Abstract
Central and peripheral tolerance is required to prevent immune responses to self-antigens. We now present a mouse model in which wild-type (WT) SH2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76) has been constitutively targeted to the membrane, where CD4+ T cells become spontaneously dysregulated and develop an inflammatory phenotype. Mice bearing membrane-targeted SLP-76 (MTS) have a partial T-cell lymphopenia and impaired signaling though the mature T-cell receptor. The CD4+ T cells that develop in these mice possess an activated-like phenotype and are skewed toward the inflammatory TH1 and TH17 lineages. MTS mice also spontaneously develop autoantibodies at an early age. To rule out abnormal thymic selection as the sole cause of the MTS phenotype, we expressed WT SLP-76 along with the MTS followed by deletion of the WT allele in peripheral T cells. The peripheral MTS-expressing T cells demonstrate skewed cytokine responses when transferred into lymphopenic hosts. Thus, the abnormal effector T-cell phenotype still occurs in the presence of preserved central and peripheral tolerance, suggesting that diminished T-cell receptor signaling can promote skewed T-cell responses.
Introduction
T-cell activation occurs when the T-cell receptor (TCR) interacts with cognate peptide and major histocompatibility complex. On TCR ligation, there is the sequential activation of the Src family kinase Lck, which in turn phosphorylates immunoreceptor tyrosine-based activation motifs present in the CD3/TCR complex.1 Phosphorylation of the immunoreceptor tyrosine-based activation motifs allows for activation and recruitment of ζ-associated protein kinase of 70 kDa (ZAP-70). ZAP-70 phosphorylates the transmembrane adaptor protein linker of activated T cells (LATs)2 and the cytosolic protein SH2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76).3 This in turn allows for recruitment of SLP-76 to LAT at the plasma membrane and the formation of a multimolecular complex composed of several other important proteins, including phospholipase Cγ1 (PLC-γ1), Vav1, noncatalytic region of tyrosine kinase, interleukin-2 (IL-2)–induced tyrosine kinase, adhesion- and degranulation-promoting adapter protein, and hematopoietic progenitor kinase 1.4 The formation of this complex is essential to propagate distal TCR signaling and successful T-cell activation.5 The importance of these proximal events is evident as the genetic deletion of LAT or SLP-76 prevents T-cell development in the thymus, and peripheral deletion results in a lack of T-cell responses to antigenic stimulation.6-8
In many cases, when TCR signaling is impaired by mutations within these signaling proteins, a partial block in T-cell development is observed. Interestingly, instead of diminished peripheral T-cell responses, mice bearing these mutations often exhibit aberrant T-cell responses in those cells that do leave the thymus, leading to immunopathology.9-14 A similar trend in abnormal T-cell-mediated responses is also observed in humans, where mutations in several of these signaling molecules have occurred naturally.15,16 These findings create a paradox in which mutations that primarily impair TCR signaling and cause immunodeficiency also lead to the initiation of autoimmunity.
Because defects in either central or peripheral tolerance are known to cause autoimmunity in different mouse models and human diseases,17-20 the focus of several investigations has been on determining the contributions of these tolerance mechanisms to the development of autoimmune diseases in mice expressing mutations in TCR signaling components. Studies of such models have typically revealed abnormalities in thymic selection, suggesting that the cause of autoimmunity may be the failure to eliminate autoreactive cells during thymic development.9,13,21 The suppressive ability of peripheral regulatory T cells has also been explored in these models often showing some degree of impairment,22,23 suggesting yet another mechanism for the observed autoimmunity. However, it remains possible that altered TCR signaling itself may predispose toward aberrant T-cell responses in the face of normal thymic selection and intact regulatory T-cell function. One such example involving the adapter LAT has recently been described.24
We recently generated a mouse expressing a chimeric protein composed of the amino-terminal membrane-targeting domain of LAT and the full-length SLP-76. This membrane-targeted SLP-76 (MTS) was found to constitutively localize to membranes of Jurkat cells and primary T cells in which a construct for this chimeric protein was knocked into the SLP-76 locus. Despite being able to rescue TCR signaling when introduced into either LAT- or SLP-76–deficient Jurkat T cells,25 this mutant was unable to rescue T-cell development when introduced into LAT−/− mice. Further investigation revealed that the homozygous MTS knock-in (MTS/MTS) mouse demonstrates impaired signaling through the TCR and a significant block in T-cell development.26
We now show that some CD4+ T cells do develop and emigrate from the thymus in MTS/MTS mice. Examination of these cells reveals that, similar to MTS thymocytes, peripheral T cells demonstrate significant defects in critical TCR-mediated signaling events. Despite the diminished TCR responses, we found that MTS CD4+ T cells possess an activated-like phenotype and are inherently skewed toward inflammatory TH1 and TH17 lineages. Furthermore, MTS mice spontaneously develop autoantibodies at an early age. The abnormal activation profile does not appear to be the result of diminished regulatory T-cell function, as MTS mice have normal frequencies of FoxP3+ T cells, and regulatory T-cell function, although variable, is largely intact. In addition, the abnormal T-cell activation in the MTS mice is not solely the result of altered thymic selection, as adoptive transfer of cells that were allowed to develop normally (because of the presence of a wild-type SLP-76 allele in the thymus) into a lymphopenic host recapitulated the skewed inflammatory phenotype. Collectively, these findings provide novel insights into the initiation of immune dysregulation in the presence of conserved central or peripheral tolerance mechanisms via impairment of TCR signals during T-cell expansion.
Methods
Mice
MTS mice were generated as previously described.26 Mice bearing floxed copies of wild-type (WT) SLP-76 were generated as previously described.27 RAG−/− mice were purchased from The Jackson Laboratory. The Cre-ERT2 transgenic mice and ROSA-YFP reporter mice were kindly provided by Dr E. Brown (University of Pennsylvania)28 and Dr F. Constantini (Columbia University),29 respectively. All mice used were between 6 and 12 weeks of age. All mice were housed under specific pathogen–free conditions at the University of Pennsylvania. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Antibodies and intracellular staining
The following antibodies were purchased from BD Biosciences: bromodeoxyuridine (BrdU; 3D4), CD4 (RM4-5), CD8 (53-6.7), B220 (RA3-6B2), TCR-γδ (GL-3), CD62L (MEL-14), CD44 (IM7), CD45RB (16A), TCR-β (H57-597), CD25 (PC61), and CD69 (H1.2F3). The following antibodies were purchased from eBioscience: IL-17A (ebio17B7), IL-2 (JES6-5H4), interferon-γ (IFN-γ; XMG1.2), IL-4 (11B11), IL-17F (ebio18F10), IL-10 (JES-16E3), and, FoxP3 (FJK-16s). Intracellular cytokine and BrdU staining was performed using kits purchased from BD Biosciences following the manufacturer's protocols. Intracellular FoxP3 staining was performed using a kit purchased from eBioscience following the manufacturer's protocol.
