Abstract
Intrinsic apoptosis defects underlie to a large extent the extended survival of malignant B cells in chronic lymphocytic leukemia (CLL). Here, we show that the Shc family adapter p66Shc uncouples the B-cell receptor (BCR) from the Erk- and Akt-dependent survival pathways, thereby enhancing B-cell apoptosis. p66Shc expression was found to be profoundly impaired in CLL B cells compared with normal peripheral B cells. Moreover, significant differences in p66Shc expression were observed in patients with favorable or unfavorable prognosis, based on the mutational status of IGHV genes, with the lowest expression in the unfavorable prognosis group. Analysis of the expression of genes implicated in apoptosis defects of CLL showed an alteration in the balance of proapoptotic and antiapoptotic members of the Bcl-2 family in patients with CLL. Reconstitution experiments in CLL B cells, together with data obtained on B cells from p66Shc−/− mice, showed that p66Shc expression correlates with a bias in the Bcl-2 family toward proapoptotic members. The data identify p66Shc as a novel regulator of B-cell apoptosis which attenuates BCR-dependent survival signals and modulates Bcl-2 family expression. They moreover provide evidence that the p66Shc expression defect in CLL B cells may be causal to the imbalance toward the antiapoptotic Bcl-2 family members in these cells.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common B-cell neoplasm in Europe and the United States, characterized by progressive accumulation of monoclonal CD5+ B cells in peripheral blood, bone marrow, and peripheral lymphoid organs. The clinical course of CLL is highly variable, ranging from an indolent disease that may never require treatment to a rapidly progressive disease.1 One of the principal prognostic features is the mutational status of the immunoglobulin heavy chain variable region genes (IGHV). CLL with poor prognosis has unmutated IGHV genes (U-CLL), whereas CLL with good prognosis carries somatic mutations in these genes (M-CLL).2,3
Neoplastic CLL cells are typically arrested in the G0/G1 phase of the cell cycle and accumulate in tissues because of prolonged survival.1 Although extrinsic factors, such as escape from immune surveillance and chemotaxis to a favorable microenvironment, contribute to the extended survival of CLL B cells,4 CLL is primarily a disease of defective apoptosis, and intrinsic defects in a number of components of the apoptotic circuitry have been identified, including overexpression of antiapoptotic proteins (eg, Bcl-2, Mcl-1, XIAP) and reduction in the expression of proapoptotic proteins (eg, Bax, DAPK-1).5-7 Other apoptosis defects, including abnormalities in the p53 and ATM pathways in the most aggressive subsets, have also been implicated in the prolonged survival of malignant cells.8-10 In this respect, apoptosis is emerging as a key therapeutic target in CLL, as witnessed by the ongoing clinical trials on Bcl-2 inhibitors.11
p66Shc, a member of the Shc family of protein adapters, acts as antagonist of mitogenic signaling and positive regulator of oxidative stress-induced apoptosis in fibroblasts.12,13 In T cells, where its expression is epigenetically controlled,14,15 p66Shc uncouples the T-cell receptor from activation of the Ras/mitogen-activated protein kinase pathway by competitively inhibiting recruitment to the T-cell receptor of the mitogenic isoform, p52Shc.16 Furthermore, p66Shc enhances T-cell susceptibility to apoptotic stimuli by increasing reactive oxygen species (ROS) production and impairing Ca2+ homeostasis.17 We have recently reported that p66Shc is expressed in murine B cells and that p66Shc deficiency results in enhanced proliferative responses of mouse B cells to B-cell receptor (BCR) engagement,18 suggesting that p66Shc may display similar activities in T and B cells.
Here, we have investigated the role of p66Shc in B-cell survival. We show that p66Shc promotes B-cell apoptosis by uncoupling the BCR from the survival pathways mediated by Akt and Erk. On the basis of these findings, we have investigated p66Shc expression and function in B cells from patients with CLL. The results identify a role for p66Shc in the imbalance among proapoptotic and antiapoptotic Bcl-2 family members in CLL and highlight an association of low p66Shc expression with unfavorable prognosis.
Methods
Patients and healthy donors
Peripheral blood mononuclear cells were obtained from 21 healthy donors, 32 patients with M-CLL, and 32 patients with U-CLL, diagnosed at the Division of Hematology and Bone Marrow Transplantation of the University of Siena according to the 2008 guidelines from the International Workshop on CLL.19 All patients had never received prior treatments and had a typical CLL phenotype.20 All patients had provided informed consent in accordance with local requirements of institutional review boards and Declaration of Helsinki.
Mononuclear cells were purified from peripheral blood by density gradient centrifugation (800g for 20 minutes at room temperature) on Ficoll-Paque (Amersham Biosciences) and were depleted of monocytes by adherence. B cells were purified from healthy donors by positive selection with the use of CD19 pan-B Dynabeads (Dynal Biotech). The purity of the resulting B-cell population was greater than 90%, as evaluated by staining with anti-CD3 phycoerythrin (PE)–conjugated antibody and anti-CD19 fluorescein (FITC)–conjugated antibody (BD Biosciences). Peripheral blood lymphocytes (PBLs) from patients with CLL were not further purified, because staining with fluorochrome-labeled anti-CD3/CD19 antibodies consistently showed a high percentage of B cells (> 85%).
Mice
p66Shc−/− mice in the 129 genetic background were previously described13 ; 129 mice were used as controls. The work was carried out on the p66Shc−/− and 129 mouse colonies bred in the animal facility at the University of Siena. Analyses were performed on 2- to 9-month-old mice. Wild-type and p66Shc−/− mice used in each experiment were matched for age and sex. All animal experiments were carried out in agreement with the Guiding Principles for Research Involving Animals and Human Beings and were approved by the University of Siena Ethical Committee.
Mice were killed by cervical dislocation. Splenic B cells were purified with the use of the SpinSep Mouse B-cell Enrichment Kit (StemCell Technologies) and subsequently checked by flow cytometry with anti-CD3 FITC-conjugated antibody and anti-CD22 PE-conjugated antibody (BD Biosciences). After the purification the percentage of B cells was greater than 85%.
