Abstract
CD146, an endothelial molecule involved in permeability and monocyte transmigration, has recently been reported to promote vessel growth. As CD146 is also detectable as a soluble form (sCD146), we hypothesized that sCD146 could stimulate angiogenesis. Experiments of Matrigel plugs in vivo showed that sCD146 displayed chemotactic activity on endogenous endothelial cells, and exogenously injected late endothelial progenitor cells (EPCs). Recruited endothelial cells participated in formation of vascular-like structures. In vitro, sCD146 enhanced angiogenic properties of EPCs, with an increased cell migration, proliferation, and capacity to establish capillary-like structures. Effects were additive with those of vascular endothelial growth factor (VEGF), and sCD146 enhanced VEGFR2 expression and VEGF secretion. Consistent with a proangiogenic role, gene expression profiling of sCD146-stimulated EPCs revealed an up-regulation of endothelial nitric oxide synthase, urokinase plasminogen activator, matrix metalloproteinase 2, and VEGFR2. Silencing membrane-bound CD146 inhibited responses. The potential therapeutic interest of sCD146 was tested in a model of hind limb ischemia. Local injections of sCD146 significantly reduced auto-amputation, tissue necrosis, fibrosis, inflammation, and increased blood flow. Together, these findings establish that sCD146 displays chemotactic and angiogenic properties and promotes efficient neovascularization in vivo. Recombinant human sCD146 might thus support novel strategies for therapeutic angiogenesis in ischemic diseases.
Introduction
CD146 is a component of the endothelial junction primarily expressed in endothelial cells. It is involved in the control of cell and tissue architecture, as demonstrated by the regulation of its expression during endothelium monolayer formation, its involvement in the control of paracellular permeability, and its colocalization with the actin cytoskeleton.1 Besides its structural role, CD146 is also involved in cell signaling.2,3 We have recently demonstrated that CD146 is involved in the regulation of monocyte transendothelial migration.4 Recent findings indicate that CD146 displays angiogenic properties. In one study, the authors showed that an anti-CD146 antibody, mAb AA98, displayed antiangiogenic properties in chicken chorioallantoic membrane assays and inhibited tumor growth in different xenografted human tumor models in mice. In a model of human umbilical vein endothelial cells (HUVECs), it was also shown that silencing CD146 with specific siRNA inhibited the proliferation and migration of the cells.5-7 Of interest, we have established that CD146 also exists in a soluble form (sCD146) as the result of metalloprotease-dependent shedding of membrane CD146.4,8 sCD146 is detectable in the human serum, and its level is modulated in different pathologies, such as inflammatory bowel diseases,9 pathologic pregnancies,10 and chronic renal failure.11 However, its exact role is still largely unknown.
Postischemic neovascularization occurs as a result of 2 mechanisms: angiogenesis, which relies on mature endothelial cells already present at the ischemic site; and vasculogenesis, which involves the homing and endothelial differentiation of endothelial progenitor cells (EPCs) mobilized from the bone marrow.12,13
Different angiogenic factors have been shown to trigger angiogenesis and/or vasculogenesis by directly or indirectly stimulating proliferation, differentiation, and migration of mature or precursor cells. Among these factors, the more effective are fibroblast growth factors (FGFs), vascular endothelial growth factor (VEGF), and angiopoietins (Ang). FGF-1 has been shown to stimulate the proliferation and differentiation of all cell types necessary for the constitution of an arterial vessel, including endothelial cells and smooth muscle cells. FGF-2 also promotes endothelial cell proliferation and organization of endothelial cells into capillary-like structures.14 In vitro studies have clearly demonstrated that VEGF is a potent stimulator of angiogenesis, stimulating endothelial cell mitogenesis and migration,15 and numerous clinical trials have been conducted to test its therapeutic effect.16 Finally, Ang1 and Ang2 have been shown to be required for the formation of mature blood vessels, as demonstrated by mouse knockout studies.17 Physiologically, these different factors act in concert to restore blood flow after ischemia. Described a decade ago by the pioneer work of Asahara et al,18 EPCs also contribute to vessel growth in both physiologic and pathologic processes. Two types of EPCs, acting together, have been described. Early EPCs of myeloid origin, represent a heterogeneous cell population, which stimulates angiogenic responses through the paracrine secretion of angiogenic factors. Late EPCs are very rare cells with a capacity of de novo vessel formation through direct incorporation into the growing vasculature.19,20 Understanding the complete mechanisms of angiogenesis/vasculogenesis, including the knowledge of the involved angiogenic factors, may provide new insights and possible approaches for the treatment of ischemic diseases.
Although recent data have demonstrated the involvement of membrane-bound CD146 in vessel growth, nothing is known about the role of sCD146 in angiogenesis. We therefore investigated whether: (1) sCD146 displayed chemotactic and angiogenic properties in vitro; and (2) sCD146 was able to promote neovascularization in a rat model of hind-limb ischemia.
Methods
rh-sCD146
A c-myc-tagged recombinant protein corresponding to the soluble form of human CD146 was obtained from Biocytex. Epitope tagging of CD146 at its N-terminus enabled us to detect specifically the recombinant molecule using an anti–c-myc peptide antibody (Abcam) and to distinguish it from endogenous CD146.
Isolation of circulating late EPCs and cell culture
EPCs were prepared and cultured in endothelial basal medium 2 (EBM-2) supplemented with EGM-2 SingleQuots (EGM-2 medium; Clonetics), as previously described.21 For EPC stimulation experiments, cells were maintained for 3 hours in EBM-2 and then stimulated with the growth factor.
Chemotactic activity in vitro
Experiments were performed on semipermeable Transwell filters (8-μm porosity; 24 wells; B&D) in EBM-2 medium. A total of 500 000 EPCs previously labeled for 30 minutes at 37°C with CM-Dil (Cell Tracker CM-Dil; Invitrogen) were seeded in the upper compartment. Recombinant human sCD146 (rh-sCD146) was added in the lower compartment, and migration of late EPC across the filter was measured after an overnight incubation at 37°C. Fluorescence intensity was measured using a cytofluor apparatus (Multi-Well Plate Reader 4000; Perspective Biosystems).
Endothelial cell tube formation in Matrigel
The 96-well plates were precoated with a 1:1 mixture of cold Matrigel (10 mg/mL; BD Biosciences): EBM-2 medium. Late EPCs were plated at 104 cells/well in EBM-2 supplemented or not with rh-sCD146 and/or VEGF. After 5 hours, pictures were taken for each condition. Capillary tube formation was evaluated by measuring the number of tubes per field. In some experiments, an anti-VEGFR2 antibody (see “Peptides, antibodies, immunoassay, and inhibitors”) was used at 50 μg/mL.