In vivo BrdU labeling
For in vivo BrdU labeling, mice were injected intraperitoneally with 1.0 mg of BrdU (Sigma-Aldrich) every 12 hours for 3 consecutive days. Cells from the spleen and peripheral LNs were subsequently harvested and stained for intracellular BrdU incorporation.
Biochemistry
T cells were purified from pooled peripheral LNs and spleen via negative selection T-cell isolation columns (Miltenyi Biotec). Isolated cells were then stimulated with anti-CD3 (5 μg/mL), lysed, and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blotting was performed using antibodies to pPLC-γ1 (Y783), pSLP-76 (Y128), pERK (T202/Y204), PLC-γ1, SLP-76, and extracellular signal-regulated kinase (ERK).
Intracellular calcium flux
Isolated T cells were loaded with 2 μg/mL Indo-1 and then incubated with biotinylated antibodies against CD3 (2C11; 0.7 μg/mL) and CD4 (RM4-5; 0.7 μg/mL) and fluorochrome-labeled antibodies against CD4 (GK1.5) for 30 minutes at 30°C. Calcium flux was triggered by cross-linking CD4 and CD3 by the administration of streptavidin (12.5 μg/mL, final concentration); ionomycin (2 μg) was used as a positive control. Calcium release was monitored by Indo-1 fluorescence.
CD25/CD69 up-regulation
Purified peripheral T cells were stimulated overnight with designated concentrations of anti-CD3 (2C11) or phorbol myristate acetate (PMA; 10 ng/mL) and ionomycin (200 ng/mL). Samples were then stained with appropriate markers and analyzed via flow cytometry.
RNA isolation and RT-PCR
RNA was obtained from purified peripheral T cells via TRIzol extraction (Invitrogen). RNA was then subjected to cDNA synthesis using Superscript Reverse Transcriptase (Invitrogen). RT-PCR was performed using commercially available TaqMan primer sets for T-bet and retinoic acid-related orphan receptor-γt (Applied Biosystems).
Autoantibody detection
Binding to dsDNA was measured by a 2-step solution phase enzyme-linked immunosorbent assay, as described previously,30 but with 2 modifications: the DNA came from calf thymus and was detected with alkaline phosphatase-labeled anti–mouse IgG (Southern Biotechnology). All serum samples were analyzed in duplicate after overnight incubation in p-nitrophenylphosphate. Sample optical densities were compared with sera from diseased MRL/lpr mice with high-titer autoantibodies (provided by Dr R. Eisenberg, University of Pennsylvania, Philadelphia). Dilutions for the standard curve ranged from 1/250 to 1/128 000 for the MRL/lpr. A dilution of 1/100 was used for B6 (negative control), MRL/lpr (positive control), and for all of the experimental samples. Antinuclear staining was performed using an antinuclear antibody test kit (Antibodies Incorporated) according to the manufacturer's protocol.
Regulatory T-cell suppression assay
Effector (CD4+CD25−) and enriched regulatory (CD4+CD25+) T cells were sort-purified from pooled LN cells and splenocytes. Effector T cells (50 000) were cultured with CD4+CD25+ T cells at various ratios in the presence of 0.5 × 106 irradiated splenocytes and 0.5 mg/mL anti-CD3 (2C11). Cultures were pulsed with tritiated thymidine after 72 hours and harvested 16 hours later.
Tamoxifen treatment
Mice were administered tamoxifen (Sigma-Aldrich) dissolved in corn oil via oral gavage for 5 consecutive days. Animals were killed for analysis after a 2-day rest period.
RAG−/− adoptive transfer
YFP+CD4+ T cells were fluorescence-activated cell sorter sort purified from pooled spleen and LNs of MTS/ΔSLP or +/ΔSLP mice, 7 days after tamoxifen treatment. Approximately 5 × 106 YFP+CD4+ T cells were transferred into each RAG−/− host via the retro-orbital sinus, and mice were killed for analysis at 2 weeks after transfer.
Immunohistochemistry staining for germinal center B cells
Spleens were frozen, sectioned, and stained as previously described31 with anti-B220-FITC (RA3-6B2; eBioscience) and biotinylated peanut agglutinin (Vector Laboratories). Secondary reagents were anti–FITC-alkaline phosphatase (Sigma-Aldrich) and streptavidin horseradish peroxidase (Southern Biotechnologies).
Statistical analyses
All data are mean plus or minus SEM, and comparisons between samples were performed using a 2-tailed Student t test or Mann-Whitney test as indicated. Statistics were determined using Prism software (GraphPad Software).
Results
MTS mice are relatively T-lymphopenic and contain peripheral CD4+ T cells with a partial activated phenotype
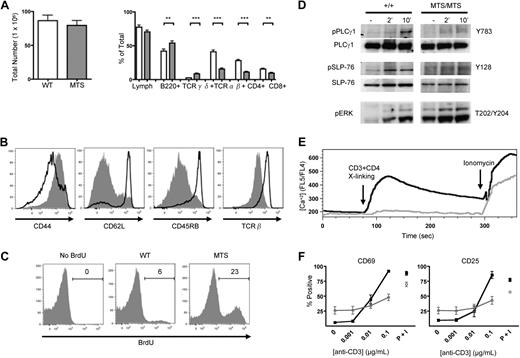
We investigated the peripheral T-cell compartment of non-TCR transgenic MTS mice. Although the total peripheral cellularity of these mice was comparable with that of WT controls, TCR-αβ–expressing lymphocytes, including both CD4+ and CD8+ T cells, were significantly reduced both in percentage and absolute number, whereas the B-cell and TCR-γδ–expressing populations were significantly increased (Figure 1A). Further analysis of the peripheral populations revealed that MTS CD4+ T cells expressed cell surface markers associated with activation (CD44-hi, CD62L-lo, CD45RB-lo, and TCR-β-lo; Figure 1B), a phenotype characteristic of T cells undergoing homeostatic proliferation in a lymphopenic environment. To examine the proliferative state of the MTS CD4+ T cells, mice were pulsed with BrdU for 3 days, and incorporation was determined by intracellular staining. We found that, compared with the WT CD4+ T-cell populations that demonstrate a mild proliferation potential over 3 days, the MTS CD4+ T cells proliferated at an increased rate (Figure 1C).