RNA isolation, reverse transcription, and real-time quantitative PCR
Total RNA was extracted from B cells from healthy donors, patients with CLL, and mice with the use of Tri Reagent (Ambion). Reverse transcription–polymerase chain reaction (RT-PCR) was carried out with the use of ImProm-II reverse transcriptase and Taq DNA polymerase (Promega Italia srl). The primer for the first strand was oligo-dT (Promega Italia srl), whereas pairs of specific primers were used for cDNA amplification. Two independent reverse transcription reactions were performed on each RNA sample. Real-time PCR was performed in duplicate on each cDNA with the use of the Sybr Green I SensiMix dT Kit according to the manufacturer's instructions (Quantace), using an Opticon 2 (MJ Research; Bio-Rad Laboratories). All duplicate samples were run on 96-well optical PCR plates (Roche Diagnostics). Duplicate cDNA samples from the same healthy donor (or the same B-cell pool from wild-type mice in the quantitative RT-PCR (qRT-PCR) analyses of p66Shc−/− B cells, or lymphocytes from the same reference patient transfected with empty vector in the reconstitution experiments) were included in all plates as a reference for normalization of the results obtained in individual experiments. After an initial denaturation for 10 minutes at 95°C, denaturation in the subsequent 40 cycles was performed for 15 seconds at 95°C, followed by 15-second primer annealing at 60°C and a final extension at 72°C for 30 seconds. The starting copy number of the unknown samples was determined using the comparative ΔΔ CT method, as previously described.21 Levels of the different transcripts were normalized to GAPDH, used as housekeeping gene. Semiquantitative RT-PCR was also performed on cDNAs from a small subset of healthy donors. PCR reactions were performed with the use of Taq DNA polymerase by Roche Diagnostics in an Eppendorf Mastercycler Thermal Cycler (Eppendorf srl). 18S was used as housekeeping control. RT-PCR products were separated by agarose gel electrophoresis, and gel images were acquired by laser densitometry (Duoscan T2500 Agfa).
The primers used to amplify cDNA fragments corresponding to human and mouse transcripts are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Cell activations and immunoblots
Ramos B cells were cultured for 2 hours in low serum (RPMI1640 supplemented with 0.5% fetal calf serum) and subsequently activated by incubation at 37°C in serum-free medium in the presence of 2.5 μg/mL goat anti–human immunoglobulin M (IgM) antibodies (Jackson Immunoresearch Laboratories). Mouse B cells were activated by adding 30 μg/mL goat F(ab)2 fragment to mouse IgM (Cappel; MP Biomedicals Europe).
Cells (5 × 106/sample) were lysed in 1% Triton X-100 in 20mM Tris-HCl (pH 8), 150mM NaCl (in the presence of a protease inhibitor cocktail). Immmunoblot analysis of postnuclear supernatants was carried out by chemiluminescence (SuperSignal; West Pico Chemiluminescent Substrate kit; Pierce Chemical) using as primary antibodies phosphospecific antibodies recognizing the active forms of Syk (pYY525/526), Erk1/2 (pT202/Y204), or Akt (pS473; Cell Signaling Technology), and secondary peroxidase-labeled antibodies (Amersham Pharmacia Biotech). Anti-Syk, anti-Erk1/2, and anti–Bcl-2 antibodies were purchased from Santa Cruz Biotechnology, anti-Akt antibodies were purchased from Cell Signaling Technology. Anti-Shc and anti-Bak antibodies were purchased from Upstate Biotechnology, anti–Bcl-x and anti-Bax antibodies were purchased from BD Biosciences. Immunoblots were scanned using a laser densitometer (Duoscan T2500 Agfa) and quantified with ImageQuant 5.0 software (Molecular Dynamics). Data were normalized to loading controls.
Flow cytometry
Apoptosis was measured by flow cytometric analysis of FITC-labeled annexin V (BD Biosciences) stained cells. Ramos cell transfectants were resuspended at 106/mL in medium supplemented with 0.5% serum and incubated at 37°C either as such or in the presence of 2.5 μg/mL goat anti–human IgM antibodies (Jackson Immunoresearch Laboratories). Splenic mouse cells were resuspended at 2 × 106/mL in serum-free medium and either incubated as such or activated with 40 μg/mL goat IgG fraction against mouse Ig (Cappel; MP Biomedicals Europe). Cells were analyzed after 24 and 48 hours, gating for the mouse experiments on CD22+ cells.
Flow cytometry was carried out with the use of a FACScan flow cytometer (BD Bioscience). Data were analyzed and plotted with the use of FlowJo software (TreeStar Inc).
Transfections of Ramos and CLL B cells
A mammalian expression vector (pcDNA3; Invitrogen srl) encoding human full-length p66Shc was introduced into Ramos B cells by electroporation. A control line was generated with the use of an empty vector. Stably transfected cells were selected in medium containing 1 mg/mL G418 (Gibco BRL, Life Technologies Italia srl) and checked for surface Ig expression by flow cytometry with the use of FITC-labeled anti–human Ig antibodies (Sigma Aldrich). sIg expression was comparable in the 2 lines (data not shown).
Fresh peripheral blood B cells from 7 patients with CLL (4 M-CLL and 3 U-CLL) were transiently cotransfected with 1 μg of green fluorescence protein (GFP) reporter/sample and 5 μg of empty vector (pcDNA3) or the same vector encoding p66Shc, using the Human B-cell Nucleofector Kit (Amaxa Biosystems) and the conditions recommended by the manufacturer. Twenty-four hours after transfection, cells were recovered, the RNA was extracted (Tri reagent protocol) and retrotranscribed, and the cDNAs were used for real-time quantitative PCR as described in “RNA isolation, reverse transcription, and real-time quantitative PCR.” For 3 of these patients, cells were also lysed to obtain postnuclear supernatants. Flow cytometric analysis of PBLs transfected with the Amaxa control GFP reporter and subsequently labeled with anti–CD19 PE-conjugated antibody (BD Biosciences) confirmed that only CD19+ cells had been transfected and showed that the transfection efficiency was consistently 40% or greater. Endotoxin-free plasmid DNA was purified with the use of the EndoFree Plasmid Maxi kit from QIAGEN Gmbh.
Analysis of promoter methylation and gene sequencing
Genomic DNA was extracted from PBLs from 11 patients with CLL (9 M-CLL and 2 U-CLL) and 5 healthy donors, using the Wizard SV Genomic DNA Purification System (Promega Italia srl) and subjected to sodium bisulfite treatment with the use of the EZ DNA methylation kit (Zymo Research). The treated DNA was subjected to PCR with the use of the GoTaq DNA polymerase (Promega Italia srl) and primers designed to amplify the p66Shc enhancer region (supplemental Table 1). The PCR products were cloned into the TOPO TA Cloning Kit Dual Promoter (Invitrogen srl); plasmid DNA was recovered from individual colonies and subjected to automated sequencing. At least 10 clones from independent PCR products were sequenced for each donor.
Exon 1 and a region upstream of the coding sequence, spanning positions −542 to +685 of p66Shc, were amplified from genomic DNA extracted from PBLs from 17 patients with CLL by PCR. PCR products were subjected to automated sequencing (Bio-Fab Research srl). The primers used for all sequence analyses are listed in supplemental Table 1.
