Epidemiologic observations and direct in vitro studies suggested that increases in fetal hemoglobin concentration might ameliorate the severity of sickle cell disease. Considerable effort has been directed toward preventing peripartum switch from γ-globin to β-globin synthesis or in reactivating γ-globin gene expression later in life. This effort has produced significant insights into the developmental regulation of hemoglobin gene expression and regulation of gene expression in general. Both cis elements and trans-acting factors that silence γ-globin gene expression and activate β-globin gene expression have been identified. DeSimone et al showed that fetal hemoglobin synthesis could be induced in anemic baboons by administration of 5-azacytadine.2 Studies from Platt et al showed that hydroxyurea also caused induction fetal globin production.3 Charache and colleagues selected hydroxyurea for phase 2 studies in persons with sickle cell disease because of the considerable clinical evidence of the safety of this drug.4
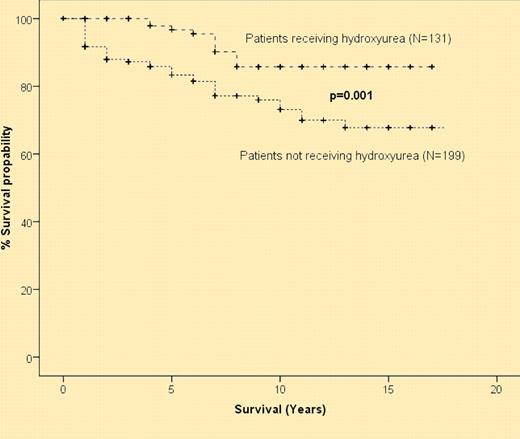
Probability of 10-year overall survival in patients with sickle cell disease who received hydroxyurea and in those who were treated conventionally: 86% versus 65% (P = .001). See the figure in the article beginning on page XXXX.
Probability of 10-year overall survival in patients with sickle cell disease who received hydroxyurea and in those who were treated conventionally: 86% versus 65% (P = .001). See the figure in the article beginning on page XXXX.
This pioneering work led to the double-blinded, randomized phase 3 Multicenter Study of Hydroxyurea in Patients With Sickle Cell Disease (MSH), which clearly established that daily administration of the drug to persons with sickle cell anemia (HbSS) resulted in statistically and clinically significant reduction in frequency of pain episodes, hospitalizations for pain episodes, incidence of acute chest syndrome, severity of anemia, and need for transfusion.5 Long-term follow-up of persons in the MSH study provided some data suggesting there was a survival advantage for persons taking hydroxyurea.6 A recent National Institutes of Health (NIH) Consensus Development Conference Statement on Hydroxyurea Treatment for Sickle Cell Disease concluded that there was high-level evidence for efficacy for these clinical outcomes and laboratory parameters including increases in hemoglobin level, percentage of fetal hemoglobin, and mean corpuscular volume with reduction of white cell count.7 Evidence for prolonged survival of persons taking hydroxyurea was considered low grade.7
Voskaridou et al here provide more compelling evidence that hydroxyurea improves survival in adults with more severe sickle cell disease.1 This prospective, nonrandomized, nonblinded study compares outcome in 131 persons on hydroxyurea with 199 persons not taking the drug followed for a median of 8 years in a single medical center. Figure 1 from their article shows that there is a significant improvement in survival for subjects taking hydroxyurea compared with controls (see figure). This study is important because it supports many of the initial observations of clinical benefits from the MSH study and extends observations to persons with sickle thalassemia. Data from this study of sickle subjects with severe disease, more than 3 pain episodes per day, suggest that persons with HbSS and sickle β plus thalassemia have the most benefit, whereas those with sickle β plus thalassemia have more modest, and not statistically significant, improvement in survival. Baseline fetal hemoglobin levels and reduction in LDH were significant predictors of response. Their results also show that hydroxyurea is well tolerated and has a very favorable safety profile.
As was concluded by the NIH Consensus Development Conference, a number of questions are left unresolved by this study. These include clinical questions about impact on fertility; healing of leg ulcers; drug effects on avascular necrosis; benefits for those with mild disease; the impact on survival and complications in pediatric patients; and the relative efficacy of hydroxyurea, transfusion, and bone marrow transplantation. Is the effect from increased fetal hemoglobin or other changes that result from hydroxyurea administration? Are there more effective ways of modifying fetal hemoglobin expression? Are changing red cell hydration, cellular adhesion, inflammatory state, and vascular tone and reactivity synergistic with modulation of fetal hemoglobin by hydroxyurea? These questions require further investigation.
After a century of study in sickle cell disease, hydroxyurea remains the single established pharmacological intervention of demonstrated clinical efficacy. Clearly the results of the study by Voskaridou et al provide more evidence that daily administration of hydroxyurea reduces complications and improves survival in sickle cell patients. Barring the presence of contraindications, all persons with sufficiently severe sickle cell anemia and sickle β-thalassemia should be offered the benefits of this therapy.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■