Abstract
Human mesenchymal stem cells (hMSCs) localized to bone marrow, nonhematopoietic organs, as well as perivascular niches are postulated to traffic through type I collagen-rich stromal tissues to first infiltrate sites of tissue damage, inflammation, or neoplasia and then differentiate. Nevertheless, the molecular mechanisms supporting the ability of hMSCs to remodel 3-dimensional (3D) collagenous barriers during trafficking or differentiation remain undefined. Herein, we demonstrate that hMSCs degrade and penetrate type I collagen networks in tandem with the expression of a 5-member set of collagenolytic matrix metalloproteinases (MMPs). Specific silencing of each of these proteases reveals that only a single membrane-tethered metalloenzyme, termed MT1-MMP, plays a required role in hMSC-mediated collagenolysis, 3D invasion, and intravasation. Further, once confined within type I collagen-rich tissue, MT1-MMP also controls hMSC differentiation in a 3D-specific fashion. Together, these data demonstrate that hMSC invasion and differentiation programs fall under the control of the pericellular collagenase, MT1-MMP.
Introduction
In response to signaling cascades initiated at sites of injury, inflammation, or neoplasia, human mesenchymal stem cells (hMSCs) are postulated to mobilize from bone marrow, nonhematopoietic organs, or perivascular niches to infiltrate affected host tissues and differentiate in a lineage-specific fashion.1-4 Despite increased interest in the potential roles played by hMSCs in regenerating injured tissues, quenching proinflammatory events, or modulating cancer cell behavior,1,4,5 the mechanisms that confer stem cell populations with the ability to traverse 3-dimensional (3D) connective tissues and differentiate remain undefined.
In vivo, type I collagen is the dominant extracellular matrix (ECM) component found in mammalian tissues.6,7 Cell types of myeloid origin are distinct in their ability to traffic through collagen-rich tissues or distort their overall shape to “squeeze” through stromal pores.7,8 By contrast, increasing evidence suggests that nonmyeloid cell types mobilize proteolytic enzymes to generate ECM-free passageways that are permissive for cell invasion.7 Although collagen is resistant to almost all forms of proteolytic attack, the triple-helical fibers can be degraded by secreted as well as a membrane-tethered members of matrix metalloproteinase (MMP) gene family.9,10 Nevertheless, the relative roles played by protease-dependent and -independent systems during hMSC invasion through collagenous barriers remain controversial. Similarly, although ECM composition, ligand density, and mechanical rigidity are known to affect hMSC differentiation programs,11,12 the regulatory roles played by collagenolytic enzymes in dictating stem cell commitment remain unexplored. Herein, we demonstrate that a single, membrane-tethered matrix metalloproteinase, termed membrane type-1 MMP (MT1-MMP), not only controls hMSC trafficking through the interstitial ECM in vitro and in vivo but also directs hMSC differentiation programs as well.
Methods
Cell culture
hMSCs (positive for CD105, CD166, CD29, and CD44 and negative for CD14, CD34, and CD45) were obtained from Lonza or obtained as a gift from D. Prockop (Tulane University). hMCSc were cultured routinely in a Mesenchymal Stem Cell Growth Medium Bullet Kit (Lonza) and maintained at 37°C in humidified air atmosphere containing 5% CO2. Cells were passaged when 90% confluent by the use of trypsin-EDTA (ethylenediaminetetraacetic acid; Lonza).