Peripheral CD4+ T cells in MTS mice are greatly decreased in number, possess an activated-like phenotype, and have impaired TCR signaling. (A) Total number of cells obtained from the spleen and peripheral LNs of WT (□) and MTS (▩) mice and percentages of lymphocyte subsets as determined by flow cytometry. Data are mean ± SEM and are representative of more than 5 independent experiments using 2 or 3 mice per group. Significance was determined using the 2-tailed Student t test. **P < .01. ***P < .001. (B) Surface expression of activation markers of WT (solid line) and MTS (shaded) mice. Histograms are gated on CD4+ T cells. Data are representative of more than 5 independent experiments using 2 or 3 mice per group. (C) Intracellular staining for the incorporation of BrdU in WT and MTS mice. Histograms are gated on CD4+ T cells. Data are representative of 2 independent experiments using 2 or 3 mice per group. (D) Purified peripheral T cells were stimulated for indicated lengths of time with a TCR cross-linking antibody, lysed, and blotted for phospho-PLC-γ1, total PLC-γ1, phospho-SLP-76, total SLP-76, and phospho-ERK. Data are representative of 3 independent experiments. (E) Peripheral cells from WT (black line) and MTS (gray line) mice were loaded with the calcium indicator Indo-1 and analyzed for their ability to flux calcium in response to CD3/CD4 cross-linking and ionomycin at the indicated time points. Histograms are gated on CD4+ T cells. Data are representative of 4 independent experiments using 1 or 2 mice per group. (F) Splenocytes isolated from either WT (black line) or MTS (gray line) mice were cultured in the presence of the indicated stimulus and stained for the early activation markers CD25 and CD69. Percentages are taken from positive gates set on the total CD4+ T-cell population. Data are mean ± SEM and are representative of 2 independent experiments. P + I indicates PMA plus ionomycin.
Peripheral CD4+ T cells in MTS mice are greatly decreased in number, possess an activated-like phenotype, and have impaired TCR signaling. (A) Total number of cells obtained from the spleen and peripheral LNs of WT (□) and MTS (▩) mice and percentages of lymphocyte subsets as determined by flow cytometry. Data are mean ± SEM and are representative of more than 5 independent experiments using 2 or 3 mice per group. Significance was determined using the 2-tailed Student t test. **P < .01. ***P < .001. (B) Surface expression of activation markers of WT (solid line) and MTS (shaded) mice. Histograms are gated on CD4+ T cells. Data are representative of more than 5 independent experiments using 2 or 3 mice per group. (C) Intracellular staining for the incorporation of BrdU in WT and MTS mice. Histograms are gated on CD4+ T cells. Data are representative of 2 independent experiments using 2 or 3 mice per group. (D) Purified peripheral T cells were stimulated for indicated lengths of time with a TCR cross-linking antibody, lysed, and blotted for phospho-PLC-γ1, total PLC-γ1, phospho-SLP-76, total SLP-76, and phospho-ERK. Data are representative of 3 independent experiments. (E) Peripheral cells from WT (black line) and MTS (gray line) mice were loaded with the calcium indicator Indo-1 and analyzed for their ability to flux calcium in response to CD3/CD4 cross-linking and ionomycin at the indicated time points. Histograms are gated on CD4+ T cells. Data are representative of 4 independent experiments using 1 or 2 mice per group. (F) Splenocytes isolated from either WT (black line) or MTS (gray line) mice were cultured in the presence of the indicated stimulus and stained for the early activation markers CD25 and CD69. Percentages are taken from positive gates set on the total CD4+ T-cell population. Data are mean ± SEM and are representative of 2 independent experiments. P + I indicates PMA plus ionomycin.
Peripheral T cells in MTS mice have impaired TCR signaling
Because peripheral CD4+ T cells in the MTS mouse possess an activated phenotype, we next investigated the ability of these cells to signal through their TCR. MTS peripheral T cells were purified and then stimulated in vitro via their TCR, followed by Western blot analysis for the presence of proteins inducibly phosphorylated on tyrosine residues. Compared with WT T cells, MTS T cells demonstrate diminished phosphorylation of PLC-γ1 and SLP-76 in response to TCR stimulation (Figure 1D). Surprisingly, we found that MTS T cells demonstrated constitutive activation of ERK, a signal that was partially enhanced with TCR engagement (Figure 1D). We next investigated the ability of TCR engagement to initiate other distal signaling events and found that peripheral MTS CD4+ T cells have a greatly diminished ability to flux calcium in response to CD3 and CD4 cross-linking compared with WT controls (Figure 1E). Concordant with impaired TCR signaling, we found that, although MTS peripheral CD4+ T cells have a higher constitutive level of the early activation markers, CD25 and CD69, these cells fail to up-regulate expression of these antigens with increasing concentrations of anti-CD3 (Figure 1F). Thus, despite possessing an activated surface phenotype, peripheral MTS CD4+ T cells demonstrate markedly impaired signaling through the TCR.
MTS T cells are poised to produce inflammatory cytokines
As peripheral CD4+ T cells in the MTS mouse possess an activated phenotype, we next investigated whether these cells had differentiated into T helper cell subsets. Because TCR signaling was impaired, we stimulated with PMA plus ionomycin directly ex vivo to examine cytokine production. Compared with WT CD4+ T cells from a naive host, which produce very low levels of cytokines ex vivo, a substantially higher percentage of MTS CD4+ T cells produced IL-17A, IL-17F, IL-2, and IFN-γ but not IL-4 (Figure 2A). Further examination revealed that purified MTS T cells express significantly increased levels of transcripts characteristic of TH17 and TH1 lineages, ROR-γt,32 and T-bet,33 respectively, when isolated directly ex vivo (Figure 2B).
