Abstract
Polyphosphorylated phosphoinositides (PIPs) are potent second messengers, which trigger a wide variety of signaling and trafficking events in most eukaryotic cells. However, the role and metabolism of PIPs in malaria parasite Plasmodium have remained largely unexplored. Our present studies suggest that PfPI3K, a novel phosphatidylinositol-3-kinase (PI3K) in Plasmodium falciparum, is exported to the host erythrocyte by the parasite in an active form. PfPI3K is a versatile enzyme as it can generate various 3′-phosphorylated PIPs. In the parasite, PfPI3K was localized in vesicular compartments near the membrane and in its food vacuole. PI3K inhibitors wortmannin and LY294002 were effective against PfPI3K and were used to study PfPI3K function. We found that PfPI3K is involved in endocytosis from the host and trafficking of hemoglobin in the parasite. The inhibition of PfPI3K resulted in entrapment of hemoglobin in vesicles in the parasite cytoplasm, which prevented its transport to the food vacuole, the site of hemoglobin catabolism. As a result, hemoglobin digestion, which is a source of amino acids necessary for parasite growth, was attenuated and caused the inhibition of parasite growth.
Introduction
Plasmodium falciparum, a protozoan parasite, is the major causative agent of human malaria. The lack of a successful vaccine and widespread drug resistance contribute heavily toward the failure in eradication of this disease. A thorough understanding of molecular mechanisms that regulate the life cycle of P falciparum may provide information useful for designing novel strategies to control malaria. Malarial parasites can propagate either asexually or undergo sexual differentiation. It is the asexual development in the erythrocytic phase of the life cycle that causes pathology of severe malaria. Subsequent to erythrocyte invasion by merozoites, parasites first adopt a ring-like morphology and develop into trophozoites, which give rise to schizonts that contain several merozoites. During the trophozoite stage, the parasite endocytoses large quantities of the erythrocyte cytosol and digests its main constituent, hemoglobin.1 The endocytosis of erythrocyte cargo occurs via cytostome invaginations and transport vesicles, which mediate the delivery of the material to the food vacuole, where hemoglobin is degraded by a battery of proteases and the residual heme is converted to hemozoin.2
Phosphoinositides are important for regulating signaling and trafficking events in most eukaryotic cells. These phospholipids are generated as a result of phosphorylation of 3′, 4′, or 5′ hydroxyls of phosphatidylinositol head group by specific phosphatidylinositol (PI) kinases.3 Phosphatidylinositol-3-kinases (PI3K) are most well characterized and catalyze the phosphorylation of phosphatidylinositol, phosphatidylinositol-4 phosphate (PI4P), phosphatidylinositol-4,5 bisphosphate [PI(4,5)P2] at 3′ position of the inositol ring to generate phosphatidylinositol-3 phosphate (PI3P), phosphatidylinositol-3,4 bisphosphate [PI(3,4)P2], and phosphatidylinositol-3,4,5 triphosphate [PI(3,4,5)P3].3,4 In contrast to class I PI3Ks, which generate PI(3,4)P2 and PI(3,4,5)P3, class III PI3Ks are known to specifically phosphorylate PI, resulting in the formation of PI3P.3 PI3P is present on vacuolar and endosomal membranes in yeast and on membranes of early endosomes and internal vesicles of multivesicular bodies in mammalian cells.5 PI3P interacts with FYVE or PX domains of proteins and recruits them to these intracellular locations and controls membrane and vesicular trafficking.6
Given the importance of PIPs in most eukaryotes, it is probable that these second messengers may also perform important functions in malaria parasite. However, the metabolism and the role of PIPs in malaria parasite are not well understood. Recently, a PI4P5 kinase was reported in Plasmodium, which is regulated by ARF7 and catalyzes the formation of PI(4,5)P2. We have identified a FYVE domain protein (FCP), which is a probable downstream target of PfPI3K as it interacts with PI3P. Studies performed on this protein resulted in identification of a novel food vacuole trafficking pathway in the parasite.8 In the present study, we report the identification of a PI3K ortholog from P falciparum, PfPI3K. This enzyme is versatile as it catalyzes the formation of PI3P, PI(3,4)P2, and PI(3,4,5)P3 from their respective precursors. PfPI3K is present in the food vacuole and in vesicular compartments at the parasite membrane (PM)/parasitophorous vacuole membrane (PVM). Surprisingly, PfPI3K is exported to the host erythrocyte by the parasite and is located at its periphery. PI3K inhibitors wortmannin and LY294002 inhibited PfPI3K activity. Using these inhibitors, we demonstrate that PfPI3K may play a key role in hemoglobin endocytosis and its trafficking in the parasite and its activity may be important for parasite development.
Methods
Most fine chemicals were purchased from Sigma-Aldrich. Wortmannin and LY294002 were purchased from Calbiochem or BIOMOL Research Laboratories. These inhibitors were dissolved in dimethyl sulfoxide (DMSO) to yield a concentration of 1 to 3 mg/mL, and serial dilutions were made in culture media or water before use. Anti-actin antibody was purchased from Calbiochem. The amino acid-deficient RPMI 1640 was custom made by HyClone. All studies were performed with the approval of the Institute's Animal Ethics Committee and Human Ethics Committee.
P falciparum cultures
Parasite growth inhibition assays
Synchronized parasites were cultured in amino acid-free RPMI 1640 supplemented with isoleucine (Ile media) for 96 hours. Subsequently, parasite cultures were diluted with either Ile medium or complete RPMI 1640 medium to approximately 1% parasitemia in 6-well (35-mm) culture plates. Six hours after plating, when parasites were in late ring/early trophozoite stage (corresponding to ∼ 12 hours after invasion), pepstatin A (30μM) or desired concentration of wortmannin or LY294002 was added to the cultures, whereas parasites were treated with DMSO/MeOH in control experiments. The maximal DMSO concentration was either 0.02% (for wortmannin experiments) or 0.05% (for LY294002 experiments), which matched its concentration in experiments done with the highest inhibitor doses. Thin blood smears were stained with Giemsa, and parasites were visualized microscopically to determine the parasitemia.
