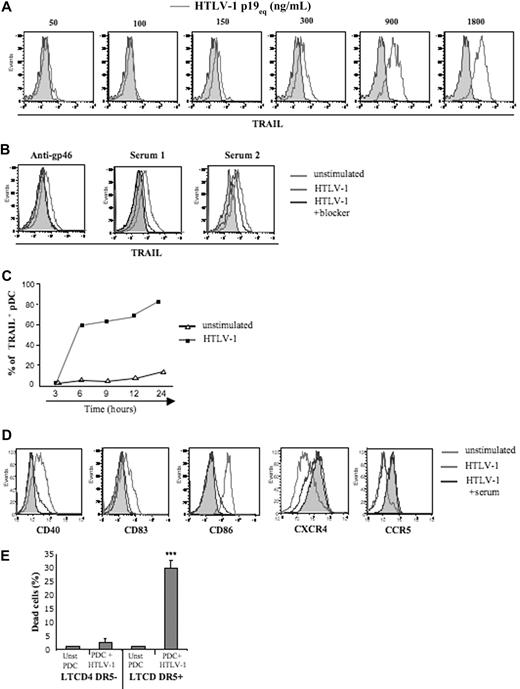
We recently demonstrated that HIV-1 induced transformation of pDCs into IFN-α–producing and TRAIL-expressing killer pDC (IKpDCs).31 Thus, we tested whether TRAIL was induced by HTLV-1–stimulated pDCs. A wide range of purified HTLV-1 concentrations was tested on pDC. TRAIL expression by pDC was strictly dependent on the quantity of HTLV-1 (p19 equivalent; Figure 2A). To validate HTLV-1 specificity, HTLV-1 blocking antibodies (HTLV-1 patients' sera or anti-gp46) significantly inhibited TRAIL expression on pDC exposed to HTLV-1 (Figure 2B). Serum from uninfected healthy blood donors did not reduce TRAIL expression by virus-exposed pDCs. As we observed for the IFN-α secretion time course, pDCs expressed TRAIL in response to HTLV-1 between 3 and 6 hours (Figure 2C).
Cell-free HTLV-1 virions induced TRAIL and activation marker expression by pDCs. (A) Purified pDCs were cultured in the absence (solid gray histograms) or presence of several concentrations of HTLV-1 (p19-equivalent, open gray histograms), and membrane TRAIL expression was measured by FACS. (B) Inhibition of membrane TRAIL expression by pDC activated by HTLV-1 virions (light gray histograms) by the use of HTLV-1 blockers (dark gray histograms) such as blocking HTLV-1 envelope antibody (anti-gp46, left) and sera containing blocking HTLV-1 antibodies from HTLV patients (Serum 1 and Serum 2, middle) and healthy donor serum (HD serum) as negative control (right). (C) Kinetics of expression of TRAIL by HTLV-1–stimulated pDCs (300 ng/mL p19-equivalent) compared with unstimulated pDCs. (D) Inhibition of activation markers expression (CD40, CD83, CD86) and chemokine receptors (CXCR4, CCR5) on pDCs stimulated by HTLV-1 virions (light gray histograms) with the use of HTLV-1 blockers (sera from infected HTLV patients and anti-gp46; dark gray histograms). (E) Apoptosis assay (annexin V/Topro-3 staining) of unstimulated (Unst pDC) and HTLV-1–activated pDCs (pDC + HTLV-1) in the presence of CD4+CD3+DR5− or CD4+CD3+DR5+ T-cell target (ratio 1:2). Data shown in each panel are representative of at least 3 independent experiments.
Cell-free HTLV-1 virions induced TRAIL and activation marker expression by pDCs. (A) Purified pDCs were cultured in the absence (solid gray histograms) or presence of several concentrations of HTLV-1 (p19-equivalent, open gray histograms), and membrane TRAIL expression was measured by FACS. (B) Inhibition of membrane TRAIL expression by pDC activated by HTLV-1 virions (light gray histograms) by the use of HTLV-1 blockers (dark gray histograms) such as blocking HTLV-1 envelope antibody (anti-gp46, left) and sera containing blocking HTLV-1 antibodies from HTLV patients (Serum 1 and Serum 2, middle) and healthy donor serum (HD serum) as negative control (right). (C) Kinetics of expression of TRAIL by HTLV-1–stimulated pDCs (300 ng/mL p19-equivalent) compared with unstimulated pDCs. (D) Inhibition of activation markers expression (CD40, CD83, CD86) and chemokine receptors (CXCR4, CCR5) on pDCs stimulated by HTLV-1 virions (light gray histograms) with the use of HTLV-1 blockers (sera from infected HTLV patients and anti-gp46; dark gray histograms). (E) Apoptosis assay (annexin V/Topro-3 staining) of unstimulated (Unst pDC) and HTLV-1–activated pDCs (pDC + HTLV-1) in the presence of CD4+CD3+DR5− or CD4+CD3+DR5+ T-cell target (ratio 1:2). Data shown in each panel are representative of at least 3 independent experiments.