MTS mice have peripheral CD4+ T cells that are skewed toward the inflammatory TH1 and TH17 lineages and develop spontaneous autoantibody production. (A) Cells were stimulated with PMA plus ionomycin for 4 hours, and cytokine production was assessed by intracellular staining for inflammatory cytokines IFN-γ, IL-2, IL-17A, and IL-17F. Populations shown are gated on CD4+ T cells. Data are representative of at least 5 independent experiments of 1 to 3 mice per group. (B) RNA isolated from unstimulated purified T cells of WT (□) and MTS (▩) mice were analyzed for mRNA encoding ROR-γt (rorc) and T-bet (tbx21) by real-time PCR analysis. Data are mean ± SEM and are representative of 3 independent experiments of 2 or 3 mice per group. (C) Sera isolated from WT (●) and MTS ( ) mice were analyzed for the presence of anti-dsDNA antibodies and antinuclear antibodies (IgG). Horizontal lines represent averages. Significance was determined using the Mann-Whitney test. ***P < .001. Images were obtained with a Nikon Eclipse E600 microscope (40× objective and 1:40 serum dilution) and captured with a Nikon DXM 1200 camera. Images were processed with IP Labs Scientific Image Processing (Scanalytics Inc).
) mice were analyzed for the presence of anti-dsDNA antibodies and antinuclear antibodies (IgG). Horizontal lines represent averages. Significance was determined using the Mann-Whitney test. ***P < .001. Images were obtained with a Nikon Eclipse E600 microscope (40× objective and 1:40 serum dilution) and captured with a Nikon DXM 1200 camera. Images were processed with IP Labs Scientific Image Processing (Scanalytics Inc).
MTS mice have peripheral CD4+ T cells that are skewed toward the inflammatory TH1 and TH17 lineages and develop spontaneous autoantibody production. (A) Cells were stimulated with PMA plus ionomycin for 4 hours, and cytokine production was assessed by intracellular staining for inflammatory cytokines IFN-γ, IL-2, IL-17A, and IL-17F. Populations shown are gated on CD4+ T cells. Data are representative of at least 5 independent experiments of 1 to 3 mice per group. (B) RNA isolated from unstimulated purified T cells of WT (□) and MTS (▩) mice were analyzed for mRNA encoding ROR-γt (rorc) and T-bet (tbx21) by real-time PCR analysis. Data are mean ± SEM and are representative of 3 independent experiments of 2 or 3 mice per group. (C) Sera isolated from WT (●) and MTS ( ) mice were analyzed for the presence of anti-dsDNA antibodies and antinuclear antibodies (IgG). Horizontal lines represent averages. Significance was determined using the Mann-Whitney test. ***P < .001. Images were obtained with a Nikon Eclipse E600 microscope (40× objective and 1:40 serum dilution) and captured with a Nikon DXM 1200 camera. Images were processed with IP Labs Scientific Image Processing (Scanalytics Inc).
) mice were analyzed for the presence of anti-dsDNA antibodies and antinuclear antibodies (IgG). Horizontal lines represent averages. Significance was determined using the Mann-Whitney test. ***P < .001. Images were obtained with a Nikon Eclipse E600 microscope (40× objective and 1:40 serum dilution) and captured with a Nikon DXM 1200 camera. Images were processed with IP Labs Scientific Image Processing (Scanalytics Inc).
Because TH17 cells are implicated in the initiation and pathogenesis of autoimmune diseases,34-37 we next investigated whether MTS mice develop evidence of autoimmunity. MTS mice display no overt symptoms of disease, and histologic examination revealed no abnormal cellular infiltrates in peripheral tissues (data not shown). However, MTS mice possess an increased frequency of splenic germinal center B cells (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) and have an increased frequency and size of germinal centers (supplemental Figure 1B). To determine whether this enhanced germinal center formation correlated with abnormal antibody responses, serum was analyzed for the presence of autoantibodies. Antinuclear antibodies were observed in nearly all MTS mice by 8 weeks of age (Figure 2C left), and significant titers of anti-dsDNA antibodies were present in a large percentage of MTS mice (Figure 2C right). In addition to abnormalities in the CD4+ T- and B-cell compartments, CD8+ T cells from MTS mice also exhibit abnormalities. In this subset of cells, we found basal high expression of CD44 (but, interestingly, high CD62L) and low expression of TCR-β, increased in vivo proliferation as evidenced by enhanced BrdU incorporation, and increased production of IFN-γ after ex vivo stimulation with PMA plus ionomycin (supplemental Figure 2A-C).
MTS mice contain normal frequencies of functional regulatory T cells
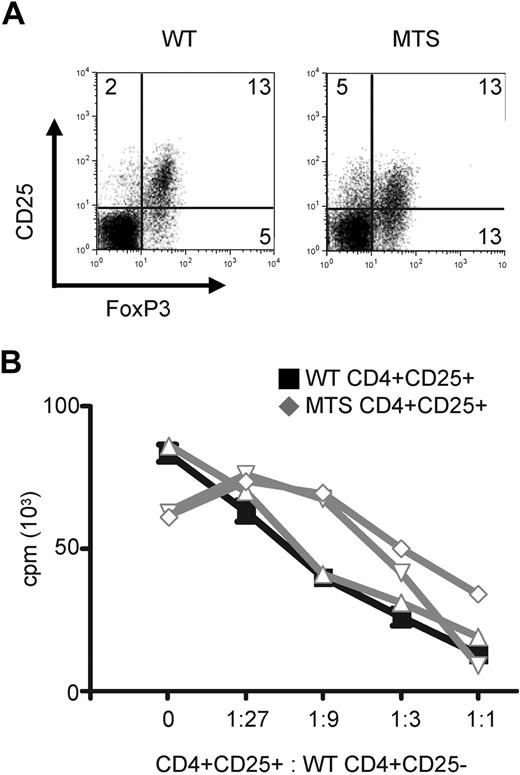
Immune responses to self can occur through a loss of peripheral or central tolerance. To determine whether regulatory T-cell (Treg) function, one mechanism of peripheral tolerance, was intact in MTS mice, we analyzed Treg populations. Tregs were identified by intracellular staining for the lineage-specific transcription factor FoxP3 and were enumerated in WT and MTS animals. Comparable percentages were found, regardless of genotype (Figure 3A). We next investigated the functional state of the Tregs by sorting CD4+CD25+ T cells from both WT and MTS mice and then comparing their ability to suppress proliferation of polyclonally stimulated WT CD4+CD25− effector T cells. We found that both WT and MTS Tregs were able to suppress effector T-cell proliferation in a ratio-dependent manner (Figure 3B). In some experiments, suppression of effector T-cell proliferation varied at intermediate ratios. This variability is probably the result of increased percentages of CD25+FoxP3− effector T cells contaminating our sorted cells from the MTS mice (Figure 3A). Collectively, our data demonstrate that MTS Tregs are present at normal frequencies and retain the capacity to suppress effector T-cell function and suggest that the dysregulated state of peripheral CD4+ T cells is not secondary to defects in the Treg compartment.