RT–PCR analysis
Total RNA was extracted from hMSCs by the use of TRIzol reagent (GIBCO-BRL). Reverse transcription (RT) was performed with 1 μg of total RNA and 10μM of specific primers as described.13 cDNAs were amplified by polymerase chain reaction (PCR) for (h)MMP-1 (sense 5′-GAGCAAACACATCTGACCTACAGGA-3′; antisense 5′-TTGTCCCGATGATCTCC CCTGACA-3′, 185-bp product), (h)MMP-2 (sense 5′-GTGCTGAAGGAC ACACTAAAGAAGA-3′; antisense 5′-TTGCCATCCTTCTCAAAGTTGTAGG-3′, 605-bp product), (h)MMP-13 (sense 5′-TCCCAGGAATTGGTGATAAAGTAGA-3′; antisense 5′-CTGG CATGACGCGAACAATA-3′, 123-bp product), (h)MT1-MMP (sense 5′-CGCTACGCCATCCAGGGTCTCAAA-3′; antisense 5′-CGGTCATCATCGGGCAGCA CAAAA-3′, 497-bp product), (h)MT2-MMP (sense 5′-ACAACCACCATCTGACCTT TAGCA-3′; antisense 5′-AGCTTGAAGTTGTCAACGTCCTTC-3′, 454-bp product), (h)MT3-MMP (sense 5′-CGGTGTACCAGACCAGACAA-3′; antisense 5′-GATTAGGATTTCCTAGTGTCC-3′, 401-bp product), or glyceraldehyde 3-phosphate dehydrogenase (GAPDH; sense 5′-ACCACAGTCCATGCCATCAC-3′; antisense 5′-TCCACCACCCTGTTGCTGTA-3′, 556-bp product).
siRNA and plasmid construct electroporation
The antisense strand of siRNAs were targeted against a 21-nt sequence in (h)MT1-MMP (5′-AACAGGCAAAGCTGAT GCAGA-3′; nt 228-248), (h)MMP-1 (5′-AAGATGTGGACTTAGTCCAGA-3′; nt 157-177), (h)MMP-2 (5′-AATACCATCGAGACCATGCGG-3′; nt 578-598), (h)MMP-13 (5′-AAGATGATTTGTCTGAGGAAG-3′; nt 111-131), and (h)MT3-MMP (5′-AAGCCAA TCACAGTCTGGAAA-3′; nt 1423-1443). An siRNA control sequence was generated by scrambling the (h)MT1-MMP siRNA (5′-AAGTGATCAAGCACCGAAGAG-3′). siRNA oligonucleotides (QIAGEN), 40nM, were introduced into hMSCs by the use of a nucleofector kit and electroporation (Amaxa Biosystems) as described previously.13,14 For rescue experiments, hMSCs were cotransfected with a control expression vector or mouse MT1-MMP expression vector (gift of M. Seiki, University of Tokyo) that escapes targeting by the (h)MT1-MMP siRNA. Silencing efficiency was established 48 hours after electroporation by RT-PCR and the hMSCs used thereafter.
Collagen-degradation assays
Type I collagen gel films (∼ 100 μg distributed uniformly over an area of 2 cm2 on glass chamber slides) were labeled with Alexa Fluor 594 or Alexa Fluor 647 (Molecular Probes) for 1 hour at 25°C. Control or siRNA-transfected hMSCs were cultured atop the collagen films in stem cell growth medium for 4 days. In selected experiments, hMSCs were cultured in the presence of 25μM GM6001 (Calbiochem) or active-site titrated recombinant human tissue inhibitor of metalloproteinases 1 (TIMP-1; 7.5 μg/mL) or recombinant human TIMP-2 (2.5 μg/mL; both from Fuji Chemicals).15 Where indicated, hMSCs were stained with CELLMask plasma membrane dye (Molecular Probes). To monitor colocalization of MT1-MMP, cortactin, and phalloidin at sites of collagen degradation, cells were cultured on Alexa Fluor 647 (Molecular Probes)–labeled collagen film for 2 days. Cell-surface MT1-MMP was stained with Alexa Fluor 532–labeled anti–MT1-MMP16 for 2 hours at 4°C. After permeabilization with 0.25% Triton X-100, cells were then incubated with rabbit polyclonal anti-cortactin 1:100 (Santa Cruz Biotechnology Inc) at 4°C overnight and/or incubated with Alexa Fluor 488–labeled phalloidin for 1 hour at 25°C. To visualize collagen degradation products, collagen gels were stained with a monoclonal antibody, 9A4 (generously provided by Pfizer, Inc), directed against a collagenase-cleavage neoepitope.17 Fluorescent images were captured by confocal laser microscopy. Collagen degradation products were quantified after a 4-day culture period as solubilized hydroxyproline-containing collagen fragments as described previously.13 Results are presented as the mean plus or minus SEM of 3 experiments.