Cell proliferation assay
Late EPCs were seeded on 96-well plates (5 × 103/well) and cultured in EGM-2 medium for 3 days. After treatment, cell proliferation was assayed using the BrdU Labeling and Detection Kit III (Roche Corporation) as indicated by the manufacturer. Results were expressed as arbitrary units. Experiments were performed in triplicate.
Wound healing assay
A reproducible wound was performed with a pipette tip on a confluent monolayer of late EPCs cultured on 24-well plates. The surface of the wound was measured and acquired with the Biocom Visiolab image analysis software. The medium was removed, and late EPCs were incubated for 6 hours with EBM-2 medium containing or not different concentrations of rh-sCD146. Results were expressed as a percentage of the area of the original wound, considered as 100%.
Western blot analysis
Western blot analysis was performed as previously described.4 Membranes were probed with specific primary antibodies (anti-VEGFR2 diluted 1/5000, anti-urokinase plasminogen activator [uPA] diluted 1/1000, anti-matrix metalloproteinase 2 [MMP-2] diluted 1/1000, anti-endothelial nitric oxide synthase [eNOS] diluted 1/5000 or anti-CD146 diluted 1/1000) followed by secondary antibodies coupled to peroxidase. Blots were revealed with the ECL substrate (Pierce). Membranes probed with various antibodies were stripped between antibodies.
RNA isolation, reverse transcription, and real-time PCR
Total cellular RNA was isolated from late EPCs, reverse transcribed into cDNA, and the resulting cDNA was subjected to quantitative polymerase chain reaction (PCR) as previously described.4 Forward and reverse specific primer sequences were: VEGFR2, forward: 5′-TGTGGGTTTGCCTAGTGTTTCT; reverse: 5′-CACTCAGTCACCTCCACCCTT. MMP-2, forward: 5′-TGATCTTGACCAGAATACCATCGA; reverse: 5′-GGCTTGCGAGGGAAGAAGTT. uPA, forward: 5′-TTTGCGGCCATCTACAGGAG; reverse: 5′-AGTTAAGCCTTGAGCGACCCA. eNOS, forward: 5′-CTCATGGGCACGGTGATG; reverse: 5′-ACCACGTCATACTCATCCATACAC. The values given refer to the number of transcript copies for a given gene for 106 GAPDH transcript copies.
Gene expression profiling
Total cellular RNA was isolated from cultured late EPCs treated or not for 3 hours with 50 ng/mL rh-sCD146. Oligoarray hybridizations were performed according to the manufacturer using angiogenesis oligoarrays (Tebu-Bio). Spot signals were quantified using the Tebu-Bio software. Subtraction of background was done for the signal mean intensities in both test and reference DNA spots. Normalization in the calculated ratios was done against the average of all ratios. The hybridizations were performed 3 times.
Matrigel plugs in vivo
Nonimmunocompromised or nude mice were anesthetized and 400 μL of ice-cold Matrigel, containing either 0.1 μg/μL c-myc peptide or 0.1 μg/μL rh-sCD146, were injected, respectively into the left and right groin area of each animal. Animals were then injected or not with 500 000 late EPCs depending on the experiment. After 12 days, the Matrigel plugs were removed and frozen. Procedures were conducted under an animal use protocol approved by the institutional review board of Unite de Formation et de Recherche Pharmacie.
Induction of hind-limb ischemia in rats
Male rats were subjected to unilateral hind-limb ischemia by complete resection of the entire left femoral artery followed by microbead injection. Laser-Doppler tissue imaging showed that obstruction of the left common femoral artery decreased blood perfusion by approximately 90% at day 1. After surgery, animals were split in 4 treatment groups: 2 control groups injected daily in ischemic adductor muscles with 10 μg of c-myc peptide for either 5 or 12 days; 2 experimental groups treated as the control groups except that the c-myc peptide was replaced with rh-sCD146. The procedures were conducted under an institutional approved animal use protocol.
Laser Doppler blood flow analysis
The ratio of the ischemic versus normal hind-limb blood flow was measured using a laser Doppler blood flow analyzer. At different time points after surgery (days 1, 5, 10, and 20), animals were subjected to 2 consecutive laser scannings over the regions of interest (leg and feet). Blood flow was expressed as the ischemic versus normal hind-limb ratio.
Morphologic, histologic, and immunochemical assessment
Histologic analysis was performed 20 days after ischemia by microscopic examination. Serial muscle sections (5 μm) were snap-frozen and stained with eosin and hematoxylin. A semiquantitative evaluation of the cell changes was performed using a 4-point scale from absence (−) to intense (++). Capillary density was determined by microscopic analysis of muscle cryosections.
In in vivo Matrigel plugs experiments or experiments on muscles, 5-μm-thick sections were used for staining. After blocking in normal serum, the sections were treated overnight at 4°C with anti-CD31 (1 of 50), anti-CD117 (1 of 50), anti-CD34 (1 of 40), anti-CD45 (1 of 200), anti–monocyte-macrophage-2 (MOMA-2; 1 of 200), anti–α-smooth muscle actin (α-SMA; 1 of 80), anti-CD33 (1 of 100), or anti-CD146 (1 of 100). Signal amplification used fluorochrome (Alexa 488 or Alexa 647)–conjugated secondary antibodies (1 of 250; Invitrogen) when noncoupled. Sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI, 1:1000; Sigma-Aldrich), rinsed, and mounted with a prolong gold antifade reagent with DAPI (Invitrogen). For the assessment of nonspecific staining, alternating sections were incubated without the primary antibody.
Pictures were taken on a Nikon Eclipse TE 2000-U microscope with a Nikon DXM 1200 F digital camera using 10×/0.30 numeric aperture (NA), 40×/0.60 NA, and 100×/1.40 NA oil objectives. Images were captured with NES-Elements AR 3.0 software.
Quantitative flow cytometry
The level of membrane expression of CD146, CD117, CD45, CD33, and CD31 was determined on human late EPCs. All detached endothelial cells were labeled with the antibody or isotype-matched control antibody coupled to fluorescent dye (10 μg/mL) for 1 hour at 4°C. After washing, samples were analyzed by flow cytometry on Gallios Flow Cytometer (Beckman Coulter).