To investigate whether HTLV-1–induced complete pDC activation, we followed expression of costimulatory molecules and chemokine receptors on pDC. CD40, CD83, and CD86 activation markers were up-regulated on HTLV-1–stimulated pDCs and were dramatically inhibited in the presence of patients' serum (Figure 2D). Similarly, HTLV-1 induced down-regulation of both CCR5 and CXCR4 on pDC that was also blocked by patients' serum (Figure 2D).
Finally, to determine whether HTLV-1–induced functional TRAIL expression by pDC, we performed a cytotoxicity assay with purified pDCs as effector and fresh CD4+ T cells as target cells. Thus, resting or HTLV-1–activated pDCs were cocultured (ratio 1:2) with DR5− or DR5+ CD4+ T cells, and apoptosis was tested by measuring Annexin-V/Topro-3 on CD4+CD3+ T cells (Figure 2E). Massive death was observed only when HTLV-1–activated pDCs were exposed to DR5+ T cells. In contrast, resting pDCs did not induce apoptosis of DR5+CD4+ T cells nor of DR5− T cells (Figure 2E). These results, therefore, demonstrated that HTLV-1 induced the transformation of pDCs into fully functional IKpDCs.
Mechanism of pDC activation by HTLV-1
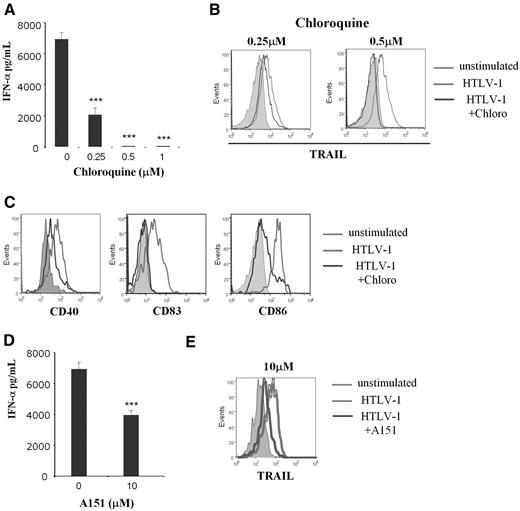
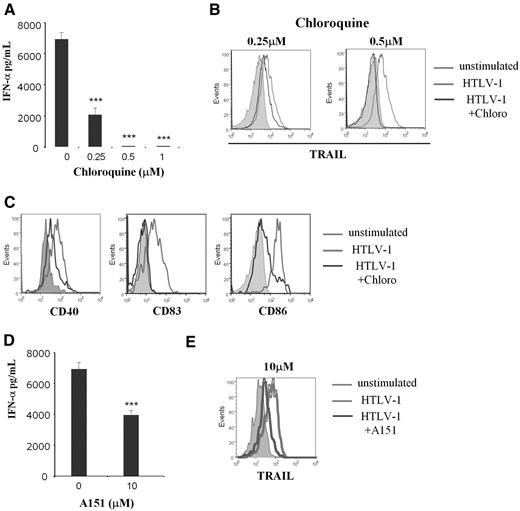
Recent studies22,25 showed that single-strain RNA viruses stimulated pDCs via the TLR7 pathway. To determine whether endocytosis is or is not required for HTLV-1 induction of IkpDC, we used the endosomal acidification inhibitor chloroquine. Chloroquine significantly inhibited IFN-α production and TRAIL expression by pDC in a dose-dependent manner (Figure 3A and B, respectively). Chloroquine also significantly reduced CD40, CD83, and CD86 expression by HTLV-1–stimulated pDCs (Figure 3C). Because the functional activity of endosome consists in viral degradation to release nucleic acid stimulating TLR, we investigated this mechanism in HTLV-1–stimulated pDCs. TLR7 is an endosomal receptor mainly expressed by pDCs that recognizes viral RNA.23,33 We used the oligodinucleotide A151, a competitive inhibitor of TLR7, to demonstrate the involvement of this pathway in HTLV-1 pDC stimulation.22 A151 significantly reduced IFN-α secretion (Figure 3D) and TRAIL expression (Figure 3E) by HTLV-1–activated pDCs. To better demonstrate the involvement of TLR7 in HTLV-1–mediated pDC activation and the specificity of the inhibitor A151, we tested whether or not TLR7-independent stimulation would be blocked by A151. Purified pDCs were cultured in the presence of –C–phosphate–G– (CpG)-A and CpG-oligodeoxynucleotide (negative control). We found that CpG-A (used at 1, 2, or 5μM) was able to induce TRAIL expression on pDC in contrast to CpG-oligodeoxynucleotide. However, A151 reduced TRAIL expression on HTLV-1–activated pDCs but not on CpG-stimulated pDCs. Greater doses of A151 (10μM) was still not able to reduce TRAIL expression on CpG-stimulated pDCs (supplemental Figure 1). Therefore, our findings demonstrate that lytic endosomal degradation and the TLR7 pathway are involved in HTLV-1–mediated transformation of pDCs into IKpDC.