Cloning, expression, and generation of antisera against PfPI3K
Total RNA was isolated from asynchronous P falciparum 3D7 parasites, and reverse transcription was performed using random hexamers provided in the Thermoscript RT-PCR kit (Invitrogen). Because the BLAST search of Plasmodium proteins using PfPI3K helical domain did not result in any significant hit other than PfPI3K, this domain was chosen to raise antisera against PfPI3K. Based on the sequence information, the following PCR primers were designed to amplify cDNA corresponding to the helical domain of PfPI3K: PfPI3KHD1 (forward) ATGTTATCACCAACTATAAACGAGATTAAAAAC and PfPI3KHD1 (reverse) GATAATTTTATTTTTTCTAATATTAGAAGTCAT. PCR products were cloned directly in pQE-UA (QIAGEN) bacterial expression vector. The helical domain was expressed in Escherichia coli as a 6xHis tagged protein (supplemental data, available on the Blood website; see the Supplemental Materials link at the top of the online article). To raise antisera against PfPI3K helical domain (PfPI3KHD), 100 μg of recombinant protein was emulsified with complete Freund adjuvant and used to raise antisera in rabbits and mice using standard protocols.
Immunoblotting and immunofluorescence
Parasites were released from infected erythrocytes by 0.05% (wt/vol) saponin treatment as described earlier.11 Unless indicated, cell-free protein extracts from specific parasite stages were prepared by suspending parasite pellets in 2% sodium dodecyl sulfate. After separation of lysate proteins on SDS-polyacrylamide gel electrophoresis gels, proteins were transferred to a nitrocellulose membrane. Immunoblotting was performed using anti-PfPI3K antisera and horseradish peroxidase (HRP)–labeled anti–rabbit IgG. West Dura enhanced chemiluminescence substrate (Pierce Chemical) was used to develop blots following the manufacturer's instructions. Hemoglobin quantitation in parasites was carried out by Western blotting with anti-hemoglobin antiserum as described previously.12,13
Immunofluorescence assays (IFAs) were performed on thin blood smears as previously described.8 Briefly, samples were fixed with methanol for 10 minutes and permeabilized with 0.05% Triton X-100 for 5 minutes. After blocking with either 3% bovine serum albumin or 2% gelatin (prepared in phosphate-buffered saline), smears were incubated with primary antibodies/antisera for 16 hours at 4°C. Control experiments were performed using preimmune bleeds or using uninfected eythrocyte lysates. After washing with phosphate-buffered saline, incubations with anti–mouse or anti–rabbit IgG conjugated to either fluorescein isothiocyanate or Texas red were performed. 4,6-Diamidino-2-phenylindole was used for nuclear localization. Immunofluorescence localization of hemoglobin in saponin-treated, aldehyde-fixed, and Triton X-100–permeabilized parasites was carried out as described previously.13,14 The procedure followed for electron microscopy is described in supplemental data.
Microscopy
For experiments reported in Figure 2Ai through B, cells were visualized using a Zeiss LSM510 confocal microscope system equipped with a 63×/1.4 oil immersion objective. For Figure 2Aii, a Zeiss AxioImager fluorescence microscope with a 100×/1.4 oil-immersion objective was used. A Zeiss AxioCam MRm camera and Zeiss AxioVision software were used to acquire images. For Figure 4A, a Nikon Eclipse E600W with Y-FL epifluorescence attachment with 100×/1.30 NA oil objective was used. A Media Cybernetics CoolSNAP-Pro monochrome-cooled charge-coupled device camera and Media Cybernetics CoolSNAP-Pro cf digital kit version 4.1 were used for image acquisition. All images were finally processed using Adobe Photoshop and Microsoft PowerPoint software.
HRP endocytosis assay
Immunoprecipitation and assay of lipid kinase activity
Parasites in mid-trophozoite stage were collected by saponin lysis and washed several times to remove red blood cell (RBC) contamination. Subsequently, parasite lysates were prepared in buffer A (10mM Tris, pH 7.5, 100mM NaCl, 5mM ethylenediaminetetraacetic acid, 1% Triton X-100, 100μM sodium orthovanadate, 20μM sodium fluoride, 20μM β-glycerophosphate, and 1× protease inhibitor cocktail, Roche Diagnostics; 10% glycerol) and were clarified by centrifugation at 20 000g at 4°C. Typically, 50 to 100 μg of parasite lysate was incubated with anti-PfPI3K antisera with end-to-end shaking at 4°C for 12 hours. Protein A/G agarose beads were added to this mix, and further incubation was done for an additional 6 hours. The protein A/G agarose beads were washed several times with buffer A and resuspended in kinase assay buffer. Typically, 10 μL of immunoprecipitate was used for performing kinase assays in a 20-μL reaction volume in a buffer containing 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.5, 5mM MgCl2, 0.45mM ethyleneglycoltetraacetic acid, 70μM adenosine triphosphate ([γ32P]- 6μCi /reaction), and 200μM substrate PI or PIP in the presence or absence of wortmannin. Reaction mix was incubated at 30°C for 30 minutes, and the reaction was terminated by the addition of 1M HCl. Lipid extraction was first done using 200 μL of CHCl3/MeOH (1:1) and then with 80 μL of 1M HCl/MeOH (1:1). Phospholipids were separated by thin-layer chromatography (TLC), which was performed on preactivated Silica Gel 60 plates (Merck) in a CHCl3/methanol/2N NH4OH (9:7:2) solvent system. Radiolabeled phosphoinositide products were detected using a Fuji FLA-5000 phosphorimager.
Results
PfPI3K, a PI3K ortholog in P falciparum
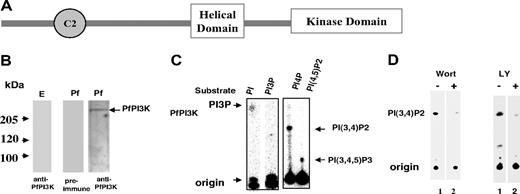
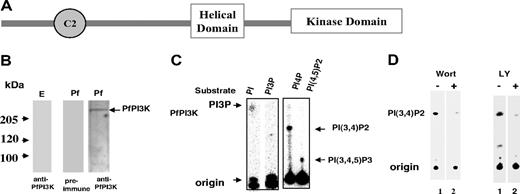
BLAST search performed with sequences of mammalian and yeast PI3Ks against P falciparum genome database resulted in only 1 significant match on chromosome 5, which was named PfPI3K. PfPI3K corresponds to PlasmoDB annotated gene PFE0765w, which is predicted to encode a 2133-amino acid protein. PfPI3K shares high homology with the catalytic and signature helical domain of PI3Ks (supplemental Table 1). PI3Ks are classified based on their domain architecture, mechanism of regulation, and substrate affinities.3 Comparison of vacuolar protein-sorting protein 34 (vps34), a class III PI3K and PI3Kγ, a class I PI3K to PfPI3K, suggests that reasonable sequence homology exists in the helical domain region and the catalytic domain of these enzymes (supplemental Table 1). The helical domain anchors the other important regions of PI3Ks in an orientation conducive for the catalytic domain to function properly.15 PfPI3K has a putative C2 domain, which is known to interact with calcium and lipids (Figure 1A). PfPI3K lacks any other obvious protein domains, such as the Ras binding domain or pleckstrin homology domain, which are found in class I or class II enzymes.3 PfPI3K contains NKNDDD repeats near its N-terminal end, which is present in several Plasmodium proteins.16 The comparison of amino acid sequence and domain organization suggests that PfPI3K may be closest to class III PI3Ks, such as vps34.