Collagen invasion assays
Type I collagen from rat tail tendons was dissolved in 0.2% acetic acid at a final concentration of 2.2 mg/mL. To initiate gelling, collagen was mixed with 10× Dulbecco modified Eagle medium and 0.34N NaOH in an 8:1:1 ratio at 4°C. For 2D invasion assays, 1 mL of the neutralized mixture was added to the upper well of a 24-mm Transwell dish (3-μm pore size; Fisher Scientific) and allowed to gel at 37°C for 1 hour. Subsequently, 105 hMSCs or siRNA-electroporated hMSCs were added to the upper chamber of the Transwells and cultured for 4 days in stem cell growth media. Where indicated, invasion assays were performed in the presence of GM6001 (25μM), human recombinant TIMP-1 (7.5 μg/mL), or TIMP-2 (2.5 μg/mL). hMSC invasion was monitored by phase-contrast microscopy and the number of invading cells determined in 5 randomly selected fields. After the 4-day culture period, samples were fixed, cross-sectioned, and stained and invasion depth was measured in 5 randomly selected fields.
For 3D invasion, 2 × 105 hMSCs were suspended in 10 μL of culture medium, mixed with 100 μL of collagen (2.2 mg/mL), and the suspension gelled in 96-well plates at 37°C for 1 hour. The collagen–hMSC plugs were then transferred to 24-well culture plates, further embedded in 0.5 mL of collagen (2.2 mg/mL), and the composite cultured for 3 days in growth medium in the absence or presence of GM6001 (25μM), TIMP-1 (7.5 μg/mL), or TIMP-2 (2.5 μg/mL). Cell migration from the central collagen plug into the surrounding collagen gel was monitored by phase-contrast microscopy, and the number of invading cells as well as the mean distance migrated plus or minus 1 SD in 5 randomly selected fields in a single representative experiment of 3 or more were performed.
Chick chorioallantoic membrane assays
hMSCs (106) were fluorescently labeled with 5% fluoresbrite carboxylate microspheres (Polysciences) and then cultured atop the chorioallantoic membrane (CAM) of 11-day-old chick embryos for 2 days as described previously.13,14 The percentage invading cells and invasion depth from the CAM surface into the underlying stromal tissues were quantified in 3 or more randomly selected fields as described,13 with results presented as the mean plus or minus SEM for 3 or more experiments. hMSC intravasation/extravasation into the lower CAM was detected as Alu-sequences by PCR on DNA extracts.13
hMSC differentiation
hMSCs (cultured in the presence or absence of 7.5 μg/mL TIMP-1 or 2.5 μg/mL TIMP-2) or hMSCs transfected with a scrambled siRNA control or MT1-MMP siRNA were either seeded atop type I collagen gels (4 × 103 cells/cm2) or mixed with collagen (2 × 105 cells) and seeded in 24-well plates. In selected experiments, acid-extracted type I collagen was replaced with pepsin-extracted type I collagen (Vitrogen; Advanced Biomatrix, Inc) to prepare 3D gels at an equivalent concentration (2.2 mg/mL).7 hMSCs were cultured in osteogenesis differentiation medium (Invitrogen) for 7 to 9 days. Alkaline phosphatase (ALP) staining was performed by the use of a mixture of napthol AS-BI alkaline solution with fast blue BB (Leukocyte Alkaline Phosphatase Kit, Sigma-Aldrich). Images were captured by phase-contrast microscopy. For ALP quantification, cells were collected from 3D collagen culture after incubation with 1% collagenase at 37°C and were lysed in buffer containing 0.05% Triton X-100 in 50mM Tris–HCl (pH 7.4). Lysate was incubated with ALP substrate, p-nitrophenyl phosphate (Sensolyte pNPP alkaline phosphatase kit, ANASPEC) at 37°C for 1 hour and absorbance determined at 405 nm.