HTLV-1 pDC stimulation was TLR7 dependent. IFN-α secretion (A), TRAIL (B), and activation marker expressions CD40, CD83, CD86 (C) by HTLV-1–activated pDCs were inhibited by the use of the inhibitor of endosomal acidification (chloroquine) in a dose-response manner (0.25-1μM). TRAIL expression and activation markers (light gray histograms in B and C) compared with unstimulated pDCs (solid gray histograms) are significantly reduced by chloroquine at 0.5μM (dark gray histograms). IFN-α secretion (D) and TRAIL expression (E) by HTLV-1–activated pDCs were blocked by use of the TLR7 inhibitor (A151) in a dose-response manner. TRAIL expression (light gray histograms in E) compared with unstimulated pDC (solid gray histograms) is significantly reduced in the presence of A151 at 10μM (dark gray histograms). Data shown in each panel are representative of at least 3 independent experiments.
HTLV-1 pDC stimulation was TLR7 dependent. IFN-α secretion (A), TRAIL (B), and activation marker expressions CD40, CD83, CD86 (C) by HTLV-1–activated pDCs were inhibited by the use of the inhibitor of endosomal acidification (chloroquine) in a dose-response manner (0.25-1μM). TRAIL expression and activation markers (light gray histograms in B and C) compared with unstimulated pDCs (solid gray histograms) are significantly reduced by chloroquine at 0.5μM (dark gray histograms). IFN-α secretion (D) and TRAIL expression (E) by HTLV-1–activated pDCs were blocked by use of the TLR7 inhibitor (A151) in a dose-response manner. TRAIL expression (light gray histograms in E) compared with unstimulated pDC (solid gray histograms) is significantly reduced in the presence of A151 at 10μM (dark gray histograms). Data shown in each panel are representative of at least 3 independent experiments.
Microscopic study of HTLV-1 viruses and TRAIL localization in pDCs
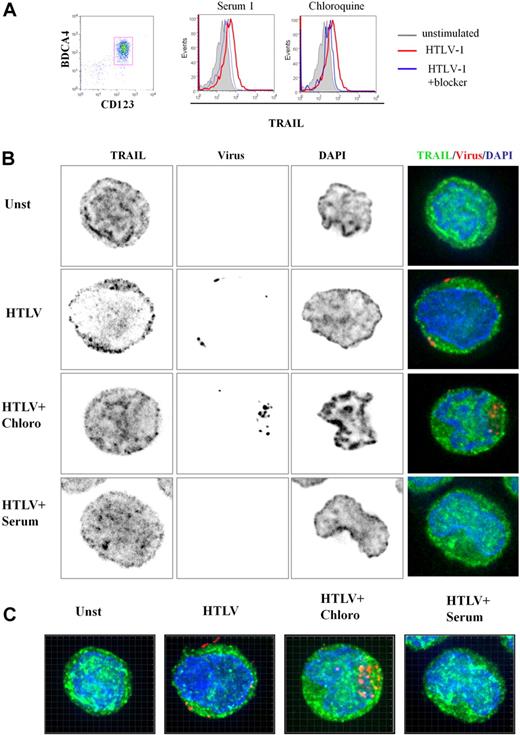
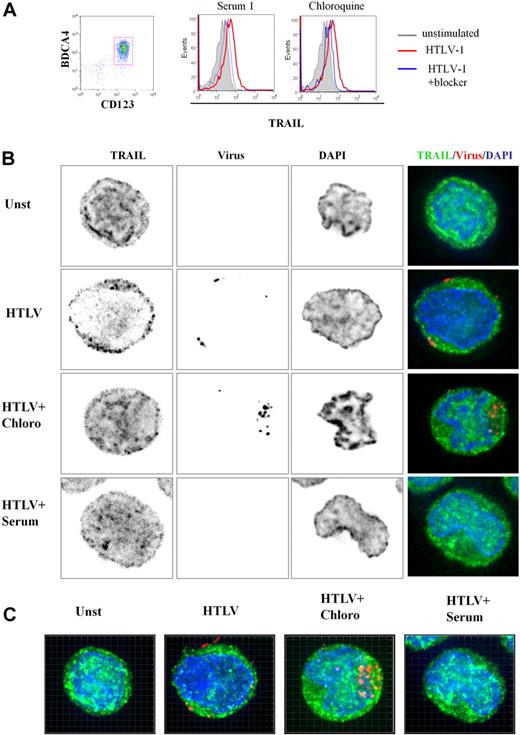
To better characterize TRAIL expression by HTLV-1–activated pDCs, we performed 3-dimensional (3D) microscopy experiments. Purified pDCs were cultured in media alone (unstimulated), with HTLV-1, HTLV-1 plus chloroquine, and HTLV-1 plus serum from HTLV-1–infected patients. pDC culture conditions used for microscopy experiments were simultaneously analyzed by FACS. As shown in Figure 4A, HTLV-1 induced TRAIL expression on pDCs (a representative experiment). Both chloroquine and patients' serum significantly inhibited TRAIL expression on the pDC cell surface. Surprisingly, we also found high levels of intracellular TRAIL expression in unstimulated pDCs (Figure 4B), suggesting that the very rapid expression of TRAIL on the cell surface of stimulated pDCs (Figure 2C) was caused by TRAIL relocalization