PfPI3K, a PI3 kinase from P falciparum. (A) Schematic diagram showing domain architecture of PfPI3K. The helical and catalytic domain of PfPI3K is separated by a linker. In addition, a C2 domain is present near its N-terminal end. (B) Equal amounts of cell lysates prepared either from either uninfected erythrocytes (E left panel) or trophozoite stage parasites (Pf right panel) were electrophoresed on 7% SDS-polyacrylamide gel electrophoresis gel, and Western blot analyses were performed using anti-PfPI3K antisera on both erythrocyte as well as the parasite lysate. A control Western blot with preimmune antisera was performed to probe the parasite lysate (middle panel). (C) PfPI3K was immunoprecipitated from trophozoite lysates, and PfPI3K-IP was used in a lipid kinase assay wherein either PI or PI3P (left panel), PI4P, or PI(4,5)P2 (right panel) was used as substrate. Phospholipids were separated on a TLC along with phosphoinositide standards, and radiolabeled lipid products were detected using a phosphorimager. (D) The activity of PfPI3K-IP from trophozoite lysates was assayed using PI4P as substrate (as described in panel C) in the presence of 2.5μM wortmannin (left panel), or 50μM LY294002 (right panel) or DMSO (−).
PfPI3K, a PI3 kinase from P falciparum. (A) Schematic diagram showing domain architecture of PfPI3K. The helical and catalytic domain of PfPI3K is separated by a linker. In addition, a C2 domain is present near its N-terminal end. (B) Equal amounts of cell lysates prepared either from either uninfected erythrocytes (E left panel) or trophozoite stage parasites (Pf right panel) were electrophoresed on 7% SDS-polyacrylamide gel electrophoresis gel, and Western blot analyses were performed using anti-PfPI3K antisera on both erythrocyte as well as the parasite lysate. A control Western blot with preimmune antisera was performed to probe the parasite lysate (middle panel). (C) PfPI3K was immunoprecipitated from trophozoite lysates, and PfPI3K-IP was used in a lipid kinase assay wherein either PI or PI3P (left panel), PI4P, or PI(4,5)P2 (right panel) was used as substrate. Phospholipids were separated on a TLC along with phosphoinositide standards, and radiolabeled lipid products were detected using a phosphorimager. (D) The activity of PfPI3K-IP from trophozoite lysates was assayed using PI4P as substrate (as described in panel C) in the presence of 2.5μM wortmannin (left panel), or 50μM LY294002 (right panel) or DMSO (−).
The crystal structure of PI3Kγ exhibits a catalytic domain that shares a fold similar to that of protein kinases, predominantly β-sheet containing N-terminal lobe and a helical C-terminal lobe.15 Structure-based sequence comparisons with PI3Kγ revealed that all 10 β-sheets and 12 α-helices found in PI3Kγ catalytic domain may be present in PfPI3K (supplemental Figure 1B). Interestingly, the catalytic domain of PfPI3K is interrupted by 3 additional insertions (supplemental Figure 1B).
The helical domain of PI3Kγ has 10 α-helices, which form 5 A/B type pairs. Although 7 of these helices may be conserved (supplemental Figure 1A) in PfPI3K, the sequence of the last 3 helices of PI3Kγ does not pair up with the corresponding region. Although helical and catalytic domains of PI3Kγ are almost contiguous, they are separated by an approximately 110-amino acid linker in PfPI3K. Despite the similarity between regions of PfPI3K with other PI3Ks, there are interesting differences, which may suggest a different mode of regulation of this enzyme.
PfPI3K expression in intraerythrocytic parasites
Attempts to express the full-length PfPI3K in either E coli or mammalian cells were not successful. The helical domain was expressed with a 6xHis tag (supplemental Figure 2) and was used to raise antisera against PfPI3K (supplemental Figure 2). An approximately 230-kDa band, which was close to the PlasmoDB predicted size of PfPI3K, was observed in trophozoite lysates and not in control uninfected erythrocyte lysates (Figure 1B).
Demonstration of PfPI3K activity in P falciparum
We were unable to express an active form of recombinant PfPI3K. However, PfPI3K was immunoprecipitated successfully from the parasite lysate, which allowed us to assay the activity of the native PfPI3K. PfPI3K immunoprecipitate (PfPI3K-IP) was used to phosphorylate PI, PI4P, or PI(4,5)P2 in a kinase assay mix. On incubation with PI, a product corresponding to the size of PIP was formed. Because this product was not observed when synthetic PI3P was used in assays, it is reasonable to attribute this to PI3P (Figure 1C left panel). When PI4P was used as a potential substrate, a radiolabeled product with slower mobility corresponding to the expected size of PI(3,4)P2 was observed. In addition, we found that PI(4,5)P2 may also be converted to PI(3,4,5)P3 by PfPI3K (Figure 1C right panel). Mock immunoprecipitates prepared from RBC lysates did not show any kinase activity (data not shown here). These data indicate that PfPI3K can generate PI3P, PI(3,4)P2, and PI(3,4,5)P3 and highlight some differences between PfPI3K and typical class III PI3Ks, which mainly form PI3P from PI.3,5
A recent study indicated that attempts to obtain P berghei lacking PI3K gene failed and suggested that this kinase may be essential for the parasite development.17 Although gene disruption has not been done in P falciparum, based on observations made in P berghei, it is probable that PfPI3K may also be indispensable for P falciparum. We used a pharmacologic approach to evaluate PfPI3K function. Wortmannin and LY294002, widely used inhibitors for PI3Ks, were tested against PfPI3K. Incubation with both these inhibitors caused a significant decrease in PfPI3K-IP activity (Figure 1D). Whereas approximately 3μM wortmannin caused almost complete inhibition of PfPI3K activity, the amount of LY294002 needed was approximately 50μM. Similar trends have been observed for other PI3Ks; most PI3Ks are more senstitive to wortmannin compared with LY294002.18,19 In this study, we have used both wortmannin and LY294002 as tools to elucidate the role of PfPI3K in the parasite life cycle.