Data analysis and statistics
Mean values, standard deviation values, and the Student t test (unpaired) were calculated with the Microsoft Excel application. Clinical and molecular categorical variables were compared by the Fisher exact test or the χ2 test, as appropriate. A level of P less than .05 was considered statistically significant. Overall survival (OS) and treatment-free interval (TFI) were chosen as indicators of clinical behavior and used to scan prognostic parameters of statistical significance. OS was measured from date of diagnosis to date of last follow-up or death. TFI was measured from date of diagnosis to date of progressive and/or symptomatic disease requiring first treatment according to guidelines from the National Cancer Institute.22 Statistical analyses were performed with the R statistical package (http://www.r-project.org/). Survival analyses were performed by the Kaplan-Meier method with the global log-rank test for significant (P < .05) associations.23
Results
p66Shc promotes B-cell apoptosis by antagonizing survival signals
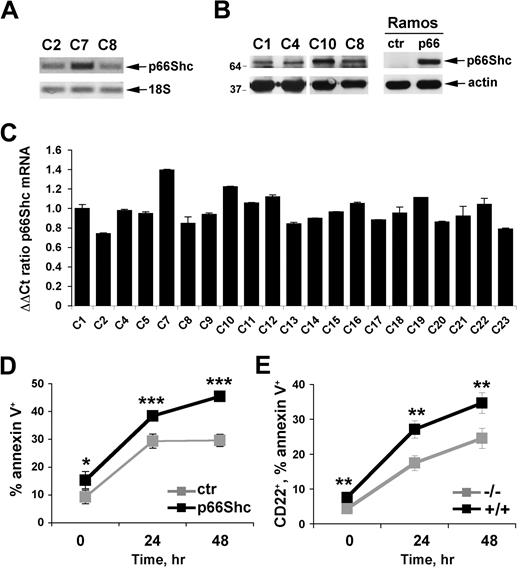
Expression of p66Shc was measured in B cells purified by immunomagnetic sorting from human PBLs. A p66Shc-specific transcript, the identity of which was confirmed by automated sequencing (data not shown), was identified by semiquantitative RT-PCR (Figure 1A). p66Shc was found to be expressed at comparable levels in purified peripheral B cells from 21 healthy donors, as assessed by real-time qRT-PCR (Figure 1C). p66Shc expression was confirmed at the protein level by immunoblot analysis of B-cell lysates with anti-Shc antibodies (Figure 1B).
p66Shc acts as a negative regulator of B-cell survival. (A) Detection of p66Shc-specific mRNA in peripheral blood B cells purified from healthy donors by semiquantitative RT-PCR. The identity of the band was confirmed by automated sequencing. (B) Immunoblot analysis of p66Shc expression in lysates of peripheral blood B cells purified from 4 representative healthy donors. Lysates of Ramos B cells stably transfected with either empty vector (ctr) or the same vector encoding human p66Shc (p66) are shown on the right. Stripped filters were reprobed with anti-actin antibodies as loading control. (C) Quantification by real-time RT-PCR of the levels of p66Shc mRNA in peripheral blood B cells purified from 21 healthy donors. Transcript levels were normalized to the expression level of GAPDH. For each donor, Syber green runs were performed on duplicate samples of cDNAs from 2 independent reverse transcription reactions, each carried out on 400 ng of total RNA. RNA from the same healthy donor (C1) was included in duplicate in each run as reference to normalize the data obtained in the individual experiments. The ΔΔCT method was applied as a comparative method of quantification, using as a reference the average of all the Ct data obtained on donor C1. The histograms show the mean ± SD of the 4 reactions carried out for each RNA. Statistical analysis (Student t test) did not show any significant difference in p66Shc mRNA levels among healthy donors. (D) Flow cytometric analysis of annexin V staining on Ramos B cells stably transfected with either empty vector (ctr) or the same vector encoding human p66Shc (p66), activated for 24 and 48 hours with anti-IgM antibodies. The graph shows the percentage (mean value ± SD) of annexin V+ cells (n = 3). (E) Flow cytometric analysis of annexin V staining of splenocytes from control (+/+) or p66Shc−/− (−/−) mice activated for 24 and 48 hours with goat IgG fraction specific for mouse immunoglobulins (40 μg/mL). The analysis was carried out on gated CD22+ cells. The graph shows the percentage (mean value ± SD) of annexin V+ splenocytes (n = 4; ***P ≤ .001, **P ≤ .01, *P ≤ .05).
p66Shc acts as a negative regulator of B-cell survival. (A) Detection of p66Shc-specific mRNA in peripheral blood B cells purified from healthy donors by semiquantitative RT-PCR. The identity of the band was confirmed by automated sequencing. (B) Immunoblot analysis of p66Shc expression in lysates of peripheral blood B cells purified from 4 representative healthy donors. Lysates of Ramos B cells stably transfected with either empty vector (ctr) or the same vector encoding human p66Shc (p66) are shown on the right. Stripped filters were reprobed with anti-actin antibodies as loading control. (C) Quantification by real-time RT-PCR of the levels of p66Shc mRNA in peripheral blood B cells purified from 21 healthy donors. Transcript levels were normalized to the expression level of GAPDH. For each donor, Syber green runs were performed on duplicate samples of cDNAs from 2 independent reverse transcription reactions, each carried out on 400 ng of total RNA. RNA from the same healthy donor (C1) was included in duplicate in each run as reference to normalize the data obtained in the individual experiments. The ΔΔCT method was applied as a comparative method of quantification, using as a reference the average of all the Ct data obtained on donor C1. The histograms show the mean ± SD of the 4 reactions carried out for each RNA. Statistical analysis (Student t test) did not show any significant difference in p66Shc mRNA levels among healthy donors. (D) Flow cytometric analysis of annexin V staining on Ramos B cells stably transfected with either empty vector (ctr) or the same vector encoding human p66Shc (p66), activated for 24 and 48 hours with anti-IgM antibodies. The graph shows the percentage (mean value ± SD) of annexin V+ cells (n = 3). (E) Flow cytometric analysis of annexin V staining of splenocytes from control (+/+) or p66Shc−/− (−/−) mice activated for 24 and 48 hours with goat IgG fraction specific for mouse immunoglobulins (40 μg/mL). The analysis was carried out on gated CD22+ cells. The graph shows the percentage (mean value ± SD) of annexin V+ splenocytes (n = 4; ***P ≤ .001, **P ≤ .01, *P ≤ .05).