from cytoplasm to the membrane. Permeabilized pDCs were stained with TRAIL-Alexa 488 (green), nuclear staining DAPI (blue), and HTLV-1 viral proteins-cy3 (red) to study protein localization by 3D microscopy. Focal plane analysis revealed that TRAIL from HTLV-1–stimulated pDCs appeared to be decreased in the cytoplasm at the expense of membrane TRAIL expression (Figure 4B) compared with unstimulated pDC (unst). This TRAIL membrane repartition induced by HTLV-1 was strongly inhibited by both chloroquine (HTLV + Chloro) and patient's serum (HTLV + serum). Free-virus HTLV-1 detected by viral staining was observed in the proximity of the cell membrane but not in the cytoplasm, in contrast to chloroquine-treated cells (HTLV + Chloro). By using chloroquine, which blocks viral degradation, we observed high virus density demonstrating that HTLV-1 entered the pDCs by endocytosis. These results were confirmed in 3D reconstruction (Z-stacks compilation; Figure 4C) and associated videos (supplemental Figure 2A-C).
3D analysis of TRAIL localization by HTLV-1–activated pDCs. (A) Freshly purified CD123+BCDA4+ pDCs (96%, left) stimulated by HTLV-1 express TRAIL on their membrane (middle and right, red histograms) that is significantly reduced by both HTLV-1–infected patient serum containing HTLV-1 blocking antibody (middle, blue histogram) and chloroquine (right, blue histogram). (B) Unstimulated pDCs (Unst), HTLV-1–stimulated pDC (HTLV-1) treated with chloroquine (HTLV-1 + Chloro) or with blocking HTLV1-infected patient serum (HTLV-1 + Serum) were stained with anti-TRAIL (green) and anti–HTLV-1 virus (red). pDC stainings (green and red) were merged with DAPI (blue)–colored nucleus (Overlay) or with phase contrast (Bright). HTLV-1–activated pDCs (HTLV-1) showed a TRAIL relocalization to cell surface in contrast to unstimulated pDCs (Unst). TRAIL surface localization (green) induced by HTLV-1 was inhibited in the presence of the HTLV-1 blockers (HTLV-1 + Chloro and HTLV-1 + Serum). Inhibition of endosomal acidification (chloroquine) allowed easier detection of HTLV-1 virions (HTLV-1 + Chloro) in contrast to few HTLV-1 particles detected in HTLV-1–stimulated pDCs. Blocking HTLV-1 serum inhibited HTLV-1 entry (HTLV-1 + Serum) and TRAIL localization on the membrane (analyzed by Metamorph software). (C) pDCs in different culture conditions (Unst, HTLV-1, HTLV1 + Chloro, HTLV-1 + Serum) were observed in several plane (Z-stack) by the use of a 3D microscope. Panels shown here were the results of image compilation allowing entire cell observation in a 2D representation to refine plane analysis. Whole 3D cell acquisition was performed and the entire Z-stack was projected in 2D (analyzed by Imaris 6.2 software).