MTS mice have normal percentages of peripheral CD4+ Tregs, which are able to efficiently suppress WT effector T-cell proliferation. (A) Spleen and LNs from WT and MTS mice were stained for the presence of intracellular FoxP3. Populations are gated on CD4+ T cells. Data are representative of 3 independent experiments of 2 or 3 mice per group. (B) Sorted CD4+CD25+ WT (black line) or MTS (gray lines) T cells were cultured in the presence of stimulated CD4+CD25− WT effector cells, and proliferation was measured by tritiated thymidine incorporation. Data are mean ± SEM and are representative of 3 independent experiments.
MTS mice have normal percentages of peripheral CD4+ Tregs, which are able to efficiently suppress WT effector T-cell proliferation. (A) Spleen and LNs from WT and MTS mice were stained for the presence of intracellular FoxP3. Populations are gated on CD4+ T cells. Data are representative of 3 independent experiments of 2 or 3 mice per group. (B) Sorted CD4+CD25+ WT (black line) or MTS (gray lines) T cells were cultured in the presence of stimulated CD4+CD25− WT effector cells, and proliferation was measured by tritiated thymidine incorporation. Data are mean ± SEM and are representative of 3 independent experiments.
Restoration of normal thymic development abrogates the activated-like phenotype of peripheral MTS CD4+ T cells
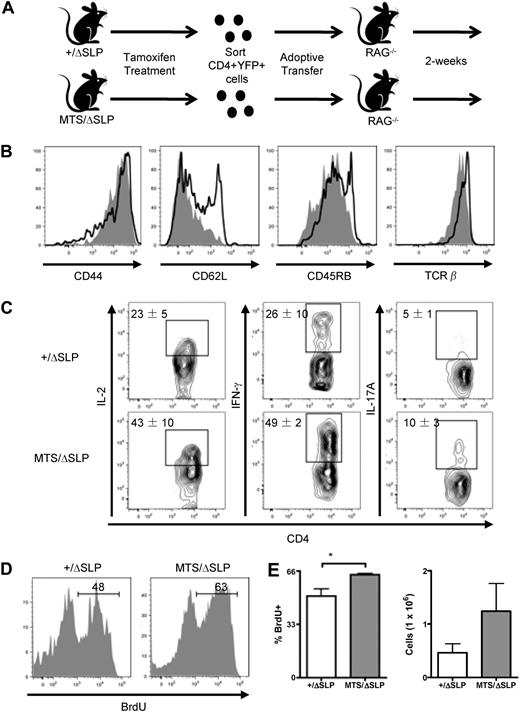
We next investigated the possibility that failed central tolerance leads to the activated phenotype of MTS T cells. It was particularly important to consider this possibility, as MTS mice expressing a restricted TCR have severe abnormalities in selection (data not shown). To eliminate thymic selection abnormalities as a cause of the peripheral CD4+ T-cell phenotype required the development of a genetic system allowing for normal thymic development before the MTS allele could exert its effects, which was possible because the MTS mutation is recessive. Mice with 1 WT and 1 MTS allele demonstrate no overt dominant-negative phenotype; specifically, no thymic selection abnormalities, peripheral T-lymphopenia, TCR signaling impairment, activated-like phenotype of peripheral CD4+ T cells, or cytokine skewing was found (data not shown). Also important for our strategy, MTS/null mice phenocopy MTS/MTS mice in each of our assays for T-cell development and function (data not shown). Therefore, we generated mice with 1 MTS allele and 1 allele encoding WT SLP-76 that can be excised using a drug-inducible Cre recombinase after normal thymic development. This inducible system is dependent on 3 sets of genes (Figure 4A). The first is the gene encoding SLP-76 in which 1 WT allele is flanked by 2 loxP sites,27 and the other allele is the MTS. The second gene is a Cre recombinase-human estrogen receptor (Cre-ERT2) chimeric molecule under control of the ubiquitin promoter, which is inactive in the absence of tamoxifen.28,38 This approach allows for constitutive expression of a Cre recombinase yet prevents it from acting on loxP sites until tamoxifen is administered. The third gene is enhanced yellow fluorescent protein (YFP) knocked into the Rosa-26 locus.29 The YFP cassette is preceded by a loxP-flanked tpA transcriptional stop signal. This construct permits expression of YFP and identification of cells that currently or previously expressed an activated Cre. When tamoxifen is administered, Cre becomes active and deletes both the WT SLP-76 gene and the transcriptional stop signal preceding the YFP reporter, marking cells that now express only the MTS allele (Figure 4A). To serve as a control for these MTS mice in which the WT allele of SLP-76 has been deleted (MTS/ΔSLP), we generated mice in which both copies of SLP-76 were WT but only one is flanked by loxP sites (+/ΔSLP).
MTS/ΔSLP CD4+ T cells do not initially possess an activated-like phenotype. (A) Diagram of constructs that permit selective induction of the MTS mutation via tamoxifen treatment. (B) Western blot analysis of total SLP-76 protein in purified T cells isolated from mice of various genotypes (left) and expression of YFP protein in CD4+ T cells 2 days after tamoxifen treatment. Populations are gated on CD4+ T cells. ΔSLP denotes a floxed WT allele of SLP-76, which was deleted after tamoxifen treatment. (C) Surface expression of activation markers using flow cytometry of +/ΔSLP (solid line) and MTS/ΔSLP (shaded) mice 2 days after tamoxifen treatment. Histograms are gated on YFP+CD4+ T cells. Data are representative of 5 independent experiments using 2 or 3 mice per group. (D) Intracellular staining for the incorporation of BrdU in +/ΔSLP and MTS/ΔSLP mice. Histograms are gated on YFP+CD4+ T cells. Data are representative of 2 independent experiments using 2 mice per group.