To gain insight into the function of p66Shc in B cells, we generated a stable B-cell transfectant overexpressing p66Shc, using as recipient Ramos B cells, which do not express p66Shc at detectable levels (Figure 1B). A control line was generated with the use of an empty vector. The potential effect of p66Shc expression on B-cell apoptosis was assessed by annexin V staining of control and p66Shc-expressing Ramos B cells stimulated to undergo apoptosis by prolonged BCR cross-linking. p66Shc expression was found to correlate with enhanced B-cell apoptosis (Figure 1D). Consistent with the impairment in cell survival, BCR-dependent phosphorylation of Akt, the central effector in the phosphatidylinositol-3 kinase survival pathway, was significantly reduced in p66Shc-expressing Ramos cells compared with controls (Figure 2A). Similar results were obtained for Erk, which mediates mitogenic and survival signals triggered by the BCR downstream of Ras activation (Figure 2A). Inhibition of BCR signaling by p66Shc occurs at an early step in the cascade, because BCR-dependent phosphorylation of the key initiating protein tyrosine kinase, Syk, was impaired in the presence of p66Shc (Figure 2A).
p66Shc attenuates BCR signaling. (A left) Immunoblot analysis of Akt, Erk1/2, and Syk phosphorylation in postnuclear supernatants from the control and p66Shc-expressing Ramos B-cell transfectants activated with anti-IgM antibodies for the indicated times. (Right) Quantification by laser densitometry of the relative levels of Akt, Erk1/2, and Syk phosphorylation in control and p66Shc-expressing Ramos cells activated with anti-IgM antibodies. Data are expressed as the percentage of maximal activation in control cells (set as 100%; n = 3; ***P ≤ .001, **P ≤ .01, *P ≤ .05), where significance refers to the differences between p66Shc-expressing and control Ramos cells at each time point. (B top) Immunoblot analysis of Akt, Erk1/2, and Syk phosphorylation in postnuclear supernatants from splenocytes from wild-type and p66Shc−/− mice activated with anti–mouse IgM antibodies for 2 minutes and 5 minutes (Syk), 5 minutes (Erk1/2), or 30 minutes (Akt). (Bottom) Quantification of the relative levels of Akt, Erk1/2, and Syk phosphorylation in splenocytes activated with anti-IgM antibodies (Syk, 2 minutes time point). Data are expressed as the percentage of maximal activation in splenocytes from wild-type mice (set as 100%; n > 3; ***P ≤ .001, **P ≤ .01, *P ≤ .05). Error bars indicate SD.
p66Shc attenuates BCR signaling. (A left) Immunoblot analysis of Akt, Erk1/2, and Syk phosphorylation in postnuclear supernatants from the control and p66Shc-expressing Ramos B-cell transfectants activated with anti-IgM antibodies for the indicated times. (Right) Quantification by laser densitometry of the relative levels of Akt, Erk1/2, and Syk phosphorylation in control and p66Shc-expressing Ramos cells activated with anti-IgM antibodies. Data are expressed as the percentage of maximal activation in control cells (set as 100%; n = 3; ***P ≤ .001, **P ≤ .01, *P ≤ .05), where significance refers to the differences between p66Shc-expressing and control Ramos cells at each time point. (B top) Immunoblot analysis of Akt, Erk1/2, and Syk phosphorylation in postnuclear supernatants from splenocytes from wild-type and p66Shc−/− mice activated with anti–mouse IgM antibodies for 2 minutes and 5 minutes (Syk), 5 minutes (Erk1/2), or 30 minutes (Akt). (Bottom) Quantification of the relative levels of Akt, Erk1/2, and Syk phosphorylation in splenocytes activated with anti-IgM antibodies (Syk, 2 minutes time point). Data are expressed as the percentage of maximal activation in splenocytes from wild-type mice (set as 100%; n > 3; ***P ≤ .001, **P ≤ .01, *P ≤ .05). Error bars indicate SD.
To further address the role of p66Shc in B-cell survival, the apoptotic response to BCR cross-linking was analyzed on splenic B cells purified from p66Shc−/− mice and wild-type controls. A significant reduction in apoptosis was observed in p66Shc−/− B cells compared with controls (Figure 1E), which correlated with enhanced BCR-dependent Akt and Erk phosphorylation (Figure 2B). Consistent with the results obtained on Ramos B cells, BCR-dependent Syk phosphorylation was enhanced in p66Shc−/− B cells (Figure 2B). Collectively, the data support the notion that p66Shc acts as an inhibitor of B-cell survival. This function stems from its capacity to uncouple the BCR from phosphatidylinositol-3 kinase/Akt and Ras/Erk activation.
p66Shc expression is impaired in B cells from patients with CLL
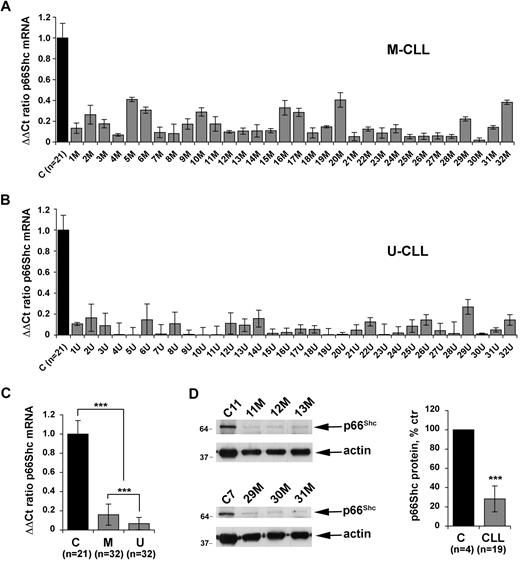
Apoptosis defects are believed to be primarily responsible for the extended life span of CLL B cells.24 Consistent with this notion, the antiapoptotic protein Bcl-2 is overexpressed in most CLL cells.25 Alterations in the expression of other proteins implicated in the control of apoptosis, such as Mlc-1, Bax, and DAPK1, have also been reported.7,26-28 Overexpression of Tcl1, a coactivator of Akt, has moreover been identified in a large proportion of CLL.29 Our finding that p66Shc antagonizes survival signals in B cells suggests that the levels of p66Shc expression may be determinant in the delicate balance of signals which ultimately determines cell fate. To test the hypothesis that a defect in p66Shc expression may contribute to the extended life span of CLL B cells, the levels of p66Shc mRNA were quantitated by qRT-PCR in leukemic B cells from a cohort of 64 patients with CLL. These included 2 subsets, of 32 patients each, with favorable (M-CLL) and unfavorable (U-CLL) prognosis, as identified by the presence or absence of somatic mutations in the tumor IGHV genes, respectively. p66Shc expression in B cells was significantly reduced in all patients analyzed compared with healthy controls (Figure 3A-B). The reduction in p66Shc expression in CLL B cells compared with B cells from healthy controls was confirmed at the protein level (Figure 3D). No difference in the levels of p52Shc, the mitogenic/prosurvival ShcA isoform, was observed between CLL and normal B cells (data not shown). Strikingly, comparison of the levels of p66Shc mRNA in the 2 groups of patients showed that p66Shc expression was significantly lower in U-CLL B cells than in M-CLL B cells (Figure 3C).