3D analysis of TRAIL localization by HTLV-1–activated pDCs. (A) Freshly purified CD123+BCDA4+ pDCs (96%, left) stimulated by HTLV-1 express TRAIL on their membrane (middle and right, red histograms) that is significantly reduced by both HTLV-1–infected patient serum containing HTLV-1 blocking antibody (middle, blue histogram) and chloroquine (right, blue histogram). (B) Unstimulated pDCs (Unst), HTLV-1–stimulated pDC (HTLV-1) treated with chloroquine (HTLV-1 + Chloro) or with blocking HTLV1-infected patient serum (HTLV-1 + Serum) were stained with anti-TRAIL (green) and anti–HTLV-1 virus (red). pDC stainings (green and red) were merged with DAPI (blue)–colored nucleus (Overlay) or with phase contrast (Bright). HTLV-1–activated pDCs (HTLV-1) showed a TRAIL relocalization to cell surface in contrast to unstimulated pDCs (Unst). TRAIL surface localization (green) induced by HTLV-1 was inhibited in the presence of the HTLV-1 blockers (HTLV-1 + Chloro and HTLV-1 + Serum). Inhibition of endosomal acidification (chloroquine) allowed easier detection of HTLV-1 virions (HTLV-1 + Chloro) in contrast to few HTLV-1 particles detected in HTLV-1–stimulated pDCs. Blocking HTLV-1 serum inhibited HTLV-1 entry (HTLV-1 + Serum) and TRAIL localization on the membrane (analyzed by Metamorph software). (C) pDCs in different culture conditions (Unst, HTLV-1, HTLV1 + Chloro, HTLV-1 + Serum) were observed in several plane (Z-stack) by the use of a 3D microscope. Panels shown here were the results of image compilation allowing entire cell observation in a 2D representation to refine plane analysis. Whole 3D cell acquisition was performed and the entire Z-stack was projected in 2D (analyzed by Imaris 6.2 software).
To better characterize HTLV-1 virion entry and TRAIL localization in pDC, 3D reconstruction (focal plane, XZ, and YZ-stacks) analysis was performed. In Figure 5A, YZ and XZ stacks showed the relocalization of TRAIL at the membrane of HTLV-1–stimulated pDCs (Figure 5Aii) in contrast to intracytoplasmic TRAIL repartition of unstimulated pDCs (Figure 5Ai). We also confirmed that the HTLV-1 blockade by chloroquine and anti–HTLV-1 serum abolished membrane TRAIL expression by pDCs (Figure 5Aiii and Aiv, respectively). Two-dimensional pixel intensity analysis with the use of interactive surface plot of ImageJ software permitted visualization with precision, internal or external localization of both TRAIL and virus, combined with phase-contrast acquisition (membrane delimitation; Figure 5B). This 3D-2D combined analysis clearly demonstrated that HTLV-1–stimulated pDCs harbored TRAIL and membrane colocalization (Figure 5Bii) in contrast to the restrictive intracellular TRAIL expression of unstimulated pDCs (Figure 5Bi). Similar intracellular TRAIL sequestration was obtained in the presence of HTLV-1 blockers (Figure 5Biii and Biv, respectively).
Microscopy analysis of TRAIL localization in pDCs. (A) Deconvolution overlays of representative 2D red cross axis XY focal plan with XZ/YZ view of focal plane and projection overlay of cell stainings (analyzed by Metamorph software). Unstimulated pDCs (i), HTLV-1–stimulated pDCs (HTLV-1; ii), treated with chloroquine (HTLV-1 + Chloro; iii), or with blocking HTLV-1–infected patient serum (HTLV-1 + Serum; iv) were stained with anti-TRAIL (green), anti-HTLV-1 virus (red), and DAPI (nucleus staining). (B) Deconvolution of representative 2D XY focal plane was treated to allow Z scaling of pixel intensity (analyzed by Metamorph and ImageJ software). Each staining (anti-TRAIL [green], anti-HTLV-1 virus]red], and DAPI [nucleus staining]) was merged together with phase contrast (gray, defined cell surface). This representation was used to enhance the observation of TRAIL localization in the cytoplasm (Cy) or its relocalization to the membrane (Mb). Unstimulated pDCs (i) or HTLV-1–stimulated pDCs (ii) treated with inhibitors (HTLV-1 + Chloroquine; iii) or with blocking HTLV-1–infected patient Serum (HTLV-1 + Serum; iv) restrictively expressed TRAIL (green) in the cytoplasm in contrast to HTLV-1–activated pDCs that expressed TRAIL at the cell surface. Inhibitor of endosomal acidification (HTLV1 + Chloroquine) allowed detection of HTLV-1 particles (red) in the cytoplasm (Cy) in contrast to the cell-surface detection of HTLV-1 virions in HTLV-1–activated pDCs. HTLV-1 virion loading was poorly detected in the presence of serum that directly blocked the virus before the pDC contact.