Localization and trafficking of PfPI3K
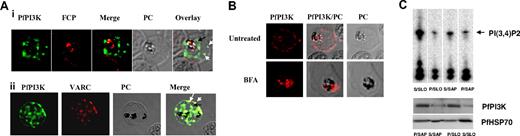
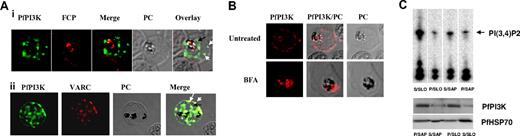
The cellular localization of PfPI3K was determined by performing IFAs. PfPI3K was detected inside the food vacuole (Figure 2A; supplemental Figure 3), which fits well with the presence of an FCP in this compartment. FCP interacts with PI3P8 and therefore is a probable effector of PfPI3K signaling. In addition, PfPI3K staining was detected in vesicular structures concentrated near the PVM/PM and the food vacuole membrane (Figure 2Ai-ii). Strikingly, PfPI3K was also found in the host erythrocyte (Figure 2A-B). It was present near the erythrocyte periphery as confirmed by costaining with an antibody against the conserved C-terminal of VAR gene products (VARC; Figure 2Aii), which are part of knob structures.20 It is important to indicate that the PfPI3K ORF seems to lack an obvious targeting sequence, such as the PEXEL/HTS, which have been shown to target parasite proteins to the host.21,22
Intracellular localization of PfPI3K and its trafficking to the host. (A) IFA was performed for localizing PfPI3K, FCP (i) and VARC (ii). FCP (red) is located inside the food vacuole of mature trophozoites. PfPI3K (green) exhibits “vesicular” staining on PVM/PM (black arrows) and the host erythrocyte (white arrows) and in the food vacuole, which can be identified by the presence of black hemozoin. (ii) PfPI3K colocalizes with VARC at the erythrocyte surface. (B) Parasites were treated either with 5μg/mL BFA or DMSO, and immunofluorescence was performed using anti-PfPI3K antisera. BFA treatment blocked the transport of PfPI3K to the erythrocyte. (C) Trophozoite stage parasites were treated with either streptolysin (SLO) or saponin (SAP). The soluble (S) and pellet (P) fractions were used for Western blot (bottom panel) and immunoprecipitation of PfPI3K. PfPI3K-IP was assayed for activity using PI4P as the substrate; the radiolabeled product PI(3,4)P2 was detected by phosphorimaging of TLC plates. Anti-PfHSP70 was used for a control Western blot.
Intracellular localization of PfPI3K and its trafficking to the host. (A) IFA was performed for localizing PfPI3K, FCP (i) and VARC (ii). FCP (red) is located inside the food vacuole of mature trophozoites. PfPI3K (green) exhibits “vesicular” staining on PVM/PM (black arrows) and the host erythrocyte (white arrows) and in the food vacuole, which can be identified by the presence of black hemozoin. (ii) PfPI3K colocalizes with VARC at the erythrocyte surface. (B) Parasites were treated either with 5μg/mL BFA or DMSO, and immunofluorescence was performed using anti-PfPI3K antisera. BFA treatment blocked the transport of PfPI3K to the erythrocyte. (C) Trophozoite stage parasites were treated with either streptolysin (SLO) or saponin (SAP). The soluble (S) and pellet (P) fractions were used for Western blot (bottom panel) and immunoprecipitation of PfPI3K. PfPI3K-IP was assayed for activity using PI4P as the substrate; the radiolabeled product PI(3,4)P2 was detected by phosphorimaging of TLC plates. Anti-PfHSP70 was used for a control Western blot.
To determine whether PfPI3K transport to the host is dependent on secretory pathways, parasites were treated with brefeldin A (BFA). BFA, which inhibits post-Golgi events, such as vesicle budding in secretion, blocks the classic secretory pathway as well as the alternate secretory pathways that send the parasite proteins to the host erythrocyte.21,22 BFA caused a loss in PfPI3K staining on the PVM/PM and also in the host erythrocyte (Figure 2B). In BFA-treated parasites, PfPI3K was retained predominantly inside the parasite. These data indicate the BFA-sensitive pathway may be responsible for exporting PfPI3K to parasite surface and to the host erythrocyte.
PfPI3K, a source of PIPs in infected erythrocytes
It was important to assess whether PfPI3K retains its ability to produce 3′-PIPs in the host. To probe this, host erythrocyte cytoplasmic proteins were separated from the parasite by streptolysin O, which may create pores in the membrane of infected RBCs (iRBCs) and cleaves it without modifying the PVM.23 In addition, PV and iRBC proteins were separated from the parasite proteins by treatment with 0.15% saponin.23 Consistent with the IFA data, Western blotting on these fractions revealed the presence of PfPI3K in iRBC as well as the parasite fraction (Figure 2C). The presence of PfPI3K in the supernatant of streptolysin-treated iRBC suggested that it is most probably anchored to the cytosolic face of iRBC membrane, which is consistent with immunofluorescence results. PfPI3K was immunoprecipitated from these fractions and assayed for kinase activity using PI4P as the substrate. PfPI3K was found to be active in the infected erythrocyte as well as the parasite fractions (Figure 2C). These observations suggest that PfPI3K secreted to the host may generate 3′-PIPs, which may be important for controlling signaling/trafficking events in the erythrocyte. Interestingly, it has been reported that the levels of PIP and PIP2 increase dramatically in erythrocytes after infection with P falciparum, and parasite machinery was speculated to contribute to this process.24 Based on our present findings, this increase in the levels of PIPs may be attributed to the parasite exported PfPI3K.