Peptides, antibodies, immunoassay, and inhibitors
rh-sCD146 was obtained from Biocytex. This peptide corresponds to an N-terminal c-myc epitope-tagged extracellular domain of human CD146. The tag was used for specific tracking of the exogenous recombinant protein and for pull down in the immunodepletion experiments. The corresponding c-myc peptide (Abcam) was used as a control. VEGF was obtained from R&D Systems. Anti–human antibodies used in this study are: anti-VEGFR2 (R&D Systems), anti-uPA (American Diagnostica), anti–MMP-2 (Calbiochem), anti-eNOS (Santa Cruz Biotechnology), and anti-CD146 (clone S-Endo-1; Biocytex), anti-CD31 (B&D), anti-CD45 (B&D), anti-CD33 (B&D), anti-CD117 (B&D). Anti–mouse antibodies used in this study are: anti-CD45, anti-CD34, anti–α-SMA, anti–MOMA-2 (Dako Denmark), Alexa Fluor 488 anti-CD31 and Alexa Fluor 647 anti-CD117 (BioLegend), anti-CD33 (Santa Cruz Biotechnology), and anti-CD146.22 Anti–rat antibodies used in this study are: anti-CD117 (Neuromics) and anti-CD146.22
An immunoassay was used to determine VEGF concentration in culture medium. Experiments were performed as described by the manufacturer (Invitrogen). An anti-VEGFR2 blocking antibody was used (r212; Acris Antibodies GmbH).
Statistical analysis
Data are expressed mean plus or minus SEM of the indicated number of experiments. Statistical analysis was performed with the Prism software (GraphPad Software). Significant differences were determined using non parametric Mann-Whitney test. A P value less than .05 was considered as significant.
Results
Recombinant human sCD146 displays chemotactic activity on endothelial cells in vitro and in vivo
To investigate the chemotactic property of sCD146, we used laminin-based gels (Matrigel plugs) filled with rh-sCD146 (0.1 μg/μL), myc peptide (0.1 μg/μL), or PBS and implanted in normal mice to test the recruitment of endogenous cells. Analyzed 14 days after implantation, rh-sCD146 Matrigel plugs contained 73 plus or minus 12 times (n = 5) more cells than control Matrigel plugs filled with c-myc peptide or PBS (Figure 1A). Interestingly, the presence of vascular-like structures could be observed. To examine the different cell types present in Matrigel plugs filled with rh-sCD146, stainings were performed with CD31, CD45, CD34, α-SMA, CD117, and MOMA-2. Results presented in Figure 1B show that hematopoietic cells (CD45+), monocytes/macrophages (MOMA-2+), smooth muscle cells and/or pericytes (α-SMA+), and endothelial cells (CD31+) could be recruited by rh-sCD146. Among the cells integrated in vascular-like structures, we observed CD34+ cells (a marker of hematopoietic stem cells and progenitor/mature endothelial cells) and immature cells stained by the undifferentiation marker CD117. To better characterize these cells, costainings were performed (Figure 1B). Results show that CD31+ cells implicated in vascular-like structures were also CD146+. Interestingly, approximately 10% to 15% of CD31+ or CD146+ cells present in vascular-like structures were costained with the undifferentiation marker CD117 (Figure 1B). Finally, double labeling CD117+/CD33− and CD117+/CD45− showed that these undifferentiated cells were not of myeloid or hematopoietic origin.
Chemotactic activity of rh-sCD146 in vitro and in vivo. (A) Microscopic examination of Matrigel plugs maintained for 12 days in normal mice. Matrigel plugs containing 0.1 μg/μL PBS (control), 0.1 μg/μL c-myc peptide, or 0.1 μg/μL rh-sCD146 were injected in the same mouse. Capillary-like structures were observed in the Matrigel plugs in the presence of rh-sCD146 (arrows) but not in control or c-myc–containing plugs. (B) Immunostainings were performed with anti-CD45, anti-CD34, anti–α-SMA, anti–MOMA-2, anti-CD31, and anti-CD117 antibodies on sections of Matrigel plugs filled with rh-sCD146 and maintained for 12 days in normal mice. Nuclei were labeled with DAPI (blue). Colabelings were also performed with CD31/CD146, CD117/CD31, CD117/CD146, CD117/CD33, and CD117/CD45. The merge pictures are given. Yellow areas correspond to a colabeling. In some pictures, these areas are better indicated with an arrow. (C) EPC characterization. EPCs were characterized by flow cytometry analysis for their expression of CD31, CD146, CD117, CD33, and CD45. Markers are represented in clear and control isotypes in dark. (D) Chemotactic effect of rh-sCD146 on EPCs in vitro. rh-sCD146, c-myc, immunodepleted rh-sCD146 (Ip rh-sCD146), control immunodepletion (IpC), and VEGF were tested. Results are the mean ± SEM values of 4 different experiments. *P < .05, **P < .01, ***P < .001, experimental versus control. (E) Immunostaining of Matrigel plugs maintained for 12 days in nude mice injected with late EPCs. Control Matrigel plugs containing 0.1 μg/μL c-myc peptide and Matrigel plugs containing 0.1 μg/μL rh-sCD146 were injected in the same mouse. Immunostaining was performed in Matrigel plugs with anti–human CD31 (red) antibody. Cell nuclei were labeled with DAPI (blue).
Chemotactic activity of rh-sCD146 in vitro and in vivo. (A) Microscopic examination of Matrigel plugs maintained for 12 days in normal mice. Matrigel plugs containing 0.1 μg/μL PBS (control), 0.1 μg/μL c-myc peptide, or 0.1 μg/μL rh-sCD146 were injected in the same mouse. Capillary-like structures were observed in the Matrigel plugs in the presence of rh-sCD146 (arrows) but not in control or c-myc–containing plugs. (B) Immunostainings were performed with anti-CD45, anti-CD34, anti–α-SMA, anti–MOMA-2, anti-CD31, and anti-CD117 antibodies on sections of Matrigel plugs filled with rh-sCD146 and maintained for 12 days in normal mice. Nuclei were labeled with DAPI (blue). Colabelings were also performed with CD31/CD146, CD117/CD31, CD117/CD146, CD117/CD33, and CD117/CD45. The merge pictures are given. Yellow areas correspond to a colabeling. In some pictures, these areas are better indicated with an arrow. (C) EPC characterization. EPCs were characterized by flow cytometry analysis for their expression of CD31, CD146, CD117, CD33, and CD45. Markers are represented in clear and control isotypes in dark. (D) Chemotactic effect of rh-sCD146 on EPCs in vitro. rh-sCD146, c-myc, immunodepleted rh-sCD146 (Ip rh-sCD146), control immunodepletion (IpC), and VEGF were tested. Results are the mean ± SEM values of 4 different experiments. *P < .05, **P < .01, ***P < .001, experimental versus control. (E) Immunostaining of Matrigel plugs maintained for 12 days in nude mice injected with late EPCs. Control Matrigel plugs containing 0.1 μg/μL c-myc peptide and Matrigel plugs containing 0.1 μg/μL rh-sCD146 were injected in the same mouse. Immunostaining was performed in Matrigel plugs with anti–human CD31 (red) antibody. Cell nuclei were labeled with DAPI (blue).