MTS/ΔSLP CD4+ T cells do not initially possess an activated-like phenotype. (A) Diagram of constructs that permit selective induction of the MTS mutation via tamoxifen treatment. (B) Western blot analysis of total SLP-76 protein in purified T cells isolated from mice of various genotypes (left) and expression of YFP protein in CD4+ T cells 2 days after tamoxifen treatment. Populations are gated on CD4+ T cells. ΔSLP denotes a floxed WT allele of SLP-76, which was deleted after tamoxifen treatment. (C) Surface expression of activation markers using flow cytometry of +/ΔSLP (solid line) and MTS/ΔSLP (shaded) mice 2 days after tamoxifen treatment. Histograms are gated on YFP+CD4+ T cells. Data are representative of 5 independent experiments using 2 or 3 mice per group. (D) Intracellular staining for the incorporation of BrdU in +/ΔSLP and MTS/ΔSLP mice. Histograms are gated on YFP+CD4+ T cells. Data are representative of 2 independent experiments using 2 mice per group.
Purified peripheral T cells from mice administered tamoxifen demonstrated efficient deletion of the WT SLP-76 allele (Figure 4B left). However, only approximately 30% of peripheral CD4+ T cells produced the YFP protein, suggesting that this system under-reports SLP-76 deletion (Figure 4B right). To be certain that deletion occurred in studied cells, all experiments were performed on cells gated for YFP+. Tamoxifen treatment in both MTS/ΔSLP and +/ΔSLP mice did not alter percentages of T cells (data not shown), and analysis of peripheral tamoxifen-induced MTS/ΔSLP CD4+ T cells revealed a naive phenotype of CD44-lo, CD62L-hi, CD45RB-hi, and TCR-β-hi, similar to that observed in +/ΔSLP controls (Figure 4C). In addition, after a 3-day pulse of BrdU, similar percentages of MTS/ΔSLP and +/ΔSLP YFP+CD4+ T cells incorporated BrdU (Figure 4D). These studies demonstrate that induction of MTS in peripheral CD4+ T cells after normal thymic development abrogates the activated-like phenotype observed in MTS CD4+ T cells that develop in the absence of WT SLP-76, at least during the 7-day time frame of these studies.
Deletion of WT SLP-76 in MTS/ΔSLP-76 peripheral CD4+ T cells results in impaired TCR signaling
We next evaluated the TCR signaling potential in cells that developed normally and then lost WT SLP-76, leaving only the MTS allele. Compared with +/ΔSLP T cells, engagement of the TCR on MTS/ΔSLP T cells resulted in significantly diminished phosphorylation of PLC-γ1 and ERK (Figure 5A). Higher basal levels of ERK phosphorylation seen in the MTS mice (Figure 1D) are lost when WT SLP-76 is present, then acutely lost, perhaps indicating that the higher level of phospho-ERK in the MTS cells may be a consequence of their activated phenotype. Note also that the PLC-γ1 phosphorylation is not as severely diminished in T cells from inducible versus MTS/MTS animals. This may reflect increased levels of TCR arising from normal thymic development in the MTS/ΔSLP animals (Figure 4C). We next examined more distal TCR signaling events and found that MTS/ΔSLP YFP+ CD4+ peripheral T cells also revealed a greatly diminished calcium flux in response to TCR stimulation (Figure 5B). Furthermore, although +/ΔSLP and MTS/ΔSLP YFP+ CD4+ T cells had equivalent basal percentages of CD25 and CD69, up-regulation of these surface markers after stimulation of the TCR was impaired in the MTS/ΔSLP T cells (Figure 5C). Thus, it appears that the TCR signaling defect observed in the MTS mice is largely, but not completely, recapitulated in cells derived from animals in which thymic development is preserved.
MTS/ΔSLP CD4+ T cells have impaired TCR signaling and are not poised to produce pro-inflammatory cytokines. (A) Purified peripheral T cells were stimulated for indicated lengths of time with a TCR cross-linking antibody, lysed, and blotted for phospho-PLC-γ1, total PLC-γ1, phospho-SLP-76, total SLP-76, and phospho-ERK. Data are representative of 3 independent experiments. (B) Peripheral cells from +/ΔSLP (black line) and MTS/ΔSLP (gray line) mice were loaded with the calcium indicator Indo-1 and analyzed for their ability to flux calcium in response to CD3/CD4 cross-linking and ionomycin at the indicated time points. Histograms are gated on YFP+CD4+ T cells. Data are representative of 3 independent experiments using 1 or 2 mice per group. (C) Splenocytes isolated from either +/ΔSLP (black line) or MTS/ΔSLP (gray line) mice were cultured in the presence of the indicated stimulus and stained for the early activation markers CD25 and CD69. Percentages are taken from positive gates set on the total YFP+CD4+ T-cell population. Data shown are representative of 3 independent experiments. P + I indicates PMA plus ionomycin. (D) Peripheral cells isolated from +/ΔSLP and MTS/ΔSLP mice after tamoxifen treatment were stimulated with PMA plus ionomycin and stained for the presence of intracellular inflammatory cytokines. Populations are gated on YFP+CD4+ T cells. Data are representative of 5 independent experiments using 2 or 3 mice per group.
MTS/ΔSLP CD4+ T cells have impaired TCR signaling and are not poised to produce pro-inflammatory cytokines. (A) Purified peripheral T cells were stimulated for indicated lengths of time with a TCR cross-linking antibody, lysed, and blotted for phospho-PLC-γ1, total PLC-γ1, phospho-SLP-76, total SLP-76, and phospho-ERK. Data are representative of 3 independent experiments. (B) Peripheral cells from +/ΔSLP (black line) and MTS/ΔSLP (gray line) mice were loaded with the calcium indicator Indo-1 and analyzed for their ability to flux calcium in response to CD3/CD4 cross-linking and ionomycin at the indicated time points. Histograms are gated on YFP+CD4+ T cells. Data are representative of 3 independent experiments using 1 or 2 mice per group. (C) Splenocytes isolated from either +/ΔSLP (black line) or MTS/ΔSLP (gray line) mice were cultured in the presence of the indicated stimulus and stained for the early activation markers CD25 and CD69. Percentages are taken from positive gates set on the total YFP+CD4+ T-cell population. Data shown are representative of 3 independent experiments. P + I indicates PMA plus ionomycin. (D) Peripheral cells isolated from +/ΔSLP and MTS/ΔSLP mice after tamoxifen treatment were stimulated with PMA plus ionomycin and stained for the presence of intracellular inflammatory cytokines. Populations are gated on YFP+CD4+ T cells. Data are representative of 5 independent experiments using 2 or 3 mice per group.