Real-time quantitative PCR analysis
hMSCs electroporated with a scrambled control siRNA or MT1 siRNA were induced in osteogenesis differentiation media in 3D collagen for 7 days, and total RNA was extracted with Trizol reagent (Invitrogen) and RNeasy kit (QIAGEN) and then reverse-transcribed into cDNA by the use of SuperScript First-Strand Synthesis System (Invitrogen). Real-time PCRs were performed by use of the SYBR Green PCR Master Mix (Applied Biosystems) and specific primers for ALP (sense 5′-AAGGCTTCTTCTTGCTGGTGG-3′; antisense 5′-GTGAAGACGTGGG AATGGTC-3′, 177-bp product), runt-related transcription factor 2, RUNX2 (sense 5′-TTTGCACTGGGTCATGTGTT-3′; antisense 5′-TGGCTGCATTGAAAAGACTG-3′, 156-bp product), BMP2 (sense 5′-ACCAGGTTGGTGAATCAGAA-3′; antisense 5′-CAATGGCCTTATCTGTGACC-3′, 210-bp product), or GAPDH (sense 5′-GAGCAACGGATTTGGTCGT-3′; antisense 5′-TTGATTTTGGAGGGATCTGG-3′, 238-bp) as a reference.
Results
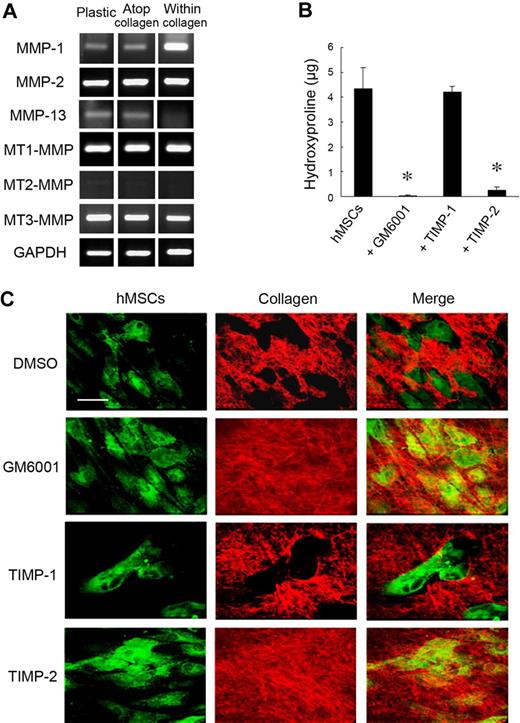
hMSCs mobilize MT1-MMP as a direct-acting type I collagenolysin
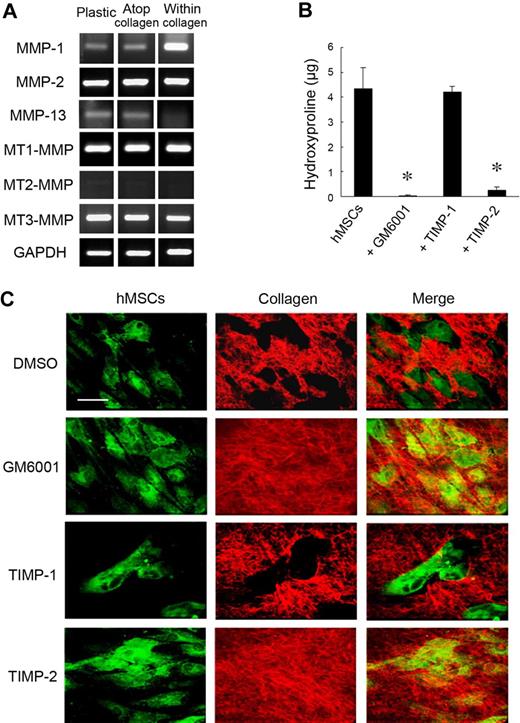
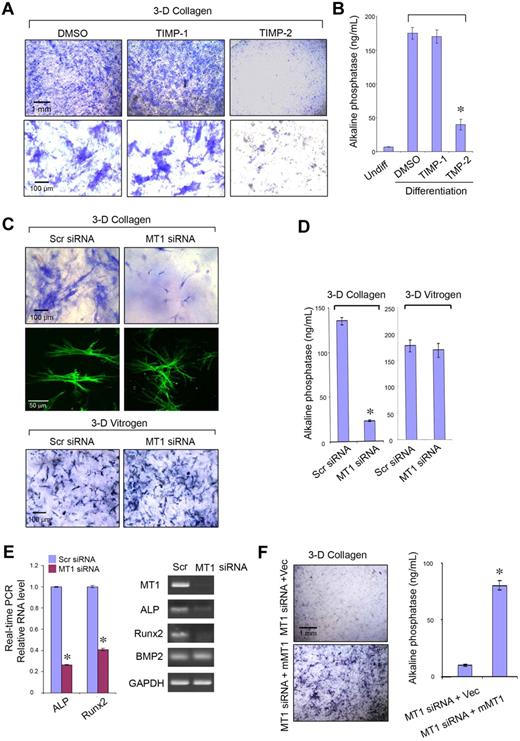
During in vitro culture, hMSCs express at least 5 MMPs with type I collagenolytic potential, ie, the secreted metalloenzymes, MMP-1, MMP-2, and MMP-13, as well as the membrane-anchored proteinases MT1-MMP and MT3-MMP (Figure 1A; MT2-MMP, a third membrane-anchored MMP than express type I collagenolytic activity,18 is not detected under these conditions).14,19 Although recent studies indicate that MMP expression may be modulated either by the composition of the underlying substratum or culture under 2D versus 3D conditions (ie, when cells are cultured either atop or embedded within a collagen gel20,21 ), hMSCs respond to the various culture conditions in a qualitatively similar fashion with regard to the repertoire of MMPs mobilized (Figure 1A). Consistent with an MMP expression pattern that includes multiple type I collagenases, hMSCs cultured atop a 3D bed of fluorescent type I collagen fibrils degrade the subjacent matrix via a process that is blocked by the synthetic MMP inhibitor, GM6001,22 as assessed quantitatively by the solubilization of hydroxyproline-containing collagen fragments (Figure 1B) or qualitatively by imaging via confocal laser microscopy (Figure 1C, ie, the green fluorescent-labeled hMSCs generate “black holes” in the underlying layer