Cardiac iron overload causes most deaths in β-thalassemia major. The efficacy of deferasirox in reducing or preventing cardiac iron overload was assessed in 192 patients with β-thalassemia in a 1-year prospective, multicenter study. The cardiac iron reduction arm (n = 114) included patients with magnetic resonance myocardial T2* from 5 to 20 ms (indicating cardiac siderosis), left ventricular ejection fraction (LVEF) of 56% or more, serum ferritin more than 2500 ng/mL, liver iron concentration more than 10 mg Fe/g dry weight, and more than 50 transfused blood units. The prevention arm (n = 78) included otherwise eligible patients whose myocardial T2* was 20 ms or more. The primary end point was the change in myocardial T2* at 1 year. In the cardiac iron reduction arm, the mean deferasirox dose was 32.6 mg/kg per day. Myocardial T2* (geometric mean ± coefficient of variation) improved from a baseline of 11.2 ms (± 40.5%) to 12.9 ms (± 49.5%) (+16%; P < .001). LVEF (mean ± SD) was unchanged: 67.4 (± 5.7%) to 67.0 (± 6.0%) (−0.3%; P = .53). In the prevention arm, baseline myocardial T2* was unchanged from baseline of 32.0 ms (± 25.6%) to 32.5 ms (± 25.1%) (+2%; P = .57) and LVEF increased from baseline 67.7 (± 4.7%) to 69.6 (± 4.5%) (+1.8%; P < .001). This prospective study shows that deferasirox is effective in removing and preventing myocardial iron accumulation. This study is registered at http://clinicaltrials.gov as NCT00171821.
Introduction
Heart failure resulting from myocardial iron deposition is the leading cause of death in patients with β-thalassemia.1,,,–5 Treatment with the iron chelator deferoxamine has improved morbidity and mortality in these patients. However, despite the availability of effective treatment, cardiac iron overload occurs in approximately half of patients maintained on long-term deferoxamine over decades.6 This may result from several poorly characterized factors, including low compliance with the demanding regimen of injections and differential heart and liver iron-loading kinetics.7 The heart iron transporters divalent metal transporter 1 (DMT1) and L-type voltage-dependent Ca2+ channel (LVDCC) are well recognized,8,9 and genetic variation in these or other proteins might also play a role in differential iron loading. This highlights the need for early detection and regular monitoring of cardiac iron overload, as well as effective iron chelation therapy with a more convenient regimen that may help improve patients' compliance.
It is now well established that some patients on long-term chelation therapy with high liver iron have little or no cardiac iron overload, and, conversely, some patients with evidence of cardiac iron overload have little liver iron loading.10 Thus, liver iron concentration (LIC) and its surrogate marker serum ferritin may not reflect myocardial iron status, particularly when used as a single cross-sectional measurement. Therefore, direct myocardial iron measurement should be regularly undertaken for confident clinical management of patients with β-thalassemia and other forms of transfusional iron overload at risk of myocardial iron loading. Noninvasive quantification of myocardial iron can be achieved using cardiovascular magnetic resonance (CMR) by measuring the myocardial T2*.10 T2* is a measure of magnetic relaxation, which shortens when particulate hemosiderin storage iron disturbs the magnetic microenvironment, and it is easier to measure in the in vivo heart than T2.11 The T2* technique has been calibrated in animals12 and humans.13 As myocardial T2* falls, there is increasing risk of left ventricular (LV) dysfunction10,14 and an increased likelihood of cardiac events.15 The use of T2* MR has been linked to reduced cardiac mortality16 and has good reproducibility.17,–19
Deferasirox (Exjade; Novartis) is a once-daily oral iron chelator that, in an extensive clinical trials program, has proven efficacious in reducing body iron burden in a variety of patients with transfusion-dependent anemias.20,–22 Deferasirox has demonstrated removal of myocardial iron in preclinical studies23 and in preliminary reports of clinical studies in patients with transfusion-dependent anemias. To evaluate the efficacy of deferasirox in removing cardiac iron and preventing cardiac iron loading in a large patient population, we prospectively assessed myocardial iron using T2* MR in a subgroup of patients with β-thalassemia who were enrolled in the large Evaluation of Patients' Iron Chelation with Exjade (EPIC) trial.24
Methods
Patient recruitment
The current study was a 1-year prospective, open-label, 16-center, single-arm study of a subgroup of 192 patients with β-thalassemia who were enrolled in the larger EPIC trial.24 The EPIC trial enrolled 1744 male or female patients (≥ 2 years of age): including thalassemia (n = 1115), myelodysplastic syndromes (n = 341), aplastic anemia (n = 116), sickle cell disease (n = 80), rare anemias (n = 43), and other transfused anemias (n = 49). Patients had transfusional iron overload as shown by a serum ferritin level of 1000 ng/mL or more, or less than 1000 ng/mL but with a history of multiple transfusions (> 20 transfusions or 100 mL/kg red blood cells) and R2 MR-confirmed LIC of 2 mg Fe/g dry weight (dw) or more. In the current study, patients aged 10 years or older with serum ferritin levels of 2500 ng/mL or more, LIC of more than 10 mg Fe/g dw, a lifetime minimum of 50 transfused blood units, and LV ejection fraction (LVEF) of at least 56% as measured by CMR were eligible for enrollment into the substudy, which comprised 2 patient populations: the cardiac iron reduction arm and the prevention arm. Patients were screened and selected for enrollment in the cardiac iron reduction arm if they had a myocardial T2* from 5 ms to less than 20 ms, reflecting mild/moderate (> 10 to < 20 ms) to severe (< 10 ms) cardiac iron overload, while those fulfilling all eligibility criteria but with myocardial T2* of 20 ms or higher were enrolled in the prevention arm. The primary end point of the study was the change in myocardial T2* from baseline to 12 months.
Patients with a contraindication to MR imaging, including incompatible metal implants or pacemakers, claustrophobia, and inability to comply with instructions, were excluded from the substudy. Additional exclusion criteria for the EPIC trial were: mean alanine aminotransferase (ALT) levels of more than 300 U/L; uncontrolled systemic hypertension; serum creatinine above the upper limit of normal (ULN); history of nephrotic syndrome; previous history of clinically relevant ocular toxicity related to iron chelation; systemic diseases (such as cardiovascular, renal, and hepatic disease) preventing treatment or surgical/medical conditions that might interfere with treatment; use of systemic investigational drugs (within 4 weeks of study) or topical investigational drugs (within 7 days of study); positive test for HIV; and life expectancy of less than 1 year. Patients with psychiatric or addictive disorders, a history of drug or alcohol abuse, and pregnant or breastfeeding women were also excluded.