Microscopy analysis of TRAIL localization in pDCs. (A) Deconvolution overlays of representative 2D red cross axis XY focal plan with XZ/YZ view of focal plane and projection overlay of cell stainings (analyzed by Metamorph software). Unstimulated pDCs (i), HTLV-1–stimulated pDCs (HTLV-1; ii), treated with chloroquine (HTLV-1 + Chloro; iii), or with blocking HTLV-1–infected patient serum (HTLV-1 + Serum; iv) were stained with anti-TRAIL (green), anti-HTLV-1 virus (red), and DAPI (nucleus staining). (B) Deconvolution of representative 2D XY focal plane was treated to allow Z scaling of pixel intensity (analyzed by Metamorph and ImageJ software). Each staining (anti-TRAIL [green], anti-HTLV-1 virus]red], and DAPI [nucleus staining]) was merged together with phase contrast (gray, defined cell surface). This representation was used to enhance the observation of TRAIL localization in the cytoplasm (Cy) or its relocalization to the membrane (Mb). Unstimulated pDCs (i) or HTLV-1–stimulated pDCs (ii) treated with inhibitors (HTLV-1 + Chloroquine; iii) or with blocking HTLV-1–infected patient Serum (HTLV-1 + Serum; iv) restrictively expressed TRAIL (green) in the cytoplasm in contrast to HTLV-1–activated pDCs that expressed TRAIL at the cell surface. Inhibitor of endosomal acidification (HTLV1 + Chloroquine) allowed detection of HTLV-1 particles (red) in the cytoplasm (Cy) in contrast to the cell-surface detection of HTLV-1 virions in HTLV-1–activated pDCs. HTLV-1 virion loading was poorly detected in the presence of serum that directly blocked the virus before the pDC contact.
HTLV-1 virion detection was limited to the cell membrane in HTLV-1–stimulated pDCs (Figure 5Aii-Bii), probably because of rapid endosomal viral degradation that abolished any viral protein detection. The detection of high intracellular density of HTLV-1 particles upon neutralization of endosomal viral degradation by chloroquine (Figure 5Aiii-Biii) strongly supports our hypothesis that cell-free HTLV-1–activated pDCs via TLR7 stimulation resulting in IFN-α and TRAIL production defining innate immune response.
Discussion
PDC innate immune response is defined by massive IFN-α production after stimulation of viral TLR7/9 receptors, leading to antiviral activity.10,11,34,35 In this study, we show that pDCs exposed to cell-free HTLV-1 particles secrete IFN-α and TNF-α. Furthermore, HTLV-1–activated pDCs rapidly express proapoptotic ligand TRAIL on the membrane, providing a killer activity, transforming them into IKpDC. Our results highlight a potential key role of pDC in primary HTLV-1 infection.
Because pDCs express high levels of BDCA-4, their activation might be mediated by 2 distinct pathways consisting in endocytosis/TLR activation versus infection. HTLV-1 is an RNA retrovirus that potentially activates the TLR7 pathway. TLR7 is a sensor molecule of the innate immune system that recognizes the ribonucleic acid of infectious agents.33,36 We found that an inhibitor of endosome activity (chloroquine) significantly reduced HTLV-1–induced IFN-α production and TRAIL expression, demonstrating that pDC stimulation is dependent on lysosomal degradation of viral particles. We then showed that TLR7 was responsible for pDC activation into IKpDC. The specific inhibitor (A151) of TLR7 largely reduced IFN-α and TRAIL mediated by HTLV-1. Similarly, activation of the TLR7 pathway by HTLV-1 induces costimulatory molecules (CD40, CD83, CD86) expression on pDC as well as down-modulation of chemokine receptors (CXCR4 and CCR5). Our results demonstrate that HTLV-1 stimulates the TLR7 pathway after endocytosis and induces innate immune response by pDC.
To better characterize HTLV-1–induced pDC activation, we developed 3D microscopy technology to simultaneously follow the localization of both viruses and TRAIL. This 3D analysis revealed an important stock of intracellular TRAIL in immature unstimulated pDCs, favoring a rapid response of these cells against viral infection reflected in the TRAIL surface expression we observed within 6 hours. We found that HTLV-1 induced relocalization of intracellular TRAIL to the cell surface. We also confirmed the involvement of an endocytotic TLR pathway by using chloroquine, which inhibited the relocalization phenomenon. By using 3D microscopy, our study visualized, for the first time to our knowledge, HTLV-1 particles in fresh human innate immune cells. This approach allowed us to generate 3D videos showing the presence of HTLV-1 and TRAIL localization in pDCs. The addition of chloroquine to our cultures facilitated HTLV-1 virus detection into pDC because of the inhibition of viral degradation. This observation contrasts with HTLV-1–exposed pDCs, in which only virions localized in close proximity to the cell membrane could be detected.