Defective p66Shc expression in CLL B cells. Quantification by real-time RT-PCR of the levels of p66Shc mRNA in monocyte-depleted peripheral blood mononuclear cells (B cells ≥ 85%, as assessed by flow cytometry) purified by density gradient centrifugation from 64 patients with CLL, of which 32 with mutated IGHV (M-CLL; A) and 32 with unmutated IGHV (U-CLL; B). Transcript levels were normalized to the expression level of GAPDH. For each patient, Sybr green runs were performed on duplicate samples of cDNAs from 2 independent reverse transcription reactions, each carried out on 400 ng of total RNA. RNA from the same healthy donor (C1) was included in duplicate in each run as reference to normalize the data obtained in the individual experiments. The ΔΔCT method was applied as a comparative method of quantification, using as a reference the average of all the Ct data obtained on healthy donors. (C) Histograms summarizing the real-time RT-PCR analysis of p66Shc mRNA levels in B cells from the 21 healthy donors and 64 patients with CLL shown in panels A and B, grouped in the M-CLL and U-CLL subsets (***P ≤ .001). Error bars indicate SD. (D) Immunoblot analysis of p66Shc expression in lysates of monocyte-depleted peripheral blood mononuclear cells purified by density gradient centrifugation from 4 representative patients with CLL and 1 representative healthy donor. Stripped filters were reprobed with anti-actin antibodies as loading control. The histogram on the right of the panel shows the quantification by laser densitometry of p66Shc immunoreactive band in lysates of PBLs from 19 patients with CLL compared with lysates of peripheral blood B cells purified from 4 healthy donors by immunomagnetic sorting, of which at least 1 was included in each gel as reference (***P ≤ .001).
Defective p66Shc expression in CLL B cells. Quantification by real-time RT-PCR of the levels of p66Shc mRNA in monocyte-depleted peripheral blood mononuclear cells (B cells ≥ 85%, as assessed by flow cytometry) purified by density gradient centrifugation from 64 patients with CLL, of which 32 with mutated IGHV (M-CLL; A) and 32 with unmutated IGHV (U-CLL; B). Transcript levels were normalized to the expression level of GAPDH. For each patient, Sybr green runs were performed on duplicate samples of cDNAs from 2 independent reverse transcription reactions, each carried out on 400 ng of total RNA. RNA from the same healthy donor (C1) was included in duplicate in each run as reference to normalize the data obtained in the individual experiments. The ΔΔCT method was applied as a comparative method of quantification, using as a reference the average of all the Ct data obtained on healthy donors. (C) Histograms summarizing the real-time RT-PCR analysis of p66Shc mRNA levels in B cells from the 21 healthy donors and 64 patients with CLL shown in panels A and B, grouped in the M-CLL and U-CLL subsets (***P ≤ .001). Error bars indicate SD. (D) Immunoblot analysis of p66Shc expression in lysates of monocyte-depleted peripheral blood mononuclear cells purified by density gradient centrifugation from 4 representative patients with CLL and 1 representative healthy donor. Stripped filters were reprobed with anti-actin antibodies as loading control. The histogram on the right of the panel shows the quantification by laser densitometry of p66Shc immunoreactive band in lysates of PBLs from 19 patients with CLL compared with lysates of peripheral blood B cells purified from 4 healthy donors by immunomagnetic sorting, of which at least 1 was included in each gel as reference (***P ≤ .001).
p66Shc expression is principally regulated by epigenetic modification of the gene promoter in T cells.14,15 To understand whether the defect in p66Shc expression in CLL B cells could result from promoter methylation, we compared the methylation state of the 8 CpG dinucleotides in the region encompassing position −139 to +66 of the p66Shc gene promoter on genomic DNA purified from B cells from 5 healthy donors and 11 patients with CLL (9 M-CLL and 2 U-CLL). Genome bisulfite sequencing showed that the overall level of CpG methylation of the p66Shc gene promoter was similar in normal and leukemic B cells. Moreover, no major differences in the methylation pattern of individual CpG dinucleotides were detectable, with the exception of CpG4 and CpG7, which were, respectively, hypomethylated and hypermethylated to a modest extent in CLL compared with normal B cells (supplemental Figure 1). Moreover, sequencing of the p66Shc gene locus on genomic DNA from 17 patients (13 M-CLL and 4 U-CLL) did not highlight any mutation either in the promoter, or in the first exon, which is unique to p66Shc, or in the first part of exon 2, where the region within the locus common to all ShcA isoforms starts (supplemental Figure 2). Collectively, these observations suggest that impaired p66Shc expression in CLL B cells is unlikely to be caused by epigenetic gene silencing or by a p66Shc gene defect intrinsic to the neoplastic B cell.
The defect in p66Shc expression in B cells from patients with CLL associates with alterations in the balance of Bcl-2 family members
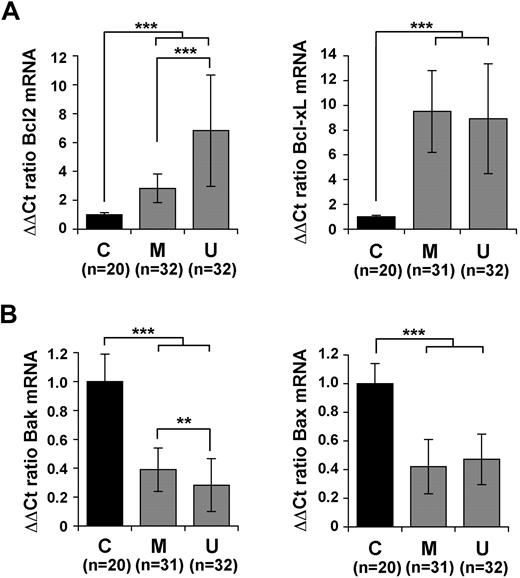
Alterations in the balance of antiapoptotic and proapoptotic members of the Bcl-2 family are believed to play a major role in the increased survival and resulting accumulation of leukemic B cells, as well as in their resistance to chemotherapy.30 We have previously reported that p66Shc expression in T cells correlates with a reduction in Bcl-xL and a concomitant increase in Bax expression,16 suggesting a link of p66Shc with expression of Bcl-2–related genes. Expression of 4 Bcl-2 family members, of which 2 antiapoptotic (Bcl-2, Bcl-xL) and 2 proapoptotic (Bax, Bak), was measured by qRT-PCR in B cells from 20 of the 21 healthy donors and the 64 patients with CLL. No significant difference in expression of these genes was found among healthy donors (supplemental Figure 3). By contrast, a significant increase in the levels of Bcl-2 and Bcl-xL mRNA was found in all patients with CLL compared with healthy donors (Figure 4A). A concomitant reduction in the levels of Bax and Bak mRNA was observed in CLL B cells (Figure 4B). These alterations were reflected at the protein level (supplemental Figure 4). A comparison of the levels of expression of these genes in M-CLL and U-CLL B cells showed statistically significant differences of Bcl-2 and Bak expression in the 2 groups, with an increase in the levels of Bcl-2 mRNA and a decrease in Bak mRNA in U-CLL compared with M-CLL (Figure 4A-B). The results obtained on the individual patients are shown in supplemental Figures 5 and 6. Expression of an unrelated regulator of apoptosis implicated in CLL, DAPK1,7 was found to be highly variable among patients (data not shown).