A total of 343 patients were screened for enrollment over 12 months (Figure 1). In total, 192 patients, 114 in the cardiac iron reduction arm and 78 in the prevention arm met eligibility criteria and received deferasirox starting at a dose of 30 mg/kg per day (cardiac iron reduction arm) or 20 to 30 mg/kg per day (prevention arm). Subsequent dose adjustments of 5 or 10 mg/kg per day (up to a maximum of 40 mg/kg per day) were based on trends in serum ferritin assessed at 3-month intervals, myocardial T2* at 6 months, and safety parameters (dose reductions for elevated levels of serum creatinine and transaminases and in response to adverse events [AEs]). Patients had to be withdrawn from the substudy and treated with appropriate standard of care if they had (1) a decrease in T2* more than 33% from baseline, or (2) an initial decrease in LVEF to less than 56% but 50% or higher and LVEF remained less than 56% on a repeat assessment within 3 months of the initial one. A deferasirox dose increase up to 45 mg/kg per day was considered in patients who had a worsening T2* of lower magnitude (≤ 33% from baseline) or no improvement in T2* (≤ 25% from baseline) at 6 months. Dose increases beyond 40 mg/kg per day had to be approved by the Study Monitoring Committee and the study sponsor. The study was ethically approved at each participating center, and all patients (or parents/guardians) provided written, informed consent. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki.
Summary of screening, completion, and withdrawal patient numbers. PP indicates per protocol. The reasons for screening failure (n = 151) were failure to meet inclusion criteria (n = 131) and failure to meet compliance criteria (n = 20). The inclusion criteria failures were: T2* outside of lower limit (n = 18), T2* outside the upper limit and not included in the prevention arm (n = 68), and LVEF outside of limit (n = 6). In addition, 39 patients were screening failures for the cardiac substudy because the sample size had been reached, but they were enrolled in the core EPIC study. The compliance criteria failures were: failed to attend for MR scan, or did not follow instructions (n = 7); failed compliance entry because of alcohol abuse (n = 1); unacceptable laboratory values (n = 10); and did not meet LIC criteria (n = 2), with one patient having LIC of less than 10 mg/kg per day and one patient with a liver mass. The 68 patients who were not included in the prevention arm after screening failed because they did not meet the eligibility criteria for the core EPIC study (unacceptable laboratory values [n = 32], unacceptable test procedure results [n = 12], did not meet diagnostic severity criteria [n = 9], consent withdrawal [n = 1], other [n = 14]). The “other” category comprised resident in Cyprus (n = 1), MR imaging not working (n = 3), noncompliance (n = 2), unfit for cardiac substudy (n = 4), and T2* more than 20 (n = 4, no other information provided).
Summary of screening, completion, and withdrawal patient numbers. PP indicates per protocol. The reasons for screening failure (n = 151) were failure to meet inclusion criteria (n = 131) and failure to meet compliance criteria (n = 20). The inclusion criteria failures were: T2* outside of lower limit (n = 18), T2* outside the upper limit and not included in the prevention arm (n = 68), and LVEF outside of limit (n = 6). In addition, 39 patients were screening failures for the cardiac substudy because the sample size had been reached, but they were enrolled in the core EPIC study. The compliance criteria failures were: failed to attend for MR scan, or did not follow instructions (n = 7); failed compliance entry because of alcohol abuse (n = 1); unacceptable laboratory values (n = 10); and did not meet LIC criteria (n = 2), with one patient having LIC of less than 10 mg/kg per day and one patient with a liver mass. The 68 patients who were not included in the prevention arm after screening failed because they did not meet the eligibility criteria for the core EPIC study (unacceptable laboratory values [n = 32], unacceptable test procedure results [n = 12], did not meet diagnostic severity criteria [n = 9], consent withdrawal [n = 1], other [n = 14]). The “other” category comprised resident in Cyprus (n = 1), MR imaging not working (n = 3), noncompliance (n = 2), unfit for cardiac substudy (n = 4), and T2* more than 20 (n = 4, no other information provided).
Measurement of myocardial T2*
To quantify changes in cardiac iron overload, myocardial T2* was measured. CMR was performed at baseline and after 12 months of treatment. An additional scan at 6 months was performed in the cardiac iron reduction arm only. All patients were scanned at each site using a 1.5T scanner. After installation of the T2* MR sequence at each site, validation was performed first by scanning a standard phantom with multiple tubes of solution with known T2* values, second by scanning 5 patients at each site twice at baseline for local interstudy reproducibility, and third by rescanning these 5 patients again at the CMR Core Laboratory in London at 3 time points (within 2 weeks of the local baseline and at 6 and 12 months) for comparison with a reference scanner to provide interscanner reproducibility.
Myocardial T2* was assessed using a single breath-hold, multiecho gradient echo sequence as previously described.17 In brief, a single 10-mm midventricular short axis slice was acquired at 6 to 9 echo times (2.6-17 ms, with 1- to 2-ms increments) in a single breath-hold. A full-thickness region of interest was chosen in the LV septum for analysis as previously detailed.25 The signal intensity of this region at each echo time was measured with CMR tools software and an exponential fit was used to derive the myocardial T2* using US Food and Drug Administration (FDA)– and European Union–approved software (Thalassemia-Tools; Cardiovascular Imaging Solutions). Analysis of all myocardial T2* images was performed centrally in the CMR Core Laboratory, and all analysis was performed blinded to patient details and scanning site.
Assessment of cardiac function
LV and right ventricular (RV) volumes, ejection fraction, and mass were measured by CMR at baseline and at 6 months (cardiac iron reduction arm only) and after 12 months of treatment. MR imaging data were acquired using steady-state free-precession cines with contiguous short-axis slices from base to apex as previously detailed.26,27 Ventricular volumes were analyzed centrally in the CMR Core Laboratory using LVtools (Cardiovascular Imaging Solutions). All analysis was performed blinded to patient details and scanning site.