of red fluorescent-tagged type I collagen). Although the endogenous MMP inhibitor, TIMP-1, effectively targets the secreted collagenases MMP-1, MMP-2, and MMP-13,9 supraphysiologic concentrations of the recombinant inhibitor in excess of those required to block these proteases in intact cell systems9 do not affect subjacent type I collagenolysis (Figure 1B-C). By contrast, TIMP-2, a second member of the TIMP family that blocks secreted as well as membrane-anchored MMPs,9 completely ablates type I collagen degradation (Figure 1B-C).
MMP-dependent collagen-degradative activity of hMSCs. (A) RT-PCR analyses of the MMP expression profile of hMSCs cultured either on a tissue-culture plastic substratum (Plastic), atop type I collagen gels (2D; Atop collagen), or embedded within type I collagen gels (3D; Within collagen) in stem cell growth medium for 2 days. GAPDH served as the loading control. (B) Degradative activity of hMSCs (5 × 104) cultured atop type I collagen films (50 μg/cm2) in the presence or absence of GM6001 (25μM), TIMP-1 (7.5 μg/mL), or TIMP-2 (2.5 μg/mL) in growth medium for 4 days. Collagenolytic activity is quantified as hydroxyproline release (mean ± SEM; n = 3). *P < .05. (C) Films of type I collagen labeled with Alexa Fluor 594 (red) were incubated with hMSCs (5 × 104) stained with CELLMask plasma membrane dye (green), and zones of degradation visualized by confocal laser microscopy after a 4-day culture period. Bar, 50 μm.
MMP-dependent collagen-degradative activity of hMSCs. (A) RT-PCR analyses of the MMP expression profile of hMSCs cultured either on a tissue-culture plastic substratum (Plastic), atop type I collagen gels (2D; Atop collagen), or embedded within type I collagen gels (3D; Within collagen) in stem cell growth medium for 2 days. GAPDH served as the loading control. (B) Degradative activity of hMSCs (5 × 104) cultured atop type I collagen films (50 μg/cm2) in the presence or absence of GM6001 (25μM), TIMP-1 (7.5 μg/mL), or TIMP-2 (2.5 μg/mL) in growth medium for 4 days. Collagenolytic activity is quantified as hydroxyproline release (mean ± SEM; n = 3). *P < .05. (C) Films of type I collagen labeled with Alexa Fluor 594 (red) were incubated with hMSCs (5 × 104) stained with CELLMask plasma membrane dye (green), and zones of degradation visualized by confocal laser microscopy after a 4-day culture period. Bar, 50 μm.