Defective p66Shc expression in CLL B cells correlates with an alteration in the balance of antiapoptotic and proapoptotic Bcl-2 family members. Quantification by real-time RT-PCR of the levels of Bcl-2 and Bcl-xL mRNA (A) or Bax and Bak (B) in PBLs from the 64 patients with CLL, grouped in the M-CLL and U-CLL subsets. Transcript levels were normalized to the expression level of GAPDH. For each patient, Syber green runs were performed on duplicate samples of cDNAs from 2 independent reverse transcription reactions, each carried out on 400 ng of total RNA. RNA from the same healthy donor (C1) was included in duplicate in each run as reference to normalize the data obtained in the individual experiments. The ΔΔCT method was applied as a comparative method of quantification, using as a reference the average of all the Ct data obtained on healthy donors. No significant variation among healthy donors was observed (see supplemental Figure 3). The histograms summarize all the data obtained on the control and CLL subsets. ***P ≤ .001, **P ≤ .01. Error bars indicate SD.
Defective p66Shc expression in CLL B cells correlates with an alteration in the balance of antiapoptotic and proapoptotic Bcl-2 family members. Quantification by real-time RT-PCR of the levels of Bcl-2 and Bcl-xL mRNA (A) or Bax and Bak (B) in PBLs from the 64 patients with CLL, grouped in the M-CLL and U-CLL subsets. Transcript levels were normalized to the expression level of GAPDH. For each patient, Syber green runs were performed on duplicate samples of cDNAs from 2 independent reverse transcription reactions, each carried out on 400 ng of total RNA. RNA from the same healthy donor (C1) was included in duplicate in each run as reference to normalize the data obtained in the individual experiments. The ΔΔCT method was applied as a comparative method of quantification, using as a reference the average of all the Ct data obtained on healthy donors. No significant variation among healthy donors was observed (see supplemental Figure 3). The histograms summarize all the data obtained on the control and CLL subsets. ***P ≤ .001, **P ≤ .01. Error bars indicate SD.
Reconstitution of p66Shc expression in B cells from patients with CLL restores the balance among Bcl-2 family members
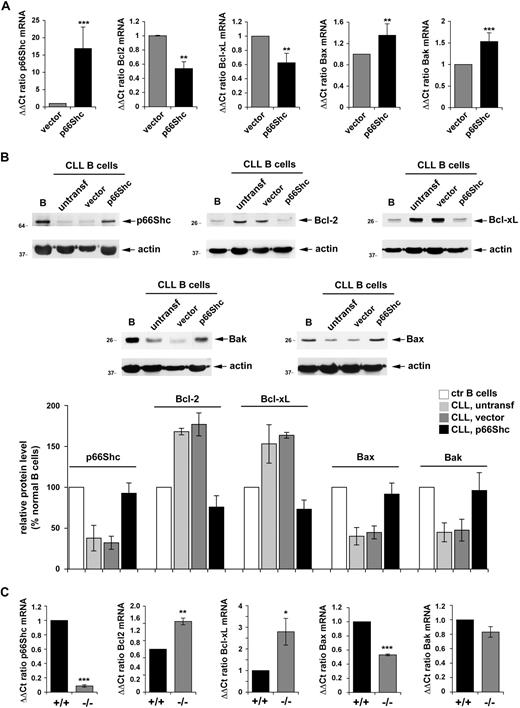
To understand whether p66Shc expression is causally related to the imbalance among Bcl-2 family members, p66Shc expression was reconstituted in B cells from 7 patients with CLL by transient transfection with a mammalian expression vector encoding human p66Shc (Figure 5A). Cells transfected with empty vector were used as controls. p66Shc expression resulted in a significant reduction in the levels of Bcl-2 and Bcl-xL mRNA and a concomitant increase in Bax and Bak mRNA, as assessed by qRT-PCR (Figure 5A). This result was confirmed at the protein level (Figure 5B). Of note, although nucleofected B cells rapidly exposed phosphatidylserine at the plasma membrane, resulting in significant annexin V staining, an approximate 30% increase in annexin V staining was observed in CLL cells transfected with the p66Shc-encoding construct compared with cells transfected with empty vector, further supporting the proapoptotic activity of p66Shc (data not shown).
Reconstitution of p66Shc expression in CLL B cells restores the balance of antiapoptotic and proapoptotic Bcl-2 family members. (A) Quantification by real-time RT-PCR of the relative levels of p66Shc, Bcl-2, Bcl-xL, Bax, and Bak mRNA in PBLs from 7 patients with CLL (29M, 30M, 31M, 32M, 30U, 31U, 32U). Cells were cotransfected with either empty vector or the same vector encoding human p66Shc. A GFP reporter was included in all samples as a transfection control. Transcript levels were normalized to the expression level of GAPDH. For each transfected sample, Syber green runs were performed on duplicate samples of cDNAs from 2 independent reverse transcription reactions, each carried out on 400 ng of total RNA. RNA from the control sample (transfection with empty vector) of patient 29M was included in duplicate in each run as reference to normalize the data obtained in the individual experiments. The ΔΔCT method was applied as a comparative method of quantification, using as a reference the average of all the Ct data obtained on the samples transfected with empty vector. (B top) Immunoblot analysis of p66Shc, Bcl-2, Bcl-xL, Bax, and Bak expression in PBL lysates from a representative patient with CLL of 3 analyzed (29M, 30M, 30U). Cells were cotransfected with either empty vector or the same vector encoding human p66Shc. Each gel included a nontransfected PBL lysate from the same patient, as well as lysates of purified normal B cells. Stripped filters were reprobed with anti-actin antibodies as loading control. (Bottom) Quantification by laser densitometry of the data obtained on the 3 patients with CLL and 4 healthy controls. (C) Quantification by real-time RT-PCR of the relative levels of p66Shc, Bcl-2, Bcl-xL, Bax, and Bak mRNA in purified splenic B cells from wild-type (+/+) or p66Shc−/− (−/−) mice. Two pools of B cells, each from 3 wild-type or 3 p66Shc−/− mice, were used. Transcript levels were normalized to the expression level of GAPDH. For each sample, Sybr green runs were performed on duplicate samples of cDNAs from 2 independent reverse transcription reactions, each carried out on 400 ng of total RNA. RNA from the same pool of B cells from wild-type mice was included in duplicate in each run as reference to normalize the data obtained in the individual experiments. The ΔΔCT method was applied as a comparative method of quantification, using as a reference the average of all the Ct data obtained on the 2 pools of B cells from wild-type mice. ***P ≤ .001, **P ≤ .01, * P ≤ .05. Error bars indicate SD.