Other iron measurements
Changes in serum ferritin and LIC from baseline to 12 months were assessed as secondary efficacy parameters. LIC was assessed by MR measurements of the transverse relaxation parameter R2 at baseline, and then at 6 months (cardiac iron reduction arm only) and 12 months after treatment initiation. R2 scans were performed using FerriScan (Resonance Health) according to standardized procedures by qualified personnel at individual sites. Before any patient assessment, all MR scanners were calibrated according to the providers' specifications, and the accuracy was verified by a central laboratory (Inner Vision Biometrics, a subsidiary of Resonance Health). The FerriScan R2 liver scan has been shown to be highly correlated with biopsy LICs.28 Axial images of the liver were acquired at all imaging sites,18 and all raw image data were transmitted electronically to a central laboratory for analysis using previously described techniques.29 Serum ferritin was also measured at baseline and then every 4 weeks until the end of the study, with all assessments carried out at a core laboratory.
Safety assessments
Safety was evaluated every 4 weeks through the monitoring and recording of all AEs and routine laboratory testing, including hematology, blood chemistry, and urine assessments.
Statistical methods
Power calculations indicated that a total sample size of 85 patients was required to achieve a study power of 99% at a one-sided α significance level of 0.025 to detect a change in the primary end point of myocardial T2* greater than 0.2 given a SD of 0.4, assuming a 10% drop-out rate. The primary efficacy end point was assessed in the per-protocol population, which included all patients who received the study medication and had a MR myocardial T2* measurement at baseline and at least one postbaseline measurement. For analysis, patients with cardiac iron overload were stratified into 2 groups according to baseline myocardial T2*: less than 10 ms and 10 to less than 20 ms. The last-observation-carried-forward (LOCF) value was used for the analysis of serum ferritin. Safety was assessed in all patients who received at least one dose of the study medication. Serum ferritin data are presented as median (range), and myocardial T2* are presented as the geometric mean (anti-log of the mean of the log data) plus or minus coefficient of variation (CV). Other data are presented as mean plus or minus SD. Statistical significance was examined using a t test at a 2-sided α level of 0.05 on the basis of log-normal distribution.
Results
Patient characterization
Patient baseline characteristics are presented in Table 1. Recruitment was initiated on May 15, 2006 and completion of 12 months of treatment was May 15, 2008. In total, 114 patients with mild to severe cardiac iron overload (baseline T2* from > 5 to < 20 ms) were enrolled in the cardiac iron reduction arm, and 78 patients with baseline T2* values of 20 ms or higher were included in the prevention arm.
Of those with cardiac iron overload, 47 (41.2%) patients had baseline T2* of less than 10 ms and 67 (58.8%) had T2* from 10 to 20 ms. Over the treatment period, patients in the cardiac iron reduction arm received a mean of 117 mL of red blood cells/kg, equivalent to 0.36 mg/kg per day of iron. In the prevention arm, patients received a mean of 96.6 mL of red blood cells/kg, equivalent to 0.29 mg/kg per day of iron. Transfusion burden did not affect responsiveness to deferasirox in T2* for the cardiac iron reduction arm (P = .11) or the prevention arm (P = .36). Nine (7.9%) patients with cardiac iron overload and 3 (3.8%) with T2* of 20 ms or longer discontinued treatment because of AEs (n = 4 [3.5%] and n = 2 [2.6%], respectively) or for other reasons (n = 5 [4.4%] and n = 1 [1.3%]).
The proportion of patients who had hepatitis C at baseline, as assessed by medical history, was 24.6% (28/114) in the cardiac iron reduction arm and 19.2% (15/78) in the prevention arm. Hepatitis C (as assessed by medical history) was not a significant predictor of baseline LIC (P = .48) or serum ferritin (P = .19).
Deferasirox dosing
The mean actual deferasirox dose received over the 1-year treatment period was 32.6 (± 4.0) mg/kg per day (range, 19.0-40.4 mg/kg per day) in patients with cardiac iron overload enrolled in the cardiac iron reduction arm, 31.8 (± 4.5) mg/kg per day (range, 19.0-38.6 mg/kg per day) in those with T2* less than 10 ms (severe cardiac iron overload), and 33.2 (± 3.6) mg/kg per day (range, 19.6-40.4 mg/kg per day) in patients with T2* from 10 to less than 20 ms (mild-to-moderate cardiac iron overload). Ten patients (8.8%) started treatment on deferasirox at 20 mg/kg per day, 102 (89.5%) on 30 mg/kg per day, and 2 (1.8%) on 40 mg/kg per day. Deferasirox dose was adjusted in 105 of 114 (92.1%) patients with cardiac iron overload. Fifty percent (57/114) of patients underwent dose increases above their starting dose. The overall median (range) time to any dose increase was 24 weeks (10-49 weeks). Doses were reduced in 13 patients (11.4%) due to laboratory abnormalities or AEs. At 12 months, 63.2% (72/114) of patients were receiving deferasirox at 40 mg/kg per day. Of the remaining patients, 5 (4.3%), 20 (17.5%), and 6 (5.3%) were receiving deferasirox at 20 to less than 30, 30, and 35 mg/kg per day, respectively. Compliance was assessed by tablet counts. Overall, 92.1% of patients in the cardiac iron reduction arm and 96.2% of patients in the prevention arm had more than 80% compliance.
In patients with myocardial T2* of 20 ms or more (prevention arm), 63 patients (80.8%) started treatment on deferasirox at 20 mg/kg per day and 15 patients (19.2%) started on 30 mg/kg per day; the mean actual deferasirox dose received over the course of the study was 27.6 (± 6.0) mg/kg per day. In total, 70 of 78 (89.7%) patients underwent dose adjustments, primarily dose increases (54/78 [69.2%]), which occurred in a median (range) of 16 weeks (4-43 weeks). Doses were reduced in 4 patients (5.1%) due to laboratory abnormalities or AEs. Almost half of patients (37/78 [47.4%]) in the prevention arm received a final deferasirox dose of 40 mg/kg per day. Final doses were less than 20, 20 to less than 30, 30, and 35 mg/kg per day in 1 (1.3%), 10 (12.8%), 19 (24.4%), and 8 (10.3%) patients, respectively.