Recruitment of EPC is an important step in the neovascularization process after ischemia. Because endothelial cells presenting characteristics of precursor endothelial cells were found in Matrigel plugs containing rh-sCD146, we evaluated the chemotactic activity of rh-sCD146 on human late EPCs defined by a CD146/CD117/CD31+ and CD33/CD45− phenotype (Figure 1C). To this end, the effect of increasing concentrations of rh-sCD146 was tested on the migratory capacity of these EPCs through semipermeable filters (Figure 1D). The migratory capacity of late EPCs increased from 0 to 50 ng/mL rh-sCD146 and reached a plateau up to 400 ng/mL. The maximal effect observed with 50 ng/mL rh-sCD146 was similar to that obtained with 20 ng/mL of VEGF. No chemotactic activity could be detected with control media containing either the c-myc peptide or immunodepleted of rh-sCD146. To confirm these results in vivo, we injected human late EPCs in nude mice containing Matrigel plugs filled with rh-sCD146 (0.1 μg/μL) or control plugs (Figure 1E). We estimated that approximately 4% to 5% of the injected EPCs were recovered in the Matrigel plugs containing rh-sCD146. Among the cells, EPCs positively stained for human CD31 were detected and accumulated in vascular-like structures (Figure 1E). A negligible number of human EPCs were detected in control Matrigel plugs implanted in the same animals.
Recombinant human sCD146 increases angiogenic capacity of late EPCs in vitro
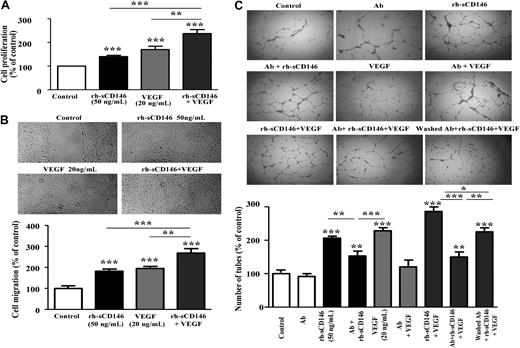
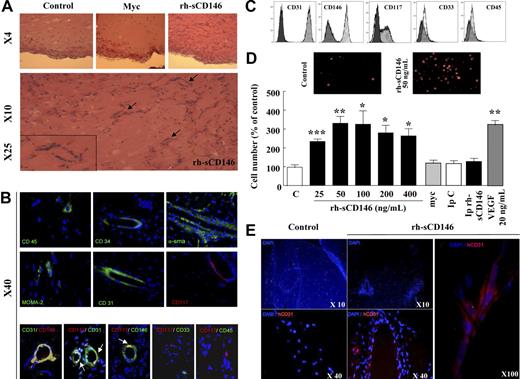
We then investigated in vitro whether rh-sCD146 was able to increase angiogenic properties of endothelial cells. To this end, we used human late EPCs. Indeed, cells with characteristics of late EPCs were found to be recruited by rh-sCD146 in Matrigel plugs in vivo. In addition, according to their endothelial features and their growth characteristics, these late EPCs constitute a useful model for ex vivo angiogenesis studies. The effects of rh-sCD146 were evaluated on late EPC migration, proliferation, and capacity to organize into capillary tubes, and compared with those of the angiogenic cytokine VEGF. The formation of capillary tubes was evaluated in Matrigel plugs in vitro (Figure 2A). When late EPCs were seeded on these plugs, spontaneous formation of endothelial tubes occurred. The addition of rh-sCD146 (25-50 ng/mL) promoted the organization of cells into a capillary-like network, as demonstrated by the increase in tube number (Figure 2A) and length (data not shown). The capillary network formed with 50 ng/mL rh-sCD146 was similar to that observed with 20 ng/mL VEGF. No increase was observed when cells were treated with the control c-myc peptide or in the absence of rh-sCD146 (buffer solution immunodepleted for rh-sCD146). The angiogenic effect of rh-sCD146 was further documented by an increase in proliferation (Figure 2B) and migration (Figure 2C) of late EPCs. Both features increased between 25 and 50 ng/mL of rh-sCD146, with the maximal effects being similar to those observed with 20 ng/mL VEGF. Both effects were prevented by immunodepletion of rh-sCD146.
Effect of rh-sCD146 on angiogenic capacity of EPCs in vitro. (A) EPC capacity to elaborate pseudocapillaries in Matrigel plugs was evaluated in different conditions (control, rh-sCD146, c-myc peptide, immunodepleted rh-sCD146 [Ip rh-sCD146] and its control [IpC], or VEGF). Number of tubes was counted after 5 hours of incubation. Results are the mean ± SEM values of 6 different experiments. (B) Proliferation capacity of late EPCs was evaluated as described in “Cell proliferation assay.” Results are the mean plus or minus SEM values of 5 different experiments. (C) Migration capacity of late EPCs was evaluated using a wound healing assay. Results are the mean ± SEM values of 4 different experiments. *P < .05, **P < .01, ***P < .001, experimental versus control.
Effect of rh-sCD146 on angiogenic capacity of EPCs in vitro. (A) EPC capacity to elaborate pseudocapillaries in Matrigel plugs was evaluated in different conditions (control, rh-sCD146, c-myc peptide, immunodepleted rh-sCD146 [Ip rh-sCD146] and its control [IpC], or VEGF). Number of tubes was counted after 5 hours of incubation. Results are the mean ± SEM values of 6 different experiments. (B) Proliferation capacity of late EPCs was evaluated as described in “Cell proliferation assay.” Results are the mean plus or minus SEM values of 5 different experiments. (C) Migration capacity of late EPCs was evaluated using a wound healing assay. Results are the mean ± SEM values of 4 different experiments. *P < .05, **P < .01, ***P < .001, experimental versus control.