Reconstitution of p66Shc expression in CLL B cells restores the balance of antiapoptotic and proapoptotic Bcl-2 family members. (A) Quantification by real-time RT-PCR of the relative levels of p66Shc, Bcl-2, Bcl-xL, Bax, and Bak mRNA in PBLs from 7 patients with CLL (29M, 30M, 31M, 32M, 30U, 31U, 32U). Cells were cotransfected with either empty vector or the same vector encoding human p66Shc. A GFP reporter was included in all samples as a transfection control. Transcript levels were normalized to the expression level of GAPDH. For each transfected sample, Syber green runs were performed on duplicate samples of cDNAs from 2 independent reverse transcription reactions, each carried out on 400 ng of total RNA. RNA from the control sample (transfection with empty vector) of patient 29M was included in duplicate in each run as reference to normalize the data obtained in the individual experiments. The ΔΔCT method was applied as a comparative method of quantification, using as a reference the average of all the Ct data obtained on the samples transfected with empty vector. (B top) Immunoblot analysis of p66Shc, Bcl-2, Bcl-xL, Bax, and Bak expression in PBL lysates from a representative patient with CLL of 3 analyzed (29M, 30M, 30U). Cells were cotransfected with either empty vector or the same vector encoding human p66Shc. Each gel included a nontransfected PBL lysate from the same patient, as well as lysates of purified normal B cells. Stripped filters were reprobed with anti-actin antibodies as loading control. (Bottom) Quantification by laser densitometry of the data obtained on the 3 patients with CLL and 4 healthy controls. (C) Quantification by real-time RT-PCR of the relative levels of p66Shc, Bcl-2, Bcl-xL, Bax, and Bak mRNA in purified splenic B cells from wild-type (+/+) or p66Shc−/− (−/−) mice. Two pools of B cells, each from 3 wild-type or 3 p66Shc−/− mice, were used. Transcript levels were normalized to the expression level of GAPDH. For each sample, Sybr green runs were performed on duplicate samples of cDNAs from 2 independent reverse transcription reactions, each carried out on 400 ng of total RNA. RNA from the same pool of B cells from wild-type mice was included in duplicate in each run as reference to normalize the data obtained in the individual experiments. The ΔΔCT method was applied as a comparative method of quantification, using as a reference the average of all the Ct data obtained on the 2 pools of B cells from wild-type mice. ***P ≤ .001, **P ≤ .01, * P ≤ .05. Error bars indicate SD.
To further substantiate the causal link of p66Shc with the Bcl-2 family, Bcl-2, Bcl-xL, Bax, and Bak expression was measured in splenic B cells purified from p66Shc−/− and wild-type mice. Similar to CLL B cells, p66Shc−/− B cells were found to express higher levels of Bcl-2 and Bcl-xL and lower levels of Bax and Bak than control B cells (Figure 5C).
Collectively, these findings support the notion that p66Shc promotes apoptosis by shifting the Bcl-2 family balance in favor of the proapoptotic members and suggest that the imbalance toward the antiapoptotic members observed in CLL B cells may result from the defect in p66Shc expression.
p66Shc may contribute to the clinical behavior of CLL B cells
The finding that the levels of p66Shc mRNA are consistently lower in CLL B cells compared with B cells from normal donors, taken together with the antagonistic role of p66Shc in B-cell survival, suggests an intrinsic critical role of p66Shc in the behavior of CLL. Moreover, comparison of p66Shc expression in U-CLL and M-CLL showed a significant difference (P < .001) between the 2 subsets (Figure 3C), with the lowest expression in the subset with unfavorable prognosis (U-CLL). Remarkably, although the levels of p66Shc transcript showed an inverse correlation with those of the antiapoptotic Bcl-2 members, Bcl-2 and Bcl-xL, and a direct correlation with those of the proapoptotic members Bak and Bax, in both the M-CLL and U-CLL subsets, the Bcl-2/p66Shc ratio was significantly higher in U-CLL than in M-CLL (Figure 6A; P < .001). A similar difference was observed for the Bcl-xL/p66Shc ratio (Figure 6A). Moreover, the product of the levels of p66Shc and either Bax or Bak was significantly lower in the U-CLL subset than in the M-CLL subset (Figure 6A). The data suggest an imbalance toward a higher antiapoptotic activity in the aggressive U-CLL subset than in the M-CLL subset with favorable prognosis.
p66Shc levels and clinical behavior of U-CLL and M-CLL. (A) Ratios of the relative levels of Bcl-2/p66Shc and Bcl-xL/p66Shc transcripts (top) and products of the relative levels of Bax*p66Shc and Bak*p66Shc transcripts (bottom) measured by qRT-PCR in B cells from the 64 patients with CLL. Each value is shown as a dot. Gray dots correspond to the patients with M-CLL, black dots to the patients with U-CLL. The line shows the median value of the ratios (top) or products (bottom). The differences between the M-CLL and U-CLL subsets (calculated on the mean values) were statistically significant, as shown in each panel. (B) TFI (left) and OS (right) in patients with CLL with Bcl-2/p66Shc transcript ratios above and below median value.
p66Shc levels and clinical behavior of U-CLL and M-CLL. (A) Ratios of the relative levels of Bcl-2/p66Shc and Bcl-xL/p66Shc transcripts (top) and products of the relative levels of Bax*p66Shc and Bak*p66Shc transcripts (bottom) measured by qRT-PCR in B cells from the 64 patients with CLL. Each value is shown as a dot. Gray dots correspond to the patients with M-CLL, black dots to the patients with U-CLL. The line shows the median value of the ratios (top) or products (bottom). The differences between the M-CLL and U-CLL subsets (calculated on the mean values) were statistically significant, as shown in each panel. (B) TFI (left) and OS (right) in patients with CLL with Bcl-2/p66Shc transcript ratios above and below median value.
The clinical effect of the opposite trend in p66Shc and Bcl-2 levels was also evident when TFI and OS were investigated (Figure 6B). In fact, the CLL subset with a high Bcl-2/p66Shc ratio had a shorter TFI (median, 29 months) and OS (median, 139 months) than the CLL subset with a low Bcl-2/p66Shc ratio (median TFI, 255 months, P < .001; median OS not reached, P = .015; Figure 6B).