Patients with cardiac iron overload (cardiac iron reduction arm)
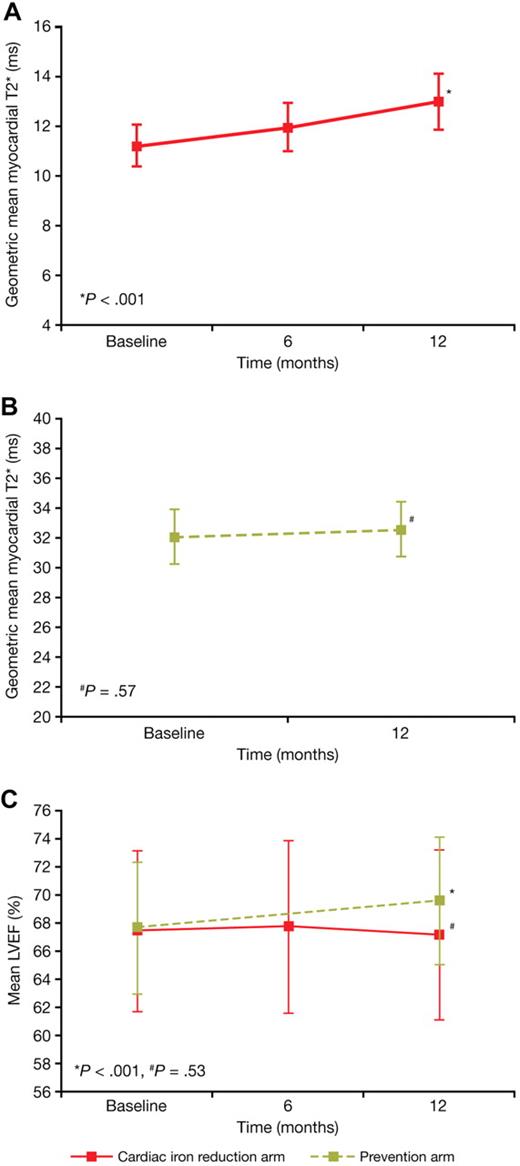
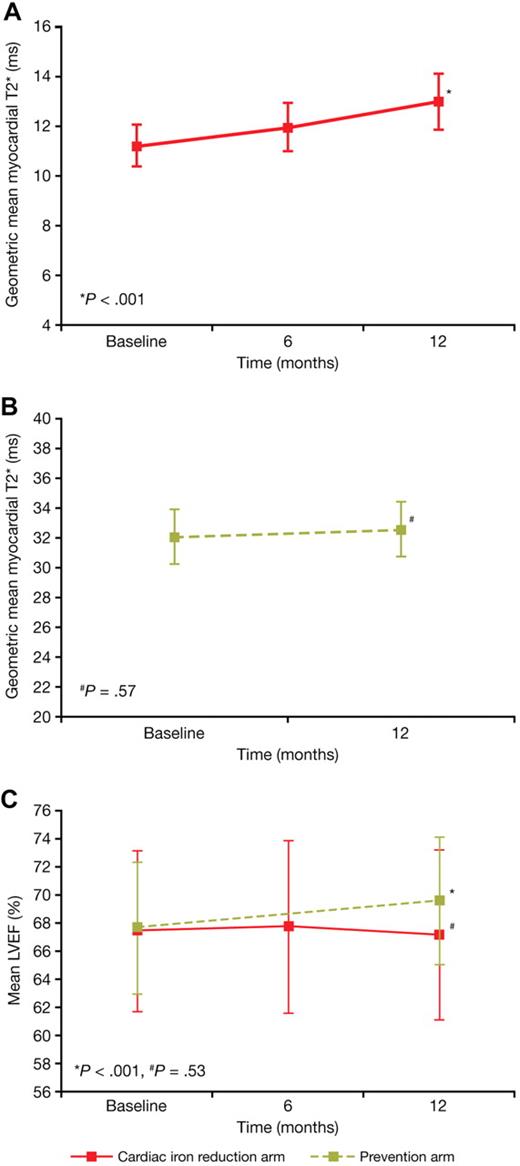
At 12 months, myocardial T2* increased significantly (indicating reduced myocardial iron) from a baseline of 11.2 ms (± 40.5%) to 12.9 ms (± 49.5%) (P < .001; Figure 2A), representing an improvement of 16% from baseline. Significant increases from 7.4 ms (± 19.4%) to 8.2 ms (± 25.6%) (P < .001) and from 14.6 ms (± 20.9%) to 17.4 ms (± 31.2%) (P < .001) were observed in the subgroups of patients with baseline T2* of less than 10 ms and from 10 to less than 20 ms, respectively. Improvement in myocardial T2* (> 4% increase) was observed in 69.5% of patients (73/105), no change in 14.3% (15/105) of patients, and worsening (> 4% decrease) in 16.2% (17/105) of patients. Twenty-five (23.8%) patients had a normal myocardial T2* of more than 20 ms at the end of 12 months of treatment.
Change in myocardial T2* and LVEF. Change in myocardial T2* (geometric mean ± 95% CI) in (A) the cardiac iron reduction arm and (B) the prevention arm. Change in LVEF (mean ± SD) (C) in both the cardiac iron reduction (red line) and prevention (green line) arms.
Change in myocardial T2* and LVEF. Change in myocardial T2* (geometric mean ± 95% CI) in (A) the cardiac iron reduction arm and (B) the prevention arm. Change in LVEF (mean ± SD) (C) in both the cardiac iron reduction (red line) and prevention (green line) arms.
LVEF remained stable at 12 months (67.0 ± 6.0%; P = .53) in patients with cardiac iron overload (Figure 2C). Similar results were observed in patients with baseline cardiac T2* of less than 10 ms (baseline, 65.8 ± 5.4%; 12 months, 65.2 ± 6.0%) and in those with T2* from 10 to less than 20 ms (baseline, 68.4 ± 5.7%; 12 months, 68.2 ± 5.8%).