Because rh-sCD146 and VEGF effects were very similar, we tested whether their effects were additive, synergistic, or not. To this end, the same experiments were performed with the addition of both factors: 50 ng/mL rh-sCD146 and 20 ng/mL VEGF (Figure 3). Results show that effects of both molecules are additive on EPC proliferation (Figure 3A), migration (Figure 3B), and ability to form capillary-like structures in Matrigel (Figure 3C). To go further in the mechanism, we performed additional experiments of capillary tube formation in Matrigel in the presence of anti-VEGFR2 antibodies. These antibodies were either incubated with the cells all along the treatment with VEGF and/or rh-sCD146 to totally block VEGFR2, or preincubated with the cells before antibodies washing and further treatment with rh-sCD146 plus VEGF. This last condition allows blocking of the VEGFR2 present on the membrane before stimulation, but not VEGFR2 eventually induced by rh-sCD146. Results (Figure 3C) show that anti-VEGFR2 antibodies: (1) had no effect in control condition, (2) totally blocked the VEGF effect, and (3) partially blocked the effect of rh-sCD146. When rh-sCD146 and VEGF were added in the presence of antibodies, the number of capillary-like structures was decreased compared with the condition without antibodies, and the number of tubes was similar to that observed with rh-sCD146 in the presence of anti-VEGFR2 antibodies. Finally, when cells were pretreated with the anti-VEGFR2 antibodies before washing of the antibodies and further stimulation with rh-sCD146 and VEGF, the number of capillary-like structures was significantly higher than in the previous condition, suggesting the induction of new VEGFR2 by rh-sCD146 at the cell surface. Altogether, these experiments indicate that rh-sCD146 response involves in the majority a sCD146-specific pathway but also, in a minor part the VEGF signaling pathway, by inducing new VEGFR2 at the cell surface.
Additive effect of rh-sCD146 and VEGF on angiogenic capacity of EPCs in vitro. (A) Proliferation capacity of late EPCs was evaluated when rh-sCD146 (50 ng/mL) and VEGF (20 ng/mL) were added together and compared with the effect of each growth factor added separately. Results are the mean ± SEM values of 4 different experiments. (B) Migration capacity of late EPCs was evaluated using a wound healing assay when rh-sCD146 (50 ng/mL) and VEGF (20 ng/mL) were added together and compared with the effect of each growth factor added separately. Results are the mean ± SEM values of 4 different experiments. (C) EPC capacity to elaborate pseudocapillaries in Matrigel plugs was evaluated in different conditions. Number of capillary-like structures was evaluated when rh-sCD146 (50 ng/mL) and VEGF (20 ng/mL) were added together and compared with the effect of each growth factor added separately. In addition, the effect of an anti-VEGFR2 antibody (Ab) preincubated before growth factor(s) addition was tested in control condition (Ab), in the presence of rh-sCD146 (Ab + rh-sCD146), in the presence of VEGF (Ab + VEGF), and in the presence of the 2 growth factors (Ab + rh-sCD146 + VEGF). In a last condition (washed Ab + rh-sCD146 + VEGF), the antibody was preincubated and then washed before addition of the 2 growth factors. Number of tubes was counted after 5 hours of incubation. Results are the mean ± SEM values of 6 different experiments. *P < .05, **P < .01, ***P < .001, experimental versus control.
Additive effect of rh-sCD146 and VEGF on angiogenic capacity of EPCs in vitro. (A) Proliferation capacity of late EPCs was evaluated when rh-sCD146 (50 ng/mL) and VEGF (20 ng/mL) were added together and compared with the effect of each growth factor added separately. Results are the mean ± SEM values of 4 different experiments. (B) Migration capacity of late EPCs was evaluated using a wound healing assay when rh-sCD146 (50 ng/mL) and VEGF (20 ng/mL) were added together and compared with the effect of each growth factor added separately. Results are the mean ± SEM values of 4 different experiments. (C) EPC capacity to elaborate pseudocapillaries in Matrigel plugs was evaluated in different conditions. Number of capillary-like structures was evaluated when rh-sCD146 (50 ng/mL) and VEGF (20 ng/mL) were added together and compared with the effect of each growth factor added separately. In addition, the effect of an anti-VEGFR2 antibody (Ab) preincubated before growth factor(s) addition was tested in control condition (Ab), in the presence of rh-sCD146 (Ab + rh-sCD146), in the presence of VEGF (Ab + VEGF), and in the presence of the 2 growth factors (Ab + rh-sCD146 + VEGF). In a last condition (washed Ab + rh-sCD146 + VEGF), the antibody was preincubated and then washed before addition of the 2 growth factors. Number of tubes was counted after 5 hours of incubation. Results are the mean ± SEM values of 6 different experiments. *P < .05, **P < .01, ***P < .001, experimental versus control.
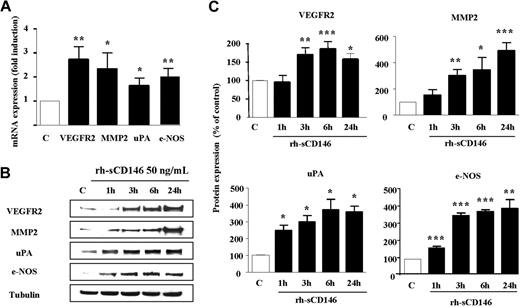
To test this hypothesis and further analyze the proangiogenic signature triggered by rh-sCD146 in late EPCs, we used an oligoarray specific for angiogenic genes (“Gene expresson profiling”). Among the 113 probed genes, eNOS, VEGFR2, MMP-2, and uPA were reproducibly up-regulated (at least 5-fold) in late EPCs treated for 3 hours with 50 ng/mL rh-sCD146 (Figure 4). Increased expression of the mRNAs for these 4 genes 3 hours after treatment was confirmed by quantitative PCR (Figure 4A). At the protein level, Western blot analysis demonstrated that expression of eNOS and uPA increased significantly 1 hour after treatment, whereas the increase in VEGFR2 and MMP-2 was observed 3 hours after treatment (Figure 4B-C). Enhancement of protein expression was sustained for 24 hours.
Up-regulation of angiogenic gene transcripts and products in EPCs in response to rh-sCD146. (A) Alterations in VEGFR2, MMP-2, uPA, and eNOS mRNA levels after rh-sCD146 treatment were analyzed by quantitative PCR. Results are the mean values of 4 different experiments. (B) Kinetics of induction of MMP-2, eNOS, uPA, and VEGFR2 proteins were performed. A representative experiment is shown for each protein. (C) Quantification of 3 to 5 experiments described in panel B. *P < .05, **P < .01, ***P < .001, experimental versus control.