PfPI3K inhibition leads to accumulation of hemoglobin in the parasite
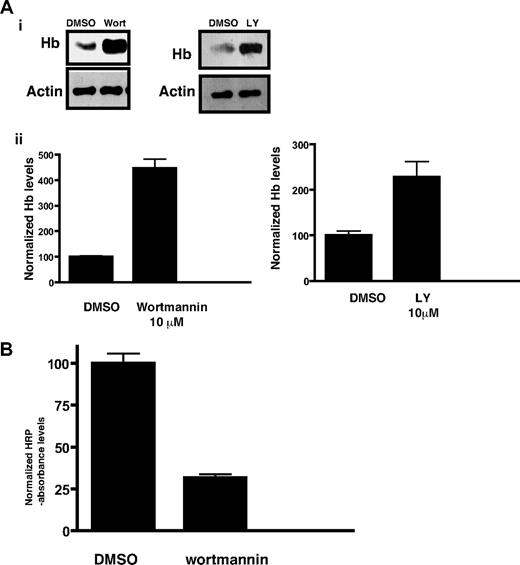
The endocytosis of hemoglobin from the host erythrocyte to the malaria parasite is a key process, which is important for parasite development. Morphologically, several cellular mechanisms have been implicated in hemoglobin trafficking to the parasite.25,26 However, molecular mechanisms that regulate hemoglobin trafficking are still unclear. Because PI3K and its products are important players in endocytosis and trafficking events in most eukaryotes, it was worth studying the role of PfPI3K in hemoglobin transport to the parasite. To investigate this, parasites were incubated with PI3K inhibitors and the amount of hemoglobin present inside the parasite was determined by Western blotting. Wortmannin- and LY294002-treated parasites exhibited significantly increased hemoglobin levels (Figure 3A) compared with the solvent control. In contrast, mefloquine, a known hemoglobin endocytosis inhibitor, significantly reduced hemoglobin levels (data not shown) as previously reported.12 Based on these observations, it is reasonable to suggest that PI3K inhibitors either cause a stimulation of hemoglobin endocytosis or attenuate hemoglobin digestion, which results in increased levels of hemoglobin in the parasite.
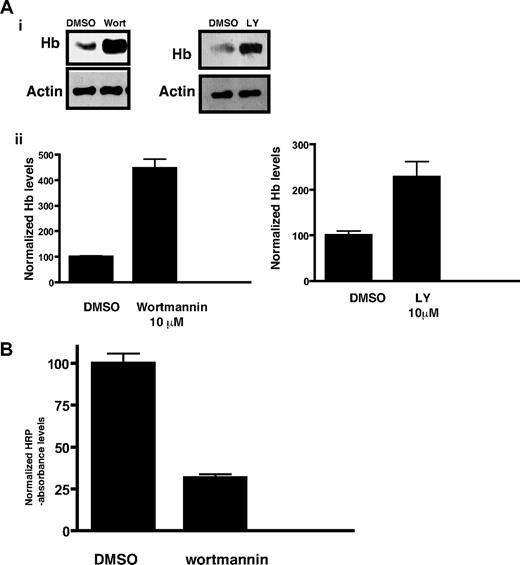
Hemoglobin accumulation in malaria parasites as result of PfPI3K inhibition. (A) Late ring stage parasites were incubated with DMSO (control), wortmannin, or LY294002 for approximately 5 hours. After releasing the parasite from the erythrocytes, parasite lysates were prepared and Western blotting performed using antihemoglobin antiserum or anti-actin antibody. (i) A representative Western blot from each experiment is shown. Actin was used as a loading control. (ii) The hemoglobin band intensity from Western blots performed on 3 independent experiments in which the parasite was treated with 10μM wortmannin or LY294002 was quantified by densitometry using Kodak 1D image analysis software and is represented by bar graphs. The intensity levels were normalized to the control set at 100, and comparisons were made. The error bars represent SEM. (B) Erythrocytes were preloaded with HRP, infected with parasites, and treated with 10μM wortmannin for 14 hours. After releasing parasites from infected erythrocytes by saponin treatment, HRP levels associated with parasites were determined with a colorimetric enzyme assay. Absorbance values were normalized to the solvent controls set at 100. Error bars represent SEM.
Hemoglobin accumulation in malaria parasites as result of PfPI3K inhibition. (A) Late ring stage parasites were incubated with DMSO (control), wortmannin, or LY294002 for approximately 5 hours. After releasing the parasite from the erythrocytes, parasite lysates were prepared and Western blotting performed using antihemoglobin antiserum or anti-actin antibody. (i) A representative Western blot from each experiment is shown. Actin was used as a loading control. (ii) The hemoglobin band intensity from Western blots performed on 3 independent experiments in which the parasite was treated with 10μM wortmannin or LY294002 was quantified by densitometry using Kodak 1D image analysis software and is represented by bar graphs. The intensity levels were normalized to the control set at 100, and comparisons were made. The error bars represent SEM. (B) Erythrocytes were preloaded with HRP, infected with parasites, and treated with 10μM wortmannin for 14 hours. After releasing parasites from infected erythrocytes by saponin treatment, HRP levels associated with parasites were determined with a colorimetric enzyme assay. Absorbance values were normalized to the solvent controls set at 100. Error bars represent SEM.
PfPI3K may be involved in uptake of material from the host
First, efforts were made to explore whether PfPI3K is involved in trafficking of material from the host. To this end, the uptake of HRP, a standard nondigestible fluid-phase endocytic tracer, was evaluated. Erythrocytes infected with mature parasites were enriched and incubated with HRP-loaded erythrocytes in culture to allow invasion of the latter. HRP uptake from the infected preloaded erythrocytes by parasite fluid-phase endocytosis was quantitated by colorimetrically measuring HRP enzymatic activity in isolated trophozoites. Wortmannin caused a significant decrease in HRP levels in parasites, indicating an inhibition of endocytosis (Figure 3B).
PI3K inhibitors block food vacuole trafficking of hemoglobin
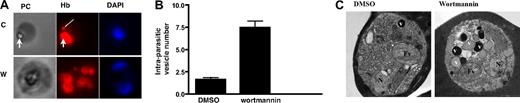
PfPI3K inhibition blocks endocytosis of HRP from the host (Figure 3B). Although this observation is indicative of PfPI3K involvement in host cell endocytosis, it does not explain the increase in the levels of hemoglobin levels on wortmannin treatment. To explore this further, changes in hemoglobin trafficking in the parasite were followed. After treatment with wortmannin, parasites were released from RBCs by saponin treatment, immobilized on coverslips, and fixed with paraformaldehyde/glutaraldehyde. Subsequently, immunofluorescence assays were performed with commercial anti-hemoglobin antiserum to determine the subcellular localization of hemoglobin in the parasite. Expectedly, hemoglobin staining was observed inside the food vacuole and occasional punctate structures in the cytoplasm, which most probably represent previously described hemoglobin-laden endocytic vesicles12,13 (Figure 4A). Treatment with wortmannin resulted in an apparent decrease in food vacuole hemoglobin. More strikingly, the inhibitor-treated parasites exhibited an increase in the number of hemoglobin-positive vesicles (Figure 4A). This was confirmed by counting the number of extra-digestive vacuolar fluorescent foci in randomly selected parasites. Parasites treated with wortmannin contained approximately 5-fold more vesicles (Figure 4B). Similar observations were made when LY294002 was used to inhibit PfPI3K activity (supplemental Figure 4). In contrast to these results, mefloquine treatment resulted in a marked decrease in the vesicle number (data not shown). The phase-contrast images indicated that hemozoin is still present in the food vacuoles of wortmannin-treated parasites. The hemozoin may represent crystals formed before the addition of wortmannin. In addition, it may suggest that the block in hemoglobin trafficking to the food vacuole is not complete and that hemoglobin is still delivered to the food vacuole for digestion and hemozoin formation, which may happen via additional processes that may operate independent of PfPI3K.