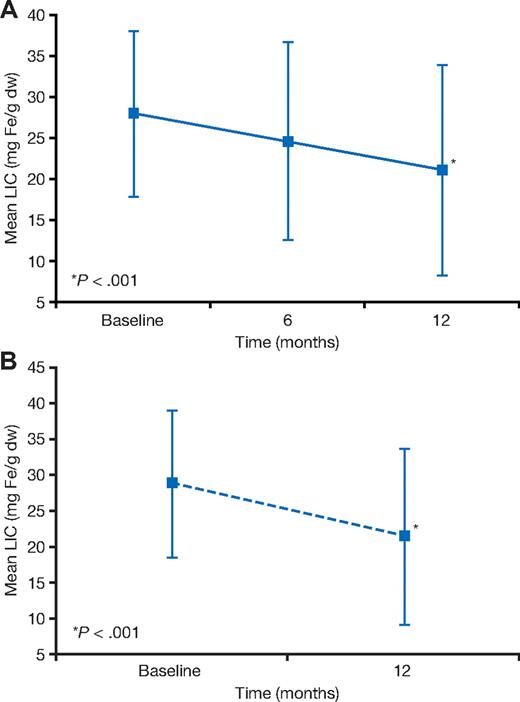
At 12 months, mean LIC was significantly reduced from baseline (28.2 ± 10.0 to 21.4 ± 12.7 mg Fe/g dw at 12 months) by −6.6 (± 9.9) mg Fe/g dw (P < .001; Figure 3A), including patients with baseline T2* of less than 10 ms (28.4 ± 10.0 at baseline and 23.4 ± 12.6 mg Fe/g dw at 12 months; −4.9 ± 9.7 mg Fe/g dw; P < .003) and those with baseline T2* from 10 to less than 20 ms (28.1 ± 10.0 at baseline and 20.0 ± 12.7 mg Fe/g dw at 12 months; −7.7 ± 10.0 mg Fe/g dw; P < .001). In 25 of 97 patients who had LIC more than 15 mg Fe/g dw at baseline, the LIC fell below 15 mg Fe/g dw at 12 months. In 13 of 15 patients who had LIC less than 15 mg Fe/g dw at baseline, the LIC remained below 15 mg Fe/g dw at 12 months.
Changes in LIC. Change in LIC (mean ± SD) in patients in the cardiac iron reduction arm (A) and the prevention arm (B).
Changes in LIC. Change in LIC (mean ± SD) in patients in the cardiac iron reduction arm (A) and the prevention arm (B).
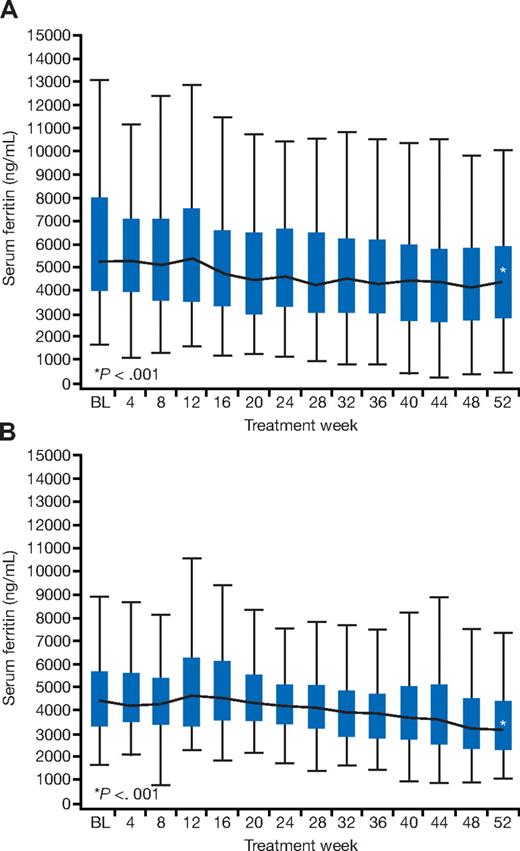
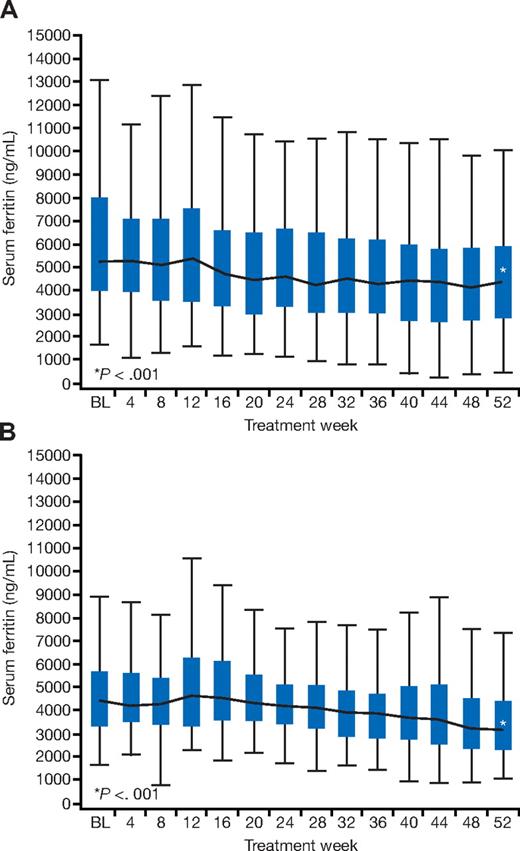
Median serum ferritin decreased significantly from baseline to 12 months by −1257 ng/mL (range, −10 282 to 7680 ng/mL; P < .001; Figure 4A). Significant decreases were also observed in patients with baseline T2* of less than 10 ms (7155 at baseline and 4810 ng/mL at 12 months; −1626 [range, −10 282 to 2970] ng/mL; P < .001) and in those with baseline T2* from 10 to less than 20 ms (4893 at baseline and 3926 ng/mL at 12 months; −1090 [range, −7264 to 7680] ng/mL; P < .001). Serum ferritin values after 1 year of treatment were seen to increase in 17% of patients at the end of the study compared with baseline. The 12-month median serum ferritin was 4345 ng/mL (range, 405-15 939 ng/mL).
Changes in median serum ferritin. Change in median serum ferritin in patients in the cardiac iron reduction arm (A) and the prevention arm (B). BL indicates baseline. Boxes represent the 25th/75th percentiles, and the whiskers represent the most extreme point within 1.5 interquartile ranges.
Changes in median serum ferritin. Change in median serum ferritin in patients in the cardiac iron reduction arm (A) and the prevention arm (B). BL indicates baseline. Boxes represent the 25th/75th percentiles, and the whiskers represent the most extreme point within 1.5 interquartile ranges.
Patients with normal myocardial iron (prevention arm)
After 12 months of treatment, the T2* was 32.5 ms (± 25.1%) compared with a baseline value of 32.0 ms (± 25.6%) (ratio of geometric mean, 1.02; P = .57; Figure 2B). None of the patients from this cohort with myocardial T2* of more than 20 ms at baseline had a T2* value below 20 ms at 12 months. LVEF significantly increased from 67.7% (± 4.7%) at baseline to 69.6% (± 4.5%) at 12 months (mean change, 1.8%; P < .001; Figure 2C).