Apoptotic induction of virus-infected cells may serve as a beneficial host defense mechanism to limit virus spread.37 TRAIL plays an important role in virus-induced apoptosis of CD4+ T cells in acute HIV-1 infection.38,39 HTLV-1 induces continuous cell mutation, providing emergence of clones as the causal viral mechanism for ATLL.40 Conversely, because HTLV-1 replicates mainly through clonal expansion, it is advantageous for the virus to prolong infected cell life. The HTLV-1 Tax protein is the viral transforming factor that confers clone emergence.41 Tax has been shown to confer resistance to the TRAIL apoptotic pathway in HTLV-1–infected T cells.42,43 Our study suggests that IKpDCs may induce the death of uninfected CD4+ T cells in the acute phase and may consequently participate in clone emergence of TRAIL-resistant Tax-positive cells, leading to ATLL. An important challenge would be to link the pDC phenotype to the different HTLV-1–associated pathologies (ATLL or HTLV-1–associated myelopathy/tropical spastic paraparesis). It would be interesting to determine whether IKpDCs persist during chronic infection to generate new HTLV-1 progression markers.
The characterization of IKpDCs in vivo opens a new area of DC research in HTLV-1 and other retrovirus-induced immunopathogenesis and in tumor cell biology. Considered together, our data highlight a dual role for pDCs in HTLV-1 disease. pDCs that become infected may participate in viral spread in the host8 and concomitantly express TRAIL, which may select the transformed CD4+ T-cell clone, leading to ATLL years later. In this context, it will be of great interest to test TRAIL sensitivity of the persistent clones after HTLV-1 infection that may subsequently be transformed to lymphoma/leukemia. Thus, pDC investigation in HTLV-1 disease will be crucial for understanding complex HTLV-1–associated pathologies. However, detection of primary infection in humans is currently not feasible because of the high latency of HTLV-1 virus before disease symptoms appearance. An alternative way to characterize and understand the early steps of HTLV-1 infection is the development of the pathogenic simian model (simian T-lymphotropic virus-1). However, in addition to selection of TRAIL-resistant clones, one could hypothesize that similar to HIV-1 infection, pDCs may participate in and contribute to the immune suppression that occurs in ATLL.
In this report we demonstrate that HTLV-1–free particles generate an immune response by professional virus “sentinel” pDC. We then identify and describe the mechanism by which purified HTLV-I virions stimulate pDCs and transform them into functional killer cells. We show that pDC response and activation to HTLV-1 is strictly virus dose dependent. Finally, purified HTLV-1 particles induced TLR7-mediated relocalization of intracellular TRAIL to the pDC membrane. In conclusion, the physiologic function of pDC during the different stages of HTLV-1 infection will represent a new field of investigation and may lead to new therapeutic strategies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Gene Shearer (Experimental Immunology Branch, National Cancer Institute, National Institutes of Health) for comments and manuscript critiques.
We also thank the Agence Nationale de la Recherche sur le SIDA (ANRS) for its financial support. We greatly acknowledge the Nikon Imaging Center@curie.fr (Institut Curie-CNRS, http://nimce.curie.fr) and the PICT-IBiSA Imaging Facility (http://pict-ibisa.curie.fr).
Authorship
Contribution: J.-P.H. and Y.L. designed research; R.C., L.B., and C.G. performed research; R.C., L.B., J.-P.H., Y.L., and C.G. analyzed research; R.H.S, F.R., and C.P. contributed new reagents; O.H. contributed to writing the paper; and J.-P.H. and Y.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Jean-Philippe Herbeuval, UMR CNRS 8147, Université Paris Descartes, Hôpital Necker, 149-161 rue de Sèvres, 75743 Paris cedex 15, France; e-mail: herbeuval@necker.fr.
References
Author notes
R.C., L.B., Y.L., and J.-P.H. contributed equally to this study.