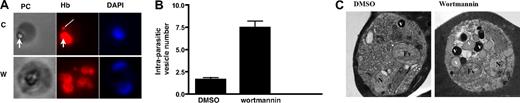
The effect of PfPI3K inhibition on hemoglobin trafficking. (A) Parasites were treated with DMSO or wortmannin. Saponin treatment was used to release excess nonparasitic hemoglobin, followed by attachment of parasites onto poly-lysine-coated glass cover slips. After fixation in paraformaldehyde/glutaraldehyde and Triton X-100 permeabilization, IFA was performed using anti-hemoglobin antibody, and 4,6-diamidino-2-phenylindole was used to stain the parasite nucleus. A single erythrocyte-free parasite can be seen in each phase-contrast image (PC) with its food vacuole. The thick arrow represents the collection of hemozoin crystals, which indicates the position of the food vacuole. Corresponding hemoglobin immunofluorescence images (Hb) contain punctate vesicle-like structures indicated by the thin arrow. (B) Average transport vesicle counts per parasite were compared between wortmannin and DMSO control. Error bars represent SEM. (C) Transmission electron micrographs of DMSO and 10μM wortmannin-treated trophozoite stage malaria parasite. Each micrograph shows a single parasite located inside a host erythrocyte. The cytoplasm of the host cell is darkly stained because of the preponderance of electron-dense hemoglobin. Labeled structures inside the parasites are the parasite nucleus (N), food vacuole (Fv), and hemoglobin transport vesicles (V).
The effect of PfPI3K inhibition on hemoglobin trafficking. (A) Parasites were treated with DMSO or wortmannin. Saponin treatment was used to release excess nonparasitic hemoglobin, followed by attachment of parasites onto poly-lysine-coated glass cover slips. After fixation in paraformaldehyde/glutaraldehyde and Triton X-100 permeabilization, IFA was performed using anti-hemoglobin antibody, and 4,6-diamidino-2-phenylindole was used to stain the parasite nucleus. A single erythrocyte-free parasite can be seen in each phase-contrast image (PC) with its food vacuole. The thick arrow represents the collection of hemozoin crystals, which indicates the position of the food vacuole. Corresponding hemoglobin immunofluorescence images (Hb) contain punctate vesicle-like structures indicated by the thin arrow. (B) Average transport vesicle counts per parasite were compared between wortmannin and DMSO control. Error bars represent SEM. (C) Transmission electron micrographs of DMSO and 10μM wortmannin-treated trophozoite stage malaria parasite. Each micrograph shows a single parasite located inside a host erythrocyte. The cytoplasm of the host cell is darkly stained because of the preponderance of electron-dense hemoglobin. Labeled structures inside the parasites are the parasite nucleus (N), food vacuole (Fv), and hemoglobin transport vesicles (V).
These data suggest that the inhibition of PfPI3K may cause defects in endocytic vesicle trafficking of hemoglobin to the food vacuole, the site of hemoglobin digestion. Hemoglobin may consequently be trapped in endocytic vesicles on PfPI3K inhibition, which prevents efficient transport to the food vacuole resulting in impaired catabolism.
To confirm the hemoglobin IFA results, wortmannin-treated parasites were further visualized by electron microscopy. As suggested by the IFA images (Figure 4A), treated parasites contained increased numbers of hemoglobin transport vesicles, often strikingly so in individual parasites (Figure 4C). The average vesicle number per parasite profile increased from 1.58 (± 0.12) to 3.83 (± 0.23). There appeared to be a concurrent increase in vesicle diameter, from an average of 322 nm (± 33 nm) in controls to 543 nm (± 26 nm) in treated parasites. Vesicle size increase was also suggested by the IFA images (Figure 4A, compare “C” with “W”). Closer inspection of the electron microscope images presented here shows that the food vacuoles in the parasites are completely filled with densely packed, parallel elongated hemozoin crystals. The presence of less electron dense or relatively void vesicles in the wortmannin-treated parasites is an interesting phenomenon that was also observed in parasites treated with actin-disrupting compounds (cytochalasin) that similarly impede vesicle delivery to the food vacuole.13 One possible explanation is that hemoglobin digestion is already initiated in hemoglobin vesicles and proceeds in individual wortmannin-induced stationary vesicles to the extent where hemoglobin content is visibly reduced.
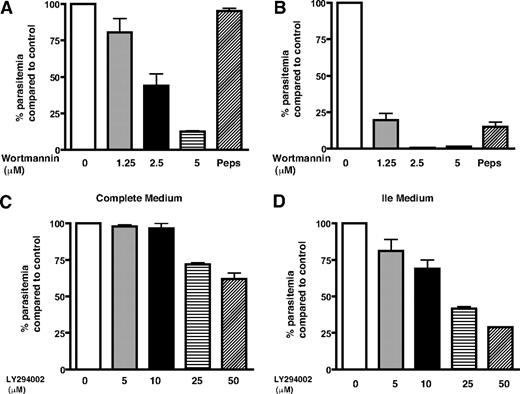
Inhibition of PfPI3K-mediated transport blocks parasite growth
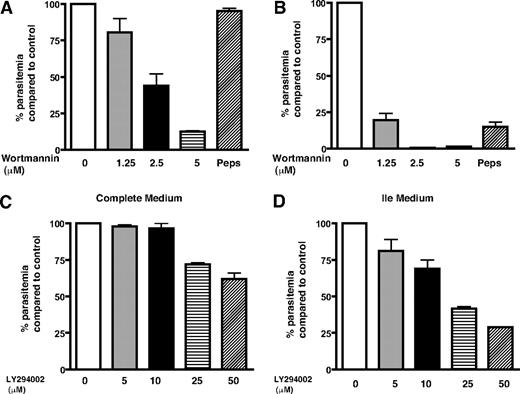
The endocytosis of hemoglobin to the food vacuole and its catabolism is important for parasite growth because it is a major source of most amino acids for the parasite. When hemoglobin proteolysis is blocked, the parasite can get the supply of amino acids from extracellular milieu.27 Isoleucine is the only amino acid that is not present in human hemoglobin, and the parasite relies almost completely on extracellular medium for its acquisition.27,28 Because hemoglobin endocytosis is a prerequisite for its degradation in the parasite food vacuole and PfPI3K regulates hemoglobin uptake, we checked whether wortmannin and LY294002 alter the parasite growth. These experiments were done simultaneously in 2 sets; in one set, the parasite was cultured in complete RPMI 1640 medium; in the other, a medium lacking all amino acids except Ile (Ile medium) was used. In the latter situation, parasite was largely dependent on hemoglobin as a source for other amino acids.27 The parasites were cultured in the presence of wortmannin, LY294002, or pepstatin A, an aspartic protease inhibitor that is known to stall parasite growth.27 It was evident that the growth of parasites, when cultured in the complete medium for 6 days in the presence of wortmannin, was inhibited significantly (Figure 5A); approximately 2.5μM wortmannin caused approximately 50% reduction in parasite growth. When parasites were treated with wortmannin in Ile medium, a strikingly higher sensitivity toward wortmannin was observed. The parasite growth was almost completely blocked by approximately 2.5μM wortmannin (Figure 5B). Similar trends were observed with LY294002, and approximately 25μM of this inhibitor could cause approximately 60% inhibition in parasite growth in Ile medium (Figure 5C-D). The amount of LY294002 needed to inhibit parasite growth was higher, which corroborates with its lower affinity toward PfPI3K (Figure 1D). As observed for wortmannin, LY294002 was also more potent in Ile medium. Treatment with protease inhibitor pepstatin A resulted in similar trends and indicated that this inhibitor was also more effective in Ile medium as reported previously.27 The parasite relies on hemoglobin degradation for amino acid supply in Ile medium, which may be a plausible reason for higher inhibitor sensitivity exhibited by the parasite in this medium. Based on these data, we conclude that PfPI3K may control trafficking pathway(s) that are involved in hemoglobin transport to the parasite food vacuole (Figure 6), a crucial step for its catabolism and therefore may be necessary for parasite growth.