Mean LIC significantly decreased from baseline (28.8 ± 10.2 to 21.6 ± 12.2 Fe/g dw at 12 months) by −7.2 (± 10.5) mg Fe/g dw (P < .001; Figure 3B), representing a 30% reduction. There was a significant reduction in median serum ferritin from 4367 ng/mL at baseline to 3145 ng/mL at 12 months, representing an overall decrease from baseline of −1048 (range, −4502 to 3710) ng/mL (P < .001; Figure 4B) and a relative reduction of 20%. Serum ferritin values after 1 year of treatment were seen to increase in 20% of patients at the end of the study compared with baseline. Change from baseline in T2* was inversely correlated with change in serum ferritin (r = −0.2, P = .05). In 18 of 64 patients who had LIC of more than 15 mg Fe/g dw at baseline, LIC fell below 15 mg Fe/g dw at 12 months. In 12 of 12 patients who had LIC of less than 15 mg Fe/g dw at baseline, LIC remained below 15 mg Fe/g dw at 12 months. The 12-month serum ferritin was 3145 ng/mL (range, 1078-9483 ng/mL).
Safety
In the cardiac iron reduction arm, AEs were reported by 88 of 114 (77.2%) patients, with the most common being infections (n = 44, 38.6%), gastrointestinal disorders (n = 35, 30.7%), and skin and subcutaneous tissue disorders (n = 27, 23.7%). Drug-related AEs were mostly of mild to moderate severity and reported in 56 of 114 (49.1%) patients, with skin rash (n = 15, 13.2%) being the most common. Serious AEs were reported in 12 of 114 (10.5%) patients, of which 2 were drug related: 1 poststreptococcal nephritis leading to acute renal failure, and 1 renal tubular disorder; both eventually resolved after drug discontinuation. Serious AEs unrelated to drug were each reported in 1 patient (except where shown): autoimmune hemolytic anemia, cardiomyopathy, ulcerative keratitis, abdominal pain (n = 2), noncardiac chest pain, pyrexia, appendicitis (n = 2), viral infection, increased β-N-acetyl-d-glucosaminidase, reduced creatinine clearance, increased urine potassium and urine sodium, hyperglycemia, bone cyst, musculoskeletal pain, calculus ureteric, microalbuminuria, and proteinuria. No patients with cardiac iron overload died during the study. The 1 patient (0.9%) reported to have cardiomyopathy had a baseline cardiac T2* of 8 ms and discontinued treatment before 6 months. This was assessed to be unrelated to drug and did not lead to death. Five patients (4.4%) had an increase in serum creatinine more than 33% above baseline and ULN on 2 consecutive visits. Two patients (1.8%) had an increase in ALT more than 10× ULN on 2 consecutive occasions; levels were already elevated at baseline in these patients, but neither had hepatitis C.
In the prevention arm, 53 patients (67.9%) reported AEs, the most common of which were infections (n = 32, 41.0%), gastrointestinal disorders (n = 24, 30.8%), and skin and subcutaneous tissue disorders (n = 19, 24.4%). Mild to moderate drug-related AEs were reported in 31 of 78 (39.7%) patients, with diarrhea (n = 8), rash (n = 7), nausea (n = 4), and urticaria (n = 4). Eleven serious AEs were reported unrelated to drug in 7 of 78 (9.0%) patients. Serious AEs, each in 1 patient only, were RV failure, abdominal pain, intestinal obstruction, acute pancreatitis, pyrexia, acute cholecystitis, cholelithiasis, gastroenteritis, pharyngotonsillitis, pulmonary tuberculosis, and pulmonary hypertension. In addition, there were no deaths in this patient group. In the 1 patient (1.3%) reported to have RV failure, the baseline T2* was 36.7 ms and was 29.3 ms after 281 days, with RV failure recorded on day 205; this did not lead to death. One patient (1.3%) had an increase in serum creatinine more than 33% above baseline and ULN on 2 consecutive visits; there were no progressive increases. No patient had an increase in ALT of more than 10× ULN on 2 consecutive visits.
Discussion
This is the first prospective, multicenter study in a large group of iron-overloaded patients with β-thalassemia to evaluate the efficacy of deferasirox in removing myocardial iron. Deferasirox over 1 year at a mean dose of 32.6 mg/kg per day removed iron from the heart in patients with cardiac iron overload and prevented myocardial iron accumulation in patients with normal baseline myocardial iron levels at a dose of 27.6 mg/kg per day. These data in a large cohort of 114 thalassemia patients confirm the efficacy of deferasirox in removing cardiac iron shown in preliminary reports, and they show for the first time that iron chelation therapy with deferasirox in 78 patients with normal baseline cardiac iron levels can prevent accumulation of cardiac iron. The improvement in myocardial T2* in the current study was associated with maintained ejection fraction in the patients with cardiac iron overload. In patients with normal baseline cardiac iron levels, there was a significant improvement in LVEF. In both patient groups, the effects on myocardial iron were associated with a concomitant significant decrease in hepatic and body iron burden.
The efficacy of cardiac iron chelation has been shown with other drugs in patients with thalassemia major using myocardial T2*. A randomized controlled trial showed deferiprone monotherapy to be effective in removing cardiac iron, and at a significantly faster rate than deferoxamine.30 A further randomized controlled trial showed the combination of deferiprone and deferoxamine to be more effective than deferoxamine alone.31 Deferiprone has also been shown to be effective in severe cardiac iron overload in an uncontrolled study.32 A randomized controlled trial comparing deferasirox against deferoxamine is underway. No prospective data exist comparing deferiprone and deferasirox.