Discussion
p66Shc has been identified as a central regulator in the p53-dependent apoptosis pathway triggered by oxidative stress both in vitro and in vivo.31 Recent evidence shows that p66Shc acts as a promoter of apoptosis also in T cells, where its expression correlates with mitochondrial dysfunction that ultimately results in dissipation of membrane potential, cytochrome-c release, and initiation of the caspase cascade.17 Alterations in the capacity of the cell to extrude Ca2+ further contribute to the apoptogenic activity of this protein in T cells.17 The data presented in this study, obtained both on a Ramos B-cell transfectant overexpressing p66Shc and on B cells from p66Shc−/− mice, identify p66Shc as a novel regulator of B-cell survival. This activity probably contributes to the development of lupuslike autoimmunity observed in aging p66Shc−/− mice.18 The proapoptotic activity of p66Shc stems, at least in part, from its ability to uncouple the BCR from Akt- and Erk-mediated survival signals beginning from the earliest step in the cascade, activation of the initiating kinase Syk. Consistent with these findings, we have recently reported that B cells from p66Shc−/− mice display enhanced proliferative responses to BCR engagement both in vitro and in vivo.18 A potential mechanism involves competitive inhibition of recruitment to the activated BCR of the mitogenic isoform, p52Shc, which is known to interact with the phosphorylated immunoreceptor tyrosine-based activation motifs in the Igα/Igβ signaling module.16 The ROS-elevating activity of p66Shc32 may also contribute to the reduction in B-cell survival observed in the presence of p66Shc.
At variance with fibroblasts, where p66Shc impairs mitochondrial integrity and promotes apoptosis by inducing opening of the permeability transition pore,31 the mitochondrial dysfunction evoked by p66Shc in T cells has been related to its capacity to alter the balance in the expression of antiapoptotic and proapoptotic members of the Bcl-2 family, with a reduction in Bcl-xL and an increase in Bax expression. In the presence of low levels of antiapoptotic Bcl-2–related proteins, Bax can indeed oligomerize with Bak, resulting in the formation of pores in the outer mitochondrial membrane which allow cytochrome-c release.33 The results obtained both on CLL B cells and B cells from p66Shc−/− mice show that p66Shc expression correlates with a profound imbalance between proapoptotic (Bax, Bak) and antiapoptotic (Bcl-2, Bcl-xL) Bcl-2 family members compared with normal controls, suggesting a causal role of p66Shc in expression of the respective genes. This notion is strongly supported by the reversal of these alterations when p66Shc expression was reconstituted in CLL B cells. The enhanced activation of Akt in response to BCR engagement both in p66Shc−/− B cells and in CLL B cells probably underlies at least in part the altered balance in the Bcl-2 family, because catalytically active Akt mutants has been reported to enhance Bcl-2, Bcl-xL, and Mcl-1 expression in both normal and leukemic B cells.34,35 Moreover, Bax and Bcl-2 expression can be inversely modulated by the redox state of the cell,36-38 suggesting that p66Shc may alter the expression of Bcl-2 family members through its ROS-elevating activity. The capacity of p66Shc to tilt the Bcl-2 family balance toward apoptosis probably plays a significant role in the extended survival of CLL B cells.
It should be underlined that, at variance with mice lacking other regulators of apoptosis, such as p53,39 no increase in the frequency of spontaneous tumor development has been observed in p66Shc−/− mice.13 This suggests that p66Shc deficiency may contribute to leukemogenesis by favoring survival of B cells carrying genetic lesions affecting their proliferation and differentiation potential, as observed for example in the cooperativity of Bcl-2 with c-Myc in the development of B-cell lymphomas in the mouse.40 However, 129sv mice (the strain in which the p66Shc null mutation has been introduced) are not prone to the spontaneous development of leukemia and lymphomas,41 warranting investigations aimed at assessing the susceptibility of these mice to oncogene- or mutagen-induced carcinogenesis.
Remarkably, although a decrease in p66Shc expression was consistently observed in B cells analyzed from all patients with CLL compared with healthy controls, we found a statistically significant inverse correlation of p66Shc expression and prognosis, with the lowest levels detectable in the U-CLL subset. The dual activity of p66Shc as attenuator of BCR signaling (Figure 2) and promoter of apoptosis (Figure 1) may contribute to disease aggressiveness. U-CLL B cells are indeed not only long-lived but, as opposed to M-CLL B cells, also able to actively respond to BCR engagement.42 Hence, a drop of p66Shc expression below a critical threshold may favor growth and survival of U-CLL B cells by enhancing BCR signaling. Interestingly, inhibition of antigen receptor signaling by p66Shc in T cells involves its interaction with ZAP-70,16 a kinase whose ectopic expression in U-CLL B cells has been associated to the signaling potential of the BCR in these cells.43
In the cohort of patients included in this study the levels of Bcl-2 and Bak mRNA showed a significant correlation with prognosis (Figure 4). Although Bcl-2 has been identified by some groups as an important predictor of disease progression, in particular when analyzed in combination with Bax,44-46 this finding has been challenged by others.26,47,48 The immunoblot analysis carried out on a subset of our patients showed that the variations in mRNA levels were consistently reflected at the protein level. This was the case not only for Bcl-2 but also for the other family members analyzed, including Bax, whose levels are controlled also posttranscriptionally in CLL B cells through increased proteasomal degradation.49 Interestingly, p66Shc was found to be a good predictor of disease progression when used in combination with Bcl-2. The implication of the Bcl-2 family in CLL has prompted the recent development of Bcl-2 inhibitors (siRNA, small molecule compounds) as novel drugs for the treatment of this leukemia, particularly in patients in whom conventional first-line treatment has failed or who have developed chemoresistance, a condition associated to Bcl-2 overexpression.50 The results of the clinical trials of Bcl-2 inhibitors appear, however, not to fully meet the expectations raised by the preclinical studies in a significant proportion of patients with CLL, unless used in combination therapy with conventional chemotherapeutic drugs (eg, fludarabine, cyclophosphamide).51 On the basis of our findings, it would be of interest to take into account p66Shc expression in the response of patients with CLL relapsing/refractory to Bcl-2 inhibitors.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank S. Grassini for technical assistance.
This work was supported by Italian Association for Cancer Research (AIRC) and Istituto Toscano Tumori (ITT) Regione Toscana. F. Finetti is the recipient of a Federazione Italiana per la Ricerca sul Cancro (FIRC) fellowship.
Authorship
Contribution: N.C., O.M.L., E.S., M.F., N.G., F. Finetti, G.D.F., E.C., F.L., F. Forconi, and C.T.B. designed the research and analyzed and interpreted data; N.C., O.M.L., E.S., M.F., N.G., F. Finetti, D.R., and F. Forconi performed the research; P.G.P. contributed vital reagents; and N.C., F. Forconi, and C.T.B. drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cosima T. Baldari, Department of Evolutionary Biology, University of Siena, Via Aldo Moro 2, 53100 Siena, Italy; e-mail: baldari@unisi.it.