PfPI3K inhibition stalls parasite growth. Parasite cultures in complete medium (A,C) or Ile medium (B,D) were incubated with DMSO/MeOH (control), indicated concentrations of wortmannin (A-B), LY294002 (C-D), or 30μM pepstatin A (Peps). Parasite growth was monitored by counting the parasite infected erythrocytes every 48 hours, the data obtained after 4 (C-D) or 6 (A-B) days of treatment are shown. The percentage growth of parasite in drug-treated cultures compared with control (100%) is indicated. Error bars represent SEM.
PfPI3K inhibition stalls parasite growth. Parasite cultures in complete medium (A,C) or Ile medium (B,D) were incubated with DMSO/MeOH (control), indicated concentrations of wortmannin (A-B), LY294002 (C-D), or 30μM pepstatin A (Peps). Parasite growth was monitored by counting the parasite infected erythrocytes every 48 hours, the data obtained after 4 (C-D) or 6 (A-B) days of treatment are shown. The percentage growth of parasite in drug-treated cultures compared with control (100%) is indicated. Error bars represent SEM.
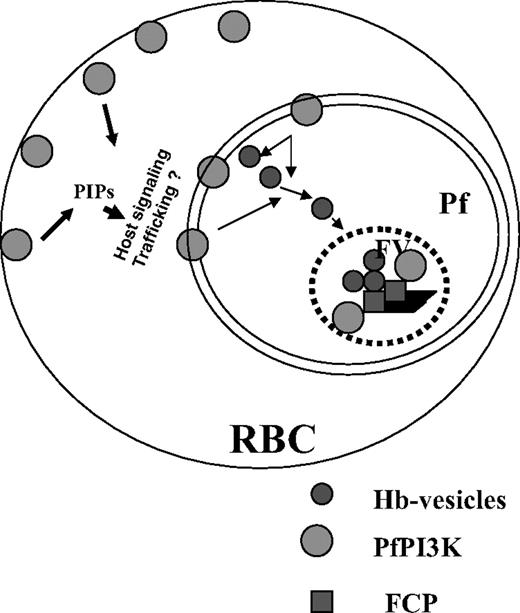
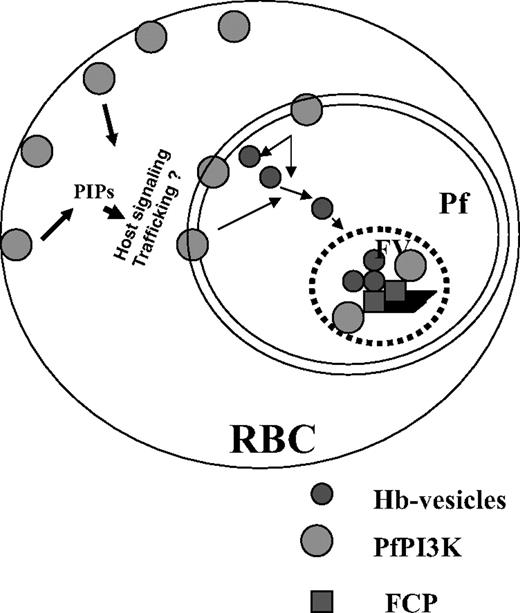
A model for the role and regulation of PfPI3K in malaria parasite. PfPI3K is trafficked to vesicular compartments at PM/PVM and the food vacuole, and exported to the host RBCs. It may regulate the function of FCP, a PI3P binding protein, which is present in the food vacuole.8 PfPI3K inhibition blocks endocytosis of hemoglobin to the food vacuole and blocks parasite growth. Because PfPI3K exported to the host erythrocyte is active, it may trigger signaling and trafficking pathways that may regulate additional events.
A model for the role and regulation of PfPI3K in malaria parasite. PfPI3K is trafficked to vesicular compartments at PM/PVM and the food vacuole, and exported to the host RBCs. It may regulate the function of FCP, a PI3P binding protein, which is present in the food vacuole.8 PfPI3K inhibition blocks endocytosis of hemoglobin to the food vacuole and blocks parasite growth. Because PfPI3K exported to the host erythrocyte is active, it may trigger signaling and trafficking pathways that may regulate additional events.