In the current study, the improvement in myocardial T2* in patients with cardiac iron overload was associated with maintained LVEF, while in the prevention arm (patients without cardiac iron overload), the ejection fraction improved significantly. In both the cardiac iron reduction and the prevention arm, only patients with baseline LVEF within the reference range for healthy adults were included, and therefore further studies would be required to evaluate the efficacy and safety of deferasirox for patients with lower ejection fraction values. Changes in ejection fraction have been evaluated in studies with other chelators using CMR. In a randomized study in patients with mild-to-moderate cardiac iron overload, and with baseline ejection fraction values within the reference range for healthy adults, deferiprone significantly improved ejection fraction in comparison to deferoxamine, which showed no change.30 Similar results were seen in a randomized comparison of combination deferiprone with deferoxamine in comparison with deferoxamine alone.31 The explanation for the different pattern of response of ejection fraction with deferasirox, and the clinical significance of this finding, are not currently known. Deferiprone and deferasirox both access labile cardiac iron, and both appear to be superior to deferoxamine in this regard.33 One possibility for the ejection fraction improvement seen with deferiprone may be that it prevents iron-related mitochondrial damage,34 which could result in earlier restoration of mitochondrial function and ATP production, which are required for normal myocyte contraction. This accords with clinical experience that an improvement in ejection fraction may precede changes in T2*, with the former reflecting acute relief of toxicity and the latter measuring storage iron. The data from the current study suggests that the ejection fraction improvement with deferasirox occurs after myocardial iron clearance is more complete. Further studies are required to examine this issue.
Compliance to iron chelation therapy has been correlated with improved survival and a reduction in the likelihood of long-term complications of iron overload (including hepatic, cardiac, and endocrine problems).35 A recent study in 19 patients receiving deferasirox at 20 to 35 mg/kg per day showed that myocardial T2* was significantly improved in patients with 95% or higher compliance to treatment compared with those with less than 95% compliance, suggesting a relationship between good compliance to therapy and reduction in myocardial iron.36 Studies have shown that more patients treated with deferasirox are satisfied with treatment and find deferasirox to be more convenient compared with prior chelation therapy (deferoxamine and/or deferiprone), factors that may help improve treatment compliance.37,–39
The highest dose of deferasirox currently approved by most health authorities is 30 mg/kg per day. However, the FDA, European Medicines Agency, and several additional health authorities (in Canada, Switzerland, and Brazil) have recently approved doses up to 40 mg/kg per day in patients not adequately controlled with doses of 30 mg/kg per day (eg, serum ferritin levels persistently > 2500 ng/mL with no decreasing trend over time); doses above 40 mg/kg per day are not recommended. The EPIC trial24 was not part of the FDA submission for approval of doses up to 40 mg/kg per day.
Deferasirox was well tolerated, with the most common drug-related AEs being mild to moderate transient gastrointestinal disturbances (prevention arm) and rash (both study arms). Of note, during this 1-year treatment period, there were no deaths reported with deferasirox therapy. Of 192 patients included in this study, cardiac events were reported in only 2 (1%) patients, 1 with cardiomyopathy in the cardiac iron reduction arm, and 1 with RV failure in the cardiac prevention arm. Neither event was assessed by the investigator to be drug related, and neither resulted in death. Furthermore, despite receiving deferasirox at 30 to 40 mg/kg per day, few patients had increases in serum creatinine 33% above baseline and ULN. This confirms previously reported data showing that the safety profile of deferasirox at doses above 30 mg/kg per day is consistent with that observed in registration trials.40
There are some limitations to this study. Although patients with marked transfusional iron overload should be monitored by cardiac and liver MR imaging whenever possible, serum ferritin trends may prove useful in healthcare systems where serum ferritin is the most accessible indicator of iron status. Ferritin values after 1 year of treatment were seen to increase in 17% and 20% of patients in the cardiac iron reduction and prevention arms, respectively. Although this may represent acute phase responses to intercurrent infection, poor compliance, or treatment failure, a similar proportion of patients was previously reported to have an increase in LIC at comparable deferasirox doses.41 The ejection fraction increased in the prevention arm, but it is not clear whether the change is clinically significant. The cardiac T2* analysis is presented for the per-protocol population who completed 12 months of treatment, but reanalysis of the intention to treat population with LOCF for missing values showed very similar results. The optimal dose for using deferasirox for cardiac chelation is not fully defined; however, the current study suggests that doses of up to 40 mg/kg per day may be required, which is the current upper dose limit. The high dosing of deferasirox in this study has cost implications, but lower maintenance doses of deferasirox are likely to be possible once cardiac iron loading and body iron stores are controlled.
In conclusion, deferasirox treatment for 1 year removed iron from the heart and maintained LVEF in patients with β-thalassemia with mild, moderate, and severe myocardial siderosis. In patients with normal myocardial iron levels, deferasirox maintained myocardial iron levels and improved LVEF. A randomized controlled trial currently underway is comparing the cardiac efficacy of deferasirox with deferoxamine and will provide further valuable data on myocardial iron treatment.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Susan Cheer for the medical editorial assistance with this manuscript.
This study was sponsored by Novartis Pharma AG. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals.
Authorship
Contribution: D.J.P. drafted the manuscript and was responsible for interpreting the CMR results; J.B.P., A.E.-B., L.L.C., Y.A., M.S.E, P.S., C.-K.L., H.I., V.V., A.K., and A.T. served as investigators on this trial, enrolled patients, contributed to data interpretation, and reviewed and provided their comments on this manuscript; M.D.C., A.E.-B., C.-K.L., and A.K. served as Study Monitoring Committee members overseeing the conduct of the trial; D.H. and G.D. coordinated the execution of the trial and contributed to the analysis, interpretation, and reporting of the trial data; G.S. contributed to the analysis of CMR scans; and B.R. served as the trial statistician.
Conflict-of-interest disclosure: D.J.P. reports receiving consulting fees and research grant funding from Novartis Pharmaceuticals and Siemens, lecture fees from Novartis Pharmaceuticals, consulting and lecture fees from Apopharma, and is a director and equity holder in Cardiovascular Imaging Solutions. J.B.P. reports receiving consulting fees, research grant funding, and lecture fees from Novartis Pharmaceuticals and consulting fees from Vifor International and Mundipharma. M.D.C. reports receiving lecture fees from Novartis Pharmaceuticals. A.E.-B. reports receiving research grant support, consulting fees, and lecture fees from Novartis Pharmaceuticals. L.L.C. reports receiving research grant support and lecture fees from Novartis Pharmaceuticals. Y.A. reports receiving research grant support, consulting fees, and lecture fees from Novartis Pharmaceuticals. P.S. reports receiving research grant support from Novartis Pharmaceuticals and consulting fees from Novartis Pharmaceuticals and Novo Nordisk. C.-K.L. reports receiving consulting fees and lecture fees from Novartis Pharmaceuticals. V.V. reports receiving research grant support and lecture fees from Novartis Pharmaceuticals and research grant support from GPO-L-ONE. A.K. reports receiving consulting fees and lecture fees from Novartis Pharmaceuticals. A.T. reports receiving research grant support and lecture fees from Novartis Pharmaceuticals. D.H., G.D., and B.R. are employed by Novartis Pharmaceuticals, the company whose product was studied in the present work. M.S.E., H.I., and G.S. declare no competing financial interests.