Up-regulation of angiogenic gene transcripts and products in EPCs in response to rh-sCD146. (A) Alterations in VEGFR2, MMP-2, uPA, and eNOS mRNA levels after rh-sCD146 treatment were analyzed by quantitative PCR. Results are the mean values of 4 different experiments. (B) Kinetics of induction of MMP-2, eNOS, uPA, and VEGFR2 proteins were performed. A representative experiment is shown for each protein. (C) Quantification of 3 to 5 experiments described in panel B. *P < .05, **P < .01, ***P < .001, experimental versus control.
Experiments were also performed to test whether VEGF secretion was modified by rh-sCD146 treatment. EPCs treated for 24 hours with 50 ng/mL rh-sCD146 exhibited a statistically significant increase in VEGF secretion compared with nontreated EPCs (71.8 ± 6.1 vs 54.8 ± 3.2 pg/mL, n = 6; P < .05).
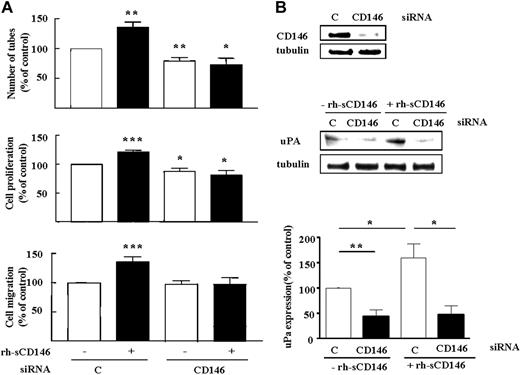
The recently described role of membrane CD146 in angiogenesis5-7 prompted us to evaluate its involvement in angiogenic properties of sCD146. We thus investigated the effect of rh-sCD146 on angiogenic functions and induction of gene expression on human late EPCs lacking CD146 (CD146 siRNA; Figure 5). CD146 silencing prevented the rh-sCD146-induced increase in late EPC tube formation, proliferation, and migration (Figure 5A) as well as uPA induction (Figure 5B), consistent with the involvement of membrane CD146 in angiogenic responses induced by sCD146.
Requirement for membrane CD146 expression in angiogenic effects of rh-sCD146. (A) Effect of CD146 siRNA on rh-sCD146–mediated effects on late EPC capacity to generate pseudocapillaries in Matrigel, proliferation, and migration. Results are mean values of 4 different experiments. (B) Effect of CD146 siRNA on rh-sCD146–mediated induction of uPA. A representative experiment and the mean values of 3 different experiments are shown. *P < .05, *P < .01, ***P < .001, experimental versus control.
Requirement for membrane CD146 expression in angiogenic effects of rh-sCD146. (A) Effect of CD146 siRNA on rh-sCD146–mediated effects on late EPC capacity to generate pseudocapillaries in Matrigel, proliferation, and migration. Results are mean values of 4 different experiments. (B) Effect of CD146 siRNA on rh-sCD146–mediated induction of uPA. A representative experiment and the mean values of 3 different experiments are shown. *P < .05, *P < .01, ***P < .001, experimental versus control.
Local injection of rh-sCD146 increases vascularization in a rat ischemic hind-limb model
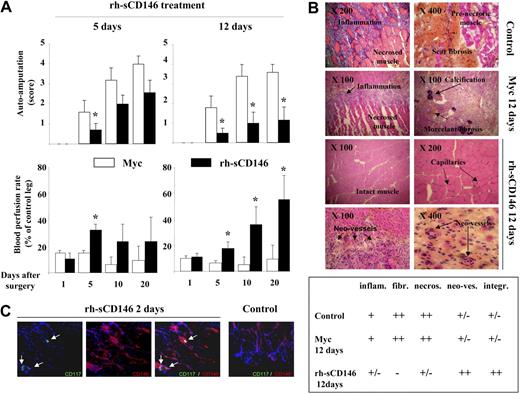
Chemotactic and angiogenic properties of rh-sCD146 led us to investigate its potential therapeutic use in a model of rat hind-limb ischemia (Figure 6). Ischemic rats were first treated with 10 μg of rh-sCD146/day for 5 days (Figure 6A). A significant decrease in auto-amputation and a significant increase in blood perfusion rate in the ischemic hind-limb were observed 5 days after surgery, compared with ischemic rats treated with the c-myc peptide (control ischemic rats). Because no further modification was detectable between the 2 groups at days 10 and 20, rats were treated for a longer period of time (12 days) with the same dose of rh-sCD146 (10 μg/day). In this condition, auto-amputation was significantly decreased up to day 20 after surgery compared with control ischemic rats. The blood perfusion rate was also significantly increased from day 5 to day 20, reaching approximately 60% of control leg in the same animals at day 20 (Figure 6A). In control ischemic rats, histochemical examination of muscle sections 20 days after surgery showed inflammation, calcification, fibrosis areas, and numerous necrotic muscular fibers in ischemic hind limbs (Figure 6B). In contrast, in ischemic rats treated with rh-sCD146 for 12 days, fibrosis, inflammation, and the number of necrotic muscular fibers were highly reduced (Figure 6B and semiquantification). In addition, the number of capillaries was increased and the majority of muscular fibers were intact (Figure 6B and semiquantification). Interestingly, we also observed a trophic effect of rh-sCD146 on healing of amputated limbs (data not shown).
Effect of local injection of rh-sCD146 in a rat ischemic hind-limb model. (A) Ischemic rats were subjected to a daily local injection of either 10 μg/mL c-myc peptide or 10 μg/mL rh-sCD146 for 5 or 12 days. Auto-amputation level and blood perfusion rate were monitored. Results are mean values of 9 different animals in each group. *P < .05, experimental versus control. (B) Histochemical examination of hind-limb muscle sections from control, c-myc peptide-treated, or rh-sCD146–treated rats 12 days after surgery. Muscle features, including inflammation and fibrosis levels, amount of necrotic fibers, angiogenesis, and muscle aspect in control animals, c-myc–treated, and rh-sCD146–treated rats (12 days) are described using a semiquantification system: − indicates absence; ±, low expression; +, intermediate expression; and ++, high expression. (C) Coimmunostainings were performed with anti-CD117 (green) and anti-CD146 (red) antibodies in muscle sections of ischemic rats treated for 2 days with rh-sCD146 or not (control). Nuclei were labeled with DAPI (blue). The merge pictures are given. Yellow areas correspond to a colabeling (indicated with an arrow).