Discussion
The presence of enzymes, such as PI synthase,24 PfPI4P5K,7 PfPI3K, and other PIP kinases, in Plasmodium suggests that PIPs may have a major role to play in parasite signaling and trafficking as is the case in most eukaryotes. 3′-Phosphorylated PIPs generated by the action of PI3Ks on PI, PI4P, and/or PI(4,5)P2 are major players in this process.3,29 PfPI3K seems to be the only PI3K in P falciparum and shares characteristics with class III PI3Ks, such as vps34. In contrast to vps34, which generates mainly PI3P from PIP,3,5 PfPI3K phosphorylates both PI and PI4P to yield PI3P, PI(3,4)P2. It was recently reported that attempts to disrupt PfPI3K homolog gene in P berghei were not successful, and it was suggested to be essential for the parasite growth.17 Based on this, it is reasonable to assume that PfPI3K may have a similar role in P falciparum, which was supported by the inhibition of parasite growth and hemoglobin trafficking by wortmannin and LY294002. It was reported that PI3P is almost absent in uninfected erythrocytes; however, its levels increase significantly in infected erythrocytes,17,24 which was attributed to the parasite but not the host machinery.24 Our results indicate that PfPI3K is exported to erythrocytes, and the detection of PfPI3K activity in the host erythrocyte may provide a probable explanation for this observation.
In Dictyostelium, PI3K is required for macromolecule uptake by macropinocytosis,30 whereas wortmannin and LY294002 have been reported to inhibit fluid-phase endocytosis within mammalian cells.31 PI3Ks also act as regulators of phagocytosis and are essential players in phagosome formation and maturation.32 Based on the reported participation of PI3K endocytic events in other eukaryotes, we investigated whether PI3K inhibition would down-regulate endocytosis of host cytoplasm in malaria parasites. Consistent with this hypothesis, parasite HRP accumulation through fluid-phase endocytosis from infected host erythrocytes was decreased by wortmannin. However, earlier Western blotting results had indicated an increase in hemoglobin content in parasites exposed to the inhibitors, suggesting additional effects on the parasite endocytic pathway and/or digestion of endocytosed material in the food vacuole. Immunofluorescence localization of hemoglobin in wortmannin-treated parasites showed a significant increase in endocytic hemoglobin transport vesicles, suggestive of a block in endocytic trafficking. LY294002, another inhibitor of PI3Ks, which also inhibits PfPI3K, caused similar defects on hemoglobin trafficking, providing further support to these observations (supplemental Figure 4). However, localization of PfPI3K itself did not seem to alter significantly on treatment with wortmannin (supplemental Figure 3). It is possible that the inhibition of hemoglobin transport and delivery to the food vacuole impair the digestion of internalized hemoglobin in the latter compartment and produce the increase in hemoglobin levels in the treated parasites. This conclusion is consistent with the reported function of PI3Ks in postendosomal sorting and degradation in mammalian cells,33 genetic evidence for the role of PI3Ks in sorting to yeast vacuoles,34 and PI3K involvement in phagosome maturation.32 In addition, the association of PfPI3K and downstream effectors, such as FCP, with the parasite food vacuole may have direct bearing on the inability of inbound hemoglobin transport vesicles to fuse with this compartment in the presence of PI3K inhibitors.
Interestingly, previous studies have reported a similar role for actin in hemoglobin trafficking in malaria parasites, evidenced by an increase in volume and number of hemoglobin-filled vesicles in parasites treated with actin disruptors.13 PfPI3K also appears to affect hemoglobin trafficking. The principal mode of hemoglobin endocytosis in trophozoites is via cytostomes, invaginations of the parasite plasma membrane characterized by an electron-dense collar at the neck. In a manner reminiscent of classic clathrin-dependent endocytosis in mammalian cells, the cytostomes presumably pinch off to form endocytic vesicles that traffic hemoglobin to the digestive vacuole in an actin and PfPI3K-dependent manner. A detailed ultrastructural study of endocytosis identified 3 additional modes of hemoglobin uptake that supplement the cytostome pathway: a “big gulp” in the ring stage that forms a nascent digestive vacuole, large hemoglobin-filled “phagotrophs” that are probably formed by a cytostome-independent mechanism and elongated “cytostomal tubes” that are morphologically distinct from the more spherical standard hemoglobin transport vesicles.25 We have not attempted to distinguish between these modes in addressing the effects of PfPI3K, but overt evidence of cytostomal tubes and phagotrophs in addition to the hemoglobin transport vesicles in our IFA and electron-microscopic images was not found. In contradiction to the aforementioned ultrastructural study, an alternative model of the hemoglobin endocytic pathway was proposed, in which the cytostomes do not pinch off to form independent vesicles but elongate and fuse directly with the digestive vacuole.26 By extension, an increase in “vesicle” number in parasites compromised in actin or PfPI3K activity would represent an increase in the convolutions of individual cytostomes. It is possible that additional PI3K or actin-independent pathways for hemoglobin trafficking may exist. It will be interesting to identify which of the aforementioned mechanisms of hemoglobin uptake is dependent on PfPI3K.
In addition to actin, hemoglobin trafficking may also be dependent on Rab5a, a known mediator of mammalian early endosome function, although the detailed underlying molecular mechanisms remain unknown.25 It is known that Rab GTPases participate in PI3K-mediated vesicular trafficking in several cell types, and several members of the Rab family are encoded in Plasmodium genome.35,36 Therefore, it will be worth testing whether PfPI3K controls hemoglobin endocytosis via Rabs.
Hemoglobin catabolism, which is important for the parasite growth, is carried out by aspartic proteases plasmepsins and cysteine protease falcipains.37 Interestingly, gene disruption of several of these enzymes only marginally slowed the parasite growth.27 It was demonstrated that the parasite could propagate in a medium containing only a single amino acid, Ile, which is missing from human hemoglobin.27 In this situation, it is dependent on hemoglobin degradation for supply of amino acids. Several protease “knockout” lines and protease inhibitor treatment exhibited marked impairment of parasite growth in this medium. Because wortmannin and LY294002 inhibit parasite growth with significantly higher efficacy in Ile medium, the hemoglobin trafficking may be a prominent PfPI3K-dependent process. Indeed, it is possible that PfPI3K may play additional roles in parasite development (summarized in Figure 6), which will be worth investigating.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Amit Sharma and Prof Nirbhay Kumar for VARC and PfHSP70 antisera, respectively, as well as Garima Verma and Justin Vijay.
This work was supported in part by the Swaranajayanti fellowship and the Wellcome Trust Senior Research Fellowship (P.S.). This work was also supported in part by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (grant R01AI075459). A.V. and R.R. were supported by the Junior/Senior Research Fellowship by the Council for Scientific and Industrial Research, India.
National Institutes of Health
Authorship
Contribution: A.V., R.R., and W.A.S planned and performed experiments and analyzed the data; and H.C.H. and P.S. planned the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pushkar Sharma, Eukaryotic Gene Expression Laboratory, National Institute of Immunology, Aruna Asaf Ali Marg, New Delhi 110067, India; e-mail: pushkar@nii.ac.in.
References
Author notes
A.V. and R.R. contributed equally to this study.