Correspondence: Dudley Pennell, Cardiovascular MR Unit, Royal Brompton Hospital, Sydney St, London SW3 6NP, United Kingdom; e-mail: d.pennell@ic.ac.uk.
Appendix
Participating centers and investigators: Y. Aydinok, Ege University Medical Faculty, Izmir, Turkey; L. L. Chan, University Malaya Medical Centre, Lembah Pantai, Kuala Lumpur, Malaysia; S. Chancharunee, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; G. Chrousos, Athens University Medical School, Athens, Greece; A. Kattamis, Athens University Medical School, Athens, Greece; A. Chuansumrit, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; M. S. Elalfy, Ain Shams University, Cairo, Egypt; A. El-Behslawy, Cairo University, Cairo, Egypt; R. Galanello, Ospedale Regionale Microcitemie, Cagliari, Italy; H. Ibrahim, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; C.-K. Li, Prince of Wales Hospital, Chinese University of Hong Kong, Hong Kong; A. Piga, Ospedale Regina Margherita, Torino, Italy; J. B. Porter, University College London, London, United Kingdom; P. Sutcharitchan, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Bangkok, Thailand; A. Taher, American University of Beirut, Beirut, Lebanon; V. Viprakasit, Faculty of Medicine Siriraj Hospital/Department of Pediatrics/Division of Haematology-Oncology, Bangkoknoi, Bangkok, Thailand; N. C. Zoumbos, Patras University Medical School, Rio, Patras, Greece.

![Figure 1. Summary of screening, completion, and withdrawal patient numbers. PP indicates per protocol. The reasons for screening failure (n = 151) were failure to meet inclusion criteria (n = 131) and failure to meet compliance criteria (n = 20). The inclusion criteria failures were: T2* outside of lower limit (n = 18), T2* outside the upper limit and not included in the prevention arm (n = 68), and LVEF outside of limit (n = 6). In addition, 39 patients were screening failures for the cardiac substudy because the sample size had been reached, but they were enrolled in the core EPIC study. The compliance criteria failures were: failed to attend for MR scan, or did not follow instructions (n = 7); failed compliance entry because of alcohol abuse (n = 1); unacceptable laboratory values (n = 10); and did not meet LIC criteria (n = 2), with one patient having LIC of less than 10 mg/kg per day and one patient with a liver mass. The 68 patients who were not included in the prevention arm after screening failed because they did not meet the eligibility criteria for the core EPIC study (unacceptable laboratory values [n = 32], unacceptable test procedure results [n = 12], did not meet diagnostic severity criteria [n = 9], consent withdrawal [n = 1], other [n = 14]). The “other” category comprised resident in Cyprus (n = 1), MR imaging not working (n = 3), noncompliance (n = 2), unfit for cardiac substudy (n = 4), and T2* more than 20 (n = 4, no other information provided).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/12/10.1182_blood-2009-04-217455/5/m_zh89990948280001.jpeg?Expires=1767810331&Signature=dOGpP67TedNrW~gomJP05n6v2IZMon6opmnB9SECUVBzWeavndLkMWmlGktxbmjfKwEql7KR3zrsOqRyT65K2QLSJcGDgu4Ye2rlifD-9625~NdD2lGkyCXm3cnx1n7x4V9mkkUgXEqaOKe9wgydrtKG6BbkpW4ePxI6n2G36EoNqDbTqqc3gibJJPzXPVfJOgxM~DHYPqofZNcDLpIzTnTwuup2Ni85sSf2HUNBa2MNZ4CA98-qnlK2VqvNC3NYnbTqn2FX1YYJoHd4tj51XNulCi9KBtr3m54~OsVTq57EpcycBdN4kkg9BrQCs~Ivad1VfQClmnIwxPGbApR08A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Summary of screening, completion, and withdrawal patient numbers. PP indicates per protocol. The reasons for screening failure (n = 151) were failure to meet inclusion criteria (n = 131) and failure to meet compliance criteria (n = 20). The inclusion criteria failures were: T2* outside of lower limit (n = 18), T2* outside the upper limit and not included in the prevention arm (n = 68), and LVEF outside of limit (n = 6). In addition, 39 patients were screening failures for the cardiac substudy because the sample size had been reached, but they were enrolled in the core EPIC study. The compliance criteria failures were: failed to attend for MR scan, or did not follow instructions (n = 7); failed compliance entry because of alcohol abuse (n = 1); unacceptable laboratory values (n = 10); and did not meet LIC criteria (n = 2), with one patient having LIC of less than 10 mg/kg per day and one patient with a liver mass. The 68 patients who were not included in the prevention arm after screening failed because they did not meet the eligibility criteria for the core EPIC study (unacceptable laboratory values [n = 32], unacceptable test procedure results [n = 12], did not meet diagnostic severity criteria [n = 9], consent withdrawal [n = 1], other [n = 14]). The “other” category comprised resident in Cyprus (n = 1), MR imaging not working (n = 3), noncompliance (n = 2), unfit for cardiac substudy (n = 4), and T2* more than 20 (n = 4, no other information provided).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/12/10.1182_blood-2009-04-217455/5/m_zh89990948280001.jpeg?Expires=1768071362&Signature=z8ZrHyN7Vs-9j0MaawMeAG1T0JazWE-JQL467g4mxorLowENGbtnkXZ6dNBwYWB2he5O5Z1nwfVeYh8UBFQPzvTcI1wClJYPmArZof0bHk~QOFAqEgfxX8MzlLPOQnAzbT0w5R4kS9BzQGQisHgvD7Kuui~uOiKraOpVXyzNy9Fver-nHhAfMDhc6v2vCu6k-g~vZKpBDNtf1g5qLR-B~~DpmJOh4966meHENyzm0SWxkHhUVY9BQ1uq4KxFfAgjtyOwkuPA0FFPAGTr5h9qgWAsiQCdIzLQkHL7EspaqT60J~RLRUTgpRcfBSydO3Nhc9Qr9d3qNM0E2v-ASGAByQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)