![Figure 5. Microscopy analysis of TRAIL localization in pDCs. (A) Deconvolution overlays of representative 2D red cross axis XY focal plan with XZ/YZ view of focal plane and projection overlay of cell stainings (analyzed by Metamorph software). Unstimulated pDCs (i), HTLV-1–stimulated pDCs (HTLV-1; ii), treated with chloroquine (HTLV-1 + Chloro; iii), or with blocking HTLV-1–infected patient serum (HTLV-1 + Serum; iv) were stained with anti-TRAIL (green), anti-HTLV-1 virus (red), and DAPI (nucleus staining). (B) Deconvolution of representative 2D XY focal plane was treated to allow Z scaling of pixel intensity (analyzed by Metamorph and ImageJ software). Each staining (anti-TRAIL [green], anti-HTLV-1 virus]red], and DAPI [nucleus staining]) was merged together with phase contrast (gray, defined cell surface). This representation was used to enhance the observation of TRAIL localization in the cytoplasm (Cy) or its relocalization to the membrane (Mb). Unstimulated pDCs (i) or HTLV-1–stimulated pDCs (ii) treated with inhibitors (HTLV-1 + Chloroquine; iii) or with blocking HTLV-1–infected patient Serum (HTLV-1 + Serum; iv) restrictively expressed TRAIL (green) in the cytoplasm in contrast to HTLV-1–activated pDCs that expressed TRAIL at the cell surface. Inhibitor of endosomal acidification (HTLV1 + Chloroquine) allowed detection of HTLV-1 particles (red) in the cytoplasm (Cy) in contrast to the cell-surface detection of HTLV-1 virions in HTLV-1–activated pDCs. HTLV-1 virion loading was poorly detected in the presence of serum that directly blocked the virus before the pDC contact.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/11/10.1182_blood-2009-06-224741/4/m_zh89990948490005.jpeg?Expires=1767696083&Signature=Rs61E7V3TOQasft7OxXruX3nI4F8PKIGjuNDPKfO82dysih3f4KUU9IkD77cwdFR2k7K67F7qFWbTnZEn5uMX5F6hyuwbPmimtAga-~LBwcjCxviGGjTDgZpurQKRImmMsSqdM5HobKY4ms1bcXpPVsdxdYCHN6b4jbJFafRW41lsFMABIJU-t1VJyfE4iWERJo~oyynPE-H4N40Vf8YEkEHOejZcgfi4yR1SiHAmq6vI02FMc3sxkQMBMFh-xK5Tt8~w8NY8SlmGza6mtFyGv6yidVTJtvPRvWvGHMcijcpYF5NlVgH64pR2HXgQ6KqYcBAmQ8fmZxgZnJ7ei0cPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. Microscopy analysis of TRAIL localization in pDCs. (A) Deconvolution overlays of representative 2D red cross axis XY focal plan with XZ/YZ view of focal plane and projection overlay of cell stainings (analyzed by Metamorph software). Unstimulated pDCs (i), HTLV-1–stimulated pDCs (HTLV-1; ii), treated with chloroquine (HTLV-1 + Chloro; iii), or with blocking HTLV-1–infected patient serum (HTLV-1 + Serum; iv) were stained with anti-TRAIL (green), anti-HTLV-1 virus (red), and DAPI (nucleus staining). (B) Deconvolution of representative 2D XY focal plane was treated to allow Z scaling of pixel intensity (analyzed by Metamorph and ImageJ software). Each staining (anti-TRAIL [green], anti-HTLV-1 virus]red], and DAPI [nucleus staining]) was merged together with phase contrast (gray, defined cell surface). This representation was used to enhance the observation of TRAIL localization in the cytoplasm (Cy) or its relocalization to the membrane (Mb). Unstimulated pDCs (i) or HTLV-1–stimulated pDCs (ii) treated with inhibitors (HTLV-1 + Chloroquine; iii) or with blocking HTLV-1–infected patient Serum (HTLV-1 + Serum; iv) restrictively expressed TRAIL (green) in the cytoplasm in contrast to HTLV-1–activated pDCs that expressed TRAIL at the cell surface. Inhibitor of endosomal acidification (HTLV1 + Chloroquine) allowed detection of HTLV-1 particles (red) in the cytoplasm (Cy) in contrast to the cell-surface detection of HTLV-1 virions in HTLV-1–activated pDCs. HTLV-1 virion loading was poorly detected in the presence of serum that directly blocked the virus before the pDC contact.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/11/10.1182_blood-2009-06-224741/4/m_zh89990948490005.jpeg?Expires=1767696084&Signature=OtqS7UAsw2sCocptWUAY3jAB9tVRE1SDU06vJ2UON3x2tL-Zko84FTsIQb8ObvScs0UykumBozLWKRoOA0vNK3uFahSTNpkHlZxQvSHA4kRvh1fDTaoXa2QhXoCnlVrmr6Kf92lF978HICFDOpOEGYN3nrcAIu5XaAVgWoqXKqTfTftaidgg8WEA8ZgX3VXLz1xprbrGdgzd1B8wkwSD4D5zaJa5TKZ4zjr6ySLHEk6JPzAajJCXz2ohARpW05j1t5C6U9kUnpx7iv3V6ZVcNyxXxLQMdZ6Hq7IU-I-BqUyPGE7b-bK-G-YzL7jENp0nQtFMP6FTlVwQZyFXuK~92Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)