Transfer of MTS/ΔSLP CD4+ T cells into a lymphopenic host recapitulates the aberrant inflammatory cytokine responses
We next investigated whether preserving normal thymic development would eliminate the skewing of MTS T cells toward inflammatory T-cell subsets. To begin this analysis, we examined cytokine production in T cells derived from MTS/ΔSLP mice 7 days after tamoxifen treatment. T cells were removed and stimulated ex vivo with PMA plus ionomycin. Both +/ΔSLP and MTS/ΔSLP YFP+CD4+ T cells produced low levels of inflammatory cytokines (Figure 5D), thus demonstrating that acute loss of WT SLP-76 leading to the MTS TCR signaling phenotype cannot by itself skew CD4+ T cells toward the inflammatory TH1 and TH17 lineages. It remained possible, however, that lymphopenia-induced proliferation of the MTS CD4+ T cells over time would lead to their aberrant phenotype. We modeled this by transferring MTS/ΔSLP YFP T cells into lymphopenic (RAG−/−) hosts.
Equal numbers of purified MTS/ΔSLP or +/ΔSLP YFP+ CD4+ T cells were transferred to RAG−/− recipients and then recovered at 14 days after transfer (Figure 6A). Both the transferred +/ΔSLP and MTS/ΔSLP CD4+YFP+ T cells were CD44-hi, CD62L-lo, and CD45RB-lo, characteristic of T cells undergoing homeostatic expansion in a lymphopenic host (Figure 6B). When stimulated directly ex vivo with PMA plus ionomycin, we found that the +/ΔSLP YFP+CD4+ T cells produced considerable amounts of IFN-γ. In contrast, however, the MTS/ΔSLP YFP+ CD4+ T cells were poised to produce markedly greater amounts of IL-2, IFN-γ, and IL-17A (Figure 6C). This was associated with a more robust proliferative response of the MTS/ΔSLP T cells as assessed by BrdU incorporation and cellular recovery in the RAG−/− hosts (Figure 6D-E). These data suggest that, even in the face of normal thymic development, when cells are forced to expand rapidly, expression of the MTS without a WT SLP-76 allele confers the dysregulated effector phenotype.
Transfer to a lymphopenic host reveals dysregulated responses of MTS/ΔSLP CD4+ T cells independent of thymic development. (A) Diagram of selective induction of the MTS mutation followed by sorting and adoptive transfer into lymphopenic hosts. (B) Surface expression of activation markers after adoptive transfer of YFP+CD4+ +/ΔSLP (solid line) or MTS/ΔSLP (shaded) T cells. Histograms are gated on YFP+CD4+ T cells. Data are representative of 2 independent experiments using 3 mice per group. (C) After adoptive transfer, peripheral cells were stimulated with PMA plus ionomycin and stained for the presence of intracellular inflammatory cytokines. Populations are gated on YFP+ CD4+ T cells. Data are mean ± SEM and are representative of 3 independent experiments using 3 mice per group. (D) Staining for the intracellular incorporation of BrdU. Histograms are gated on YFP+CD4+ T cells. (E) Total number of recovered YFP+CD4+ T cells from RAG−/− recipients. Data are mean ± SEM and are representative of 1 experiment using 3 mice per group. Significance was determined using the 2-tailed Student t test. *P < .05
Transfer to a lymphopenic host reveals dysregulated responses of MTS/ΔSLP CD4+ T cells independent of thymic development. (A) Diagram of selective induction of the MTS mutation followed by sorting and adoptive transfer into lymphopenic hosts. (B) Surface expression of activation markers after adoptive transfer of YFP+CD4+ +/ΔSLP (solid line) or MTS/ΔSLP (shaded) T cells. Histograms are gated on YFP+CD4+ T cells. Data are representative of 2 independent experiments using 3 mice per group. (C) After adoptive transfer, peripheral cells were stimulated with PMA plus ionomycin and stained for the presence of intracellular inflammatory cytokines. Populations are gated on YFP+ CD4+ T cells. Data are mean ± SEM and are representative of 3 independent experiments using 3 mice per group. (D) Staining for the intracellular incorporation of BrdU. Histograms are gated on YFP+CD4+ T cells. (E) Total number of recovered YFP+CD4+ T cells from RAG−/− recipients. Data are mean ± SEM and are representative of 1 experiment using 3 mice per group. Significance was determined using the 2-tailed Student t test. *P < .05
Discussion
In this report, we present a model of dysregulated peripheral CD4+ T-cell activation caused by constitutively targeting SLP-76 to the membrane. Interestingly, this phenotype exists; although instead of enhanced TCR signaling, the mutant SLP-76 molecule impairs critical TCR-mediated biochemical signaling events. Similar findings of diminished TCR signaling yet inappropriate T-cell activation have been reported in several previous publications describing mice with hypomorphic mutations in TCR signaling proteins, including the LAT136F 10,11, ZAP-70SKG 9, and ZAP-70mrd/mrt 14 mice. The stimulus that activates these CD4+ T cells in vivo is not clear. It is also unknown why the CD4+ T cells are skewed toward the inflammatory TH1 and TH17 lineages in the MTS and ZAP-70SKG mice39 but to the TH2 lineage in the LAT136F mouse.10,11 Although IL-17 production is dysregulated in these cells, the MTS mice still require some of the same signals required to drive TH17 differentiation in WT cells, because T cells from MTS animals crossed to IL-6–deficient mice are completely unable to produce IL-17 (supplemental Figure 3). Interestingly, with the loss of IL-17A production, there was a selective increase in the frequency of IFN-γ+ cells, suggesting a shift toward the TH1 cell lineage (supplemental Figure 3). Thus, the differentiation pathways of the MTS CD4+ T cells toward the TH17 lineage appear to have the same requirement for IL-6 reported in WT cells.40-42 This requirement has been shown as well for CD4+ T-cell responses in ZAP-70SKG mice.39 Similarly, although the activated peripheral cells from LAT136F mice produce large amounts of TH2 cytokines, this production still requires the presence of STAT6,43 a factor essential for TH2 development in WT mice.44
We were surprised to find that, given their skewed cytokine response and presence of autoantibodies, MTS mice do not develop overt symptoms of disease, even when aged to over 2 years. This finding is consistent, however, with reports of other mice in which key components of the TCR signaling apparatus are mutated. For example, in the ZAP-70SKG mouse, in which ZAP-70 function is markedly diminished, disease development is dependent on genetic background, the presence of particular gut flora, and/or induction with microbial products.39 Investigation into whether these factors promote disease development in MTS mice is a future area of research. MTS mice do develop enhanced germinal centers and significant titers of anti-dsDNA and antinuclear antibodies. This finding is fitting with recent advances demonstrating that IL-17 and TH17 cells promote germinal center formation and autoantibody production.37,45 Most recently, the importance of IL-17 regulation in the development of human autoimmune disorders has been suggested with the observation that IL-17 promotes the survival of autoreactive B cells and that serum levels of this cytokine correlate directly with severity of systemic lupus erythematosus disease in a cohort of patients.37
One attractive model for why mutations that cause diminished TCR signaling capacity result in uncontrolled T-cell activation rather than diminished T-cell responses in vivo is that altered signaling leads to defective Treg function. Although there are reports of such dysfunction in the ZAP-70SKG Tregs,22 evidence for this in the LAT136F model is controversial.23,46 We tested Treg cell function in the MTS model and found that Tregs from most MTS mice function as efficiently as WT Tregs in in vitro coculture assays. In some experiments, we found that MTS CD25+CD4+ T cells were less efficient at suppressing effector cell proliferation; however, staining showed that there is a higher proportion of CD25+Foxp3− cells in the MTS mice than in WT animals. Thus, it appears that, on a per-cell basis, CD25+Foxp3+ MTS cells function similarly to their WT counterparts. Importantly, all MTS mice have dysregulated IL-17 production, but only a proportion show diminished suppression in the coculture assays. Thus, although we cannot rule out that decreased Treg function may contribute to some of the pathologies observed in MTS mice, diminished Treg function does not appear to be the cause of the dysregulated T-cell cytokine production.