Effect of local injection of rh-sCD146 in a rat ischemic hind-limb model. (A) Ischemic rats were subjected to a daily local injection of either 10 μg/mL c-myc peptide or 10 μg/mL rh-sCD146 for 5 or 12 days. Auto-amputation level and blood perfusion rate were monitored. Results are mean values of 9 different animals in each group. *P < .05, experimental versus control. (B) Histochemical examination of hind-limb muscle sections from control, c-myc peptide-treated, or rh-sCD146–treated rats 12 days after surgery. Muscle features, including inflammation and fibrosis levels, amount of necrotic fibers, angiogenesis, and muscle aspect in control animals, c-myc–treated, and rh-sCD146–treated rats (12 days) are described using a semiquantification system: − indicates absence; ±, low expression; +, intermediate expression; and ++, high expression. (C) Coimmunostainings were performed with anti-CD117 (green) and anti-CD146 (red) antibodies in muscle sections of ischemic rats treated for 2 days with rh-sCD146 or not (control). Nuclei were labeled with DAPI (blue). The merge pictures are given. Yellow areas correspond to a colabeling (indicated with an arrow).
Because CD117/CD146+ endothelial precursor cells participating in vascular-like structures were observed in vivo in Matrigel plugs containing rh-sCD146 (Figure 1B), we tested whether such cells could also be detected in rat muscles after ischemia. To this end, we analyzed muscles of rats with hind-limb ischemia, treated or not with rh-sCD146, 2 days after the beginning of the treatment to detect early events. Results show that CD117/CD146+ cells could be detected in rh-sCD146–treated animals, whereas these cells were not found in control rats (Figure 6C).
Discussion
CD146 is a component of the endothelial junction involved in the control of vascular permeability, monocyte transmigration, and vessel growth, but very few studies have focused on the biologic functions of its soluble form. Here, we report, for the first time, that sCD146 displays chemotactic and angiogenic properties. These effects are additive with those of VEGF, require the expression of membrane CD146, and are associated with the induction of proangiogenic genes. Interestingly, administration of sCD146 promotes neovascularization in a rat model of hind-limb ischemia.
Up to now, the soluble form of CD146 has been mainly described in human serum as an endothelial biomarker that is modulated in vascular inflammatory diseases, such as idiopathic inflammatory myopathies23 or inflammatory bowel diseases.9 In patients with active Crohn disease, decreased levels of sCD146 were associated with increased expression of membrane CD146 in areas of neovascularization.9 A clinical improvement, observed in patients treated with the anti–tumor necrosis factor antibody infliximab, was associated with normalized sCD146 levels.24 To understand the significance of these variations, the biologic role of sCD146 was further investigated. Using culture endothelial cells stimulated by tumor necrosis factor, we recently reported that sCD146 increases endothelial permeability and stimulates monocyte transendothelial migration in inflammatory conditions.4 The present data indicate that, in addition to its role in inflammation, sCD146 displays angiogenic properties. One can thus hypothesize that sCD146 variations observed in pathologies could reflect not only the inflammatory but also the angiogenic status of the patient. In this way, it has been suggested that sCD146 of synovial fluid may constitute a marker for synovial membrane angiogenesis in rheumatoid arthritis.25 Various growth factors regulate in parallel angiogenesis, vascular permeability, and cell transmigration. As an example, the angiogenic factor VEGF induces vascular permeability and macrophage migration.26 Likewise, stromal-derived factor-1 is an important chemokine involved in angiogenesis and migration of human mast cells.27 In the literature, inflammation and angiogenesis have been frequently linked. Common molecular mechanisms and signaling pathways have been described in these 2 processes, and it has been shown that several proinflammatory cytokines induced during inflammation could promote a suitable microenvironment for angiogenesis.28 In the literature, different soluble receptors, as soluble EphB429 or soluble Notch1,30 have been shown to act as endogenous inhibitors of angiogenesis, acting as traps for their ligand. This is also the case for the soluble form of VEGFR2, which blocks the angiogenic effect of VEGF.31 In contrast, other soluble molecules have been shown to act as activators of angiogenesis, such as the soluble N-cadherin fragment32 or the soluble CD40 ligand.33 The reason for the observed opposite effects of soluble molecules, inhibitor or activator, is unknown but may result from distinct signaling pathways. Thus, one can hypothesize that soluble forms of receptor molecules may trap the ligand and inhibit the effect. In contrast, other soluble molecules, such as sCD146, result from a membrane protein shedding and could serve as a ligand that activates its receptor. Such an activating effect of sCD146 has recently been described on monocytes.4 Up to now, the receptor of sCD146 is still unknown. However, the membrane CD146 is not this receptor because we were unable to evidence homophilic interactions between both molecules.4
Chemotactic activity constitutes a major property of sCD146. Indeed, sCD146 induces the recruitment of different cell types, including hematopoietic cells as monocytes, smooth muscle cells, and/or pericytes. Interestingly, we recently showed that sCD146 was able to bind monocytes and that this binding resulted in an increased transendothelial monocyte transmigration.4 In addition, sCD146 diplays chemotactic activity on cells with endothelial characteristics, as attested by their insertion into vascular-like structures and their positive staining for endothelial marker as CD34, CD146, or CD31. We can thus hypothesize that the receptor of sCD146 could be present on these different cells. Interestingly, most of the cells are involved in the formation of the vessel wall. Among the endothelial cells, some cells (∼ 10%-15%) express both CD31 or CD146 and the immaturity marker CD117, suggesting the recruitment of a subpopulation of endothelial cells with precursor characteristics, which are able to participate in the vascular-like structures. Double staining of these cells shows that they are CD33− and CD45−, indicating that they are not of myeloid or hematopoietic origin. Altogether, these results are in favor of the recruitment by sCD146 of precursor endothelial cells, with phenotype of late EPCs, able to participate in the vessel formation. In agreement with these data, exogenously injected human late EPCs could be recruited in Matrigel plug containing sCD146 and were also able to organize as vascular-like structures.