A second possible explanation for why hypomorphic signaling mutations result in exuberant rather than decreased peripheral T-cell function is that altered signaling during thymocyte development allows for the emergence of highly self-reactive T cells that are not efficiently deleted during negative selection. Using TCR transgenic models, we found that, similar to mice with mutant ZAP-70 or LAT alleles,9,14,21 MTS CD4+ T cells also undergo abnormal thymic selection (data not shown). To address whether a developmental abnormality is causal for the skewed peripheral T-cell function, we engineered mice in which normal thymic development was restored but in which the MTS allele could exert its effects in the mature T-cell population by coexpressing a WT SLP-76 allele along with the MTS until thymocyte development was complete. Our initial findings demonstrated that deletion of WT SLP-76 in peripheral CD4+ T cells, leaving sole expression of the MTS allele, does not immediately result in an abnormal activated phenotype or aberrant responses. However, placement of these cells in a lymphopenic environment reveals that inflammatory subset skewing and activation can occur, although these cells developed normally within the thymus. This inflammatory subset skewing and activation occur at significantly higher levels than seen in controls, also implying that the lymphopenic state of the MTS mouse is not solely responsible for its phenotype. Taken together, these data indicate that the abnormal phenotype observed in the MTS mouse can occur in the setting of preserved central and peripheral tolerance mechanisms.
A link between lymphopenia and dysregulated T-cell responses has long been investigated.47-51 Prior work has implicated a failure of Treg cell activity and/or the selective growth of highly self-reactive clones resulting from constant proliferation as probable causes of the high degree of T-cell activation observed in models of autoimmunity. We now present a model in which a mutation causing the mislocalization of a single signaling molecule can lead to CD4+ T-cell skewing toward TH1 and TH17 inflammatory lineages as a consequence of lymphopenia, even in the setting of intact thymocyte selection and apparently normal Treg cell function. Thus, it appears that, when proliferative signals are present, cells with defects in the signaling pathways initiated by TCR engagement may respond with an exaggerated rather than diminished cytokine response. This result may be the result of imbalanced signaling via the TCR and other surface receptors. For example, in lymphopenic environments, homeostatic cytokines, such as IL-7, are present in excess.52 One may speculate that, in WT T cells, signaling mediated by the TCR and IL-7 receptor are coordinated, but that in the absence of a normally functioning TCR signaling cascade, a potent activating IL-7 response may predominate, somehow promoting aberrant cellular responses. It is probable that the spectrum of cytokines produced is a consequence of which signaling pathways are most disrupted, leading to an imbalance of second messengers produced. Additional work, comparing cells from mice with various signaling protein mutations, will be required to determine which signals predispose to which T-cell effector phenotypes. In addition, this work suggests that, as new pharmacologic agents are developed targeting T-cell signaling molecules, it will be important to consider skewed as well as diminished T-cell responses as potential undesirable consequences.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Edward Behrens, Matthew Riese, Kim Nichols, and Tao Zou for critically reading of the manuscript, the entire Koretzky laboratory for helpful discussions, and Michele Metzgar for performing the immunohistochemistry.
This work was supported by the National Institutes of Health (grants R37GM053256 and P01CA066570; G.A.K.).
National Institutes of Health
Authorship
Contribution: G.F.S. designed experiments, performed research, analyzed data, and wrote the paper; P.R.M. and M.S.J. designed experiments, performed research, and analyzed data; N.A.B. generated critical reagents, performed research, and analyzed data; D.R.S. performed key experiments; E.T.L.P. supervised key experiments; J.E. designed and supervised key experiments; J.S.M. designed experiments and generated critical reagents; and G.A.K. designed experiments, supervised the project, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gary A. Koretzky, University of Pennsylvania, 415 BRB II/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: koretzky@mail.med.upenn.edu.
References
Author notes
G.F.S. and P.R.M. contributed equally to this study.






 ) mice were analyzed for the presence of anti-dsDNA antibodies and antinuclear antibodies (IgG). Horizontal lines represent averages. Significance was determined using the Mann-Whitney test. ***P < .001. Images were obtained with a Nikon Eclipse E600 microscope (40× objective and 1:40 serum dilution) and captured with a Nikon DXM 1200 camera. Images were processed with IP Labs Scientific Image Processing (Scanalytics Inc).
) mice were analyzed for the presence of anti-dsDNA antibodies and antinuclear antibodies (IgG). Horizontal lines represent averages. Significance was determined using the Mann-Whitney test. ***P < .001. Images were obtained with a Nikon Eclipse E600 microscope (40× objective and 1:40 serum dilution) and captured with a Nikon DXM 1200 camera. Images were processed with IP Labs Scientific Image Processing (Scanalytics Inc).