Most of endothelial growth factors involved in new blood vessel formation have important features in common. They are mitogens for endothelial cells, promote endothelial cell migration, and up-regulate different proteins as matrix proteinases, cytokines, and adhesion molecules.34 In this line, VEGF is a potent angiogenic agent that regulates all the key steps of the angiogenic process, including endothelial cell proliferation and migration.35 VEGF is also important in postnatal vasculogenesis, specifically in EPC mobilization into the circulation. In this study, we show that sCD146 displays angiogenic properties, as shown by its capacity to increase proliferation, migration, and ability to form capillary-like structures in Matrigel, as well as its ability to stimulate endothelial cell recruitment. Effects observed with sCD146 were similar to that obtained with VEGF. Our experiments show that the signaling pathway of sCD146 is different from that of VEGF. Indeed, rh-sCD146 and VEGF have additive effects on EPCs. Interestingly, signaling pathways of both factors are however interconnected because our data show that sCD146 (1) induces the VEGFR2 expression and (2) enhances VEGF secretion, supporting its role as a growth factor. Concerning the sCD146 receptor, we showed in a previous work that no homophilic interaction occurred between soluble and membrane CD146.4 In the present study, we show that sCD146 did not increase angiogenic capacity in the absence of membrane CD146. Together, these data suggest that a yet unknown receptor for sCD146 could mediate a proangiogenic pathway relying on both soluble and membrane CD146 to control angiogenic responses in endothelial cells. Among the potential pathways, one could depend on AKT activation.36 Indeed, in human melanoma, overexpression of CD146 activated the AKT pathway known to regulate proliferation, migration, and invasion processes.36 Another pathway could imply the up-regulation of proangiogenic factors. Indeed, we found that sCD146 induces several proteins of importance during angiogenesis, a phenomenon abolished in EPCs silenced for CD146. Among these proteins, VEGFR2 is highly increased. It is known that the activity of this receptor is enhanced during vasculogenesis or during tumor angiogenesis, its overexpression stimulating in turn that of several proteins, such as uPA, uPAR, and some MMPs.37 Among the genes induced by sCD146, uPA and MMP-2 were found, which belong to proteolytic complexes involved both in physiologic and tumoral-associated angiogenesis through promoting the degradation of both the basal membrane and the extracellular matrix during migration and cellular invasion. This is consistent with the essential role of uPA and MMP-2 in angiogenic behavior of late EPCs in vitro.38 Consistent with our data, treatment of melanoma with anti-MUC18 antibodies decreased the capacity of HUVECs to colonize Matrigel plugs in vitro; this decrease of HUVEC invasiveness was associated with a decrease in the collagenase activity of MMP-2.6 Finally, eNOS expression is also enhanced by sCD146 treatment. This protein plays a key role in mobilization and angiogenic properties of stem cells. Accordingly, pretreatment of bone marrow mononuclear cells derived from patients with ischemic cardiomyopathy with a novel eNOS synthase transcription enhancer, AVE9488, restored the capacity of these progenitor cells to induce neovascularization.39 Likewise, it was proposed that NO-dependent vessel vasodilatation and hyperpermeability were critical for bone marrow-derived mononuclear cell infiltration in ischemic tissues and for their proangiogenic potential.40
Evidence of proangiogenic properties of sCD146 in vitro led us to investigate whether it could promote neovascularization in vivo. In an animal model of limb ischemia, administration of sCD146 induces a beneficial effect in terms of limb functionality and blood flow with an increase in neovascularization associated to a decrease in inflammation, necrosis, and fibrosis. Although future studies will now be necessary to confirm these effects in human preclinical models, we propose that sCD146 may constitute a novel angiogenic factor, able to stimulate therapeutic angiogenesis. The complete mechanism governing the favorable effects of sCD146 is unknown and remains to be established, but several pathways could be involved. Thus, sCD146 could act on local endothelial resident cells and/or monocyte infiltration because we evidenced a chemotactic effect on monocytes in Matrigel plugs in vivo. Alternatively, or additionally, sCD146 may play a role in vasculogenesis by recruiting EPCs to an area of neovascularization, as described for other growth factor, such as VEGF.41 In agreement with this last hypothesis, cells presenting characteristics of late endothelial progenitors were recruited in Matrigel plugs and organized as vascular-like structures. In addition, immature endothelial cells could be observed in muscle sections of animals after 2 days of treatment with sCD146. Future studies will now be necessary to explore these different pathways.
In conclusion, sCD146 displays chemotactic and angiogenic properties and promotes neovascularization in an animal model of hind-limb ischemia. Thus, besides its role as a biomarker, sCD146 could be used as a proangiogenic factor. sCD146 could therefore become a new tool for therapeutic angiogenesis in patients with ischemic diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr P. Gascard for critical reading of the manuscript (University of California San Francisco) and Biocytex company for providing rh-sCD146.
This work was supported by Inserm.
Authorship
Contribution: M.B.-C. designed experiments, analyzed data, and wrote the manuscript; N.B., F.S., J.S., and F.D.-G. analyzed data and wrote the manuscript; B.G. and P.P. designed experiments, performed experiments, analyzed data, and edited the manuscript; K.H. and A.K. designed and performed experiments and analyzed data; and A.F.-B., E.L., S.V., M.-D.P.-M., C.B., F.V., and L.O. performed experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcel Blot-Chabaud, UMR-S 608 Inserm-Laboratoire d'Hématologie et d'Immunologie, Unite de Formation et de Recherche de Pharmacie, 27 Bd J Moulin, 13385 Marseille Cedex 5, France; e-mail: marcel.blot-chabaud@laposte.net.

![Figure 2. Effect of rh-sCD146 on angiogenic capacity of EPCs in vitro. (A) EPC capacity to elaborate pseudocapillaries in Matrigel plugs was evaluated in different conditions (control, rh-sCD146, c-myc peptide, immunodepleted rh-sCD146 [Ip rh-sCD146] and its control [IpC], or VEGF). Number of tubes was counted after 5 hours of incubation. Results are the mean ± SEM values of 6 different experiments. (B) Proliferation capacity of late EPCs was evaluated as described in “Cell proliferation assay.” Results are the mean plus or minus SEM values of 5 different experiments. (C) Migration capacity of late EPCs was evaluated using a wound healing assay. Results are the mean ± SEM values of 4 different experiments. *P < .05, **P < .01, ***P < .001, experimental versus control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/18/10.1182_blood-2009-06-229591/4/m_zh89991051620002.jpeg?Expires=1768061245&Signature=OSH9Cs4LFhzNBiyHX2JJ5gHg0NS8WflVFjK8o8FBbYZy~gmYJwflETgsu0w6BYjPGtqqbGQ7sVUmHlySZHzSnlKJr~RSV1z8XgxuTFStZzwcwSaREDtV75HgKSmLv8UBCJteaG8eoqxyUqHiF5yhLjRYWzz1a6IS5w73gNL~uYjZiClvBDVh6C0HvPLdSbX7br2HJe7jvWE~lz3FDKVJoZsfPhZTcqj3j1Veo4ZW5TUUYHbHqstXb-kjSJ4RUt9sTNrYZVxl0tosLxA90Xg-1dUDasDKnxXFF6jPEHuzA4qPm5jzrtPatDNFsPdzChJU0jSRK0QOuZMA5WdTvY8qzQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)