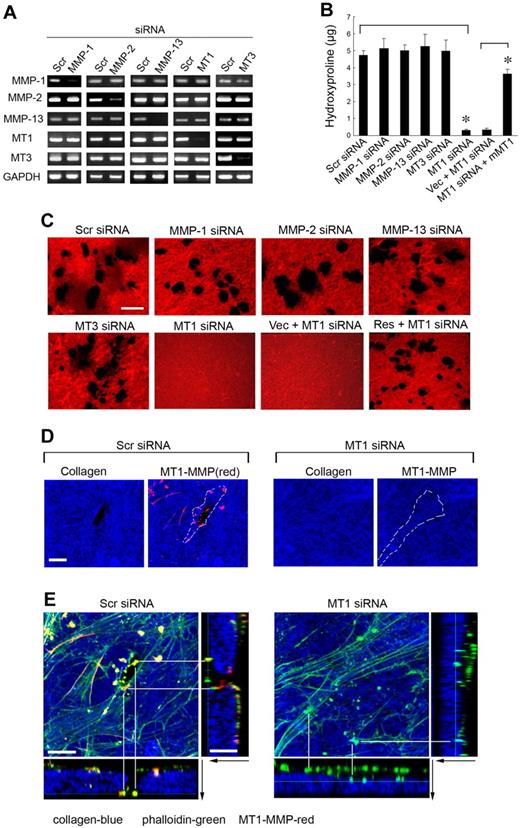
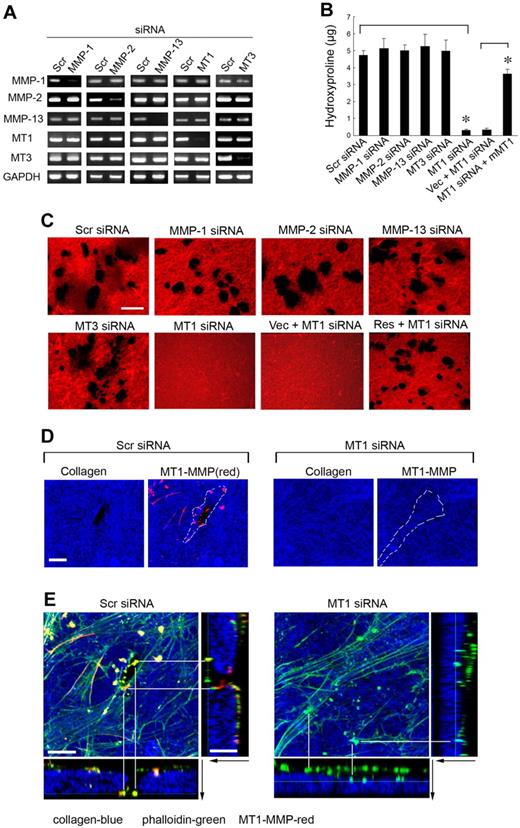
To identify the MMP(s) responsible for type I collagenolysis, hMSCs were electroporated with siRNAs directed against each of the candidate collagenases, and the impact on collagenolytic potential was assessed (Figure 2A). Under these conditions, only MT1-MMP silencing, which does not elicit compensatory changes in MMP-1, MMP-2, MMP-13, or MT3-MMP expression, abolishes subjacent collagen degradation (Figure 2A-C). Consistent with a dominant role for MT1-MMP in pericellular collagenolysis, the metalloenzyme can be localized to the interface between the basal surface of the stem cells and the degraded collagen (Figure 2D; punctate foci of MT1-MMP are detected as red “dots” on the basal surface of hMSCs electroporated with a control-scrambled siRNA construct which lie above the layer of blue-colored type I collagen). Further, within subjacent zones of collagenolytic activity, laser confocal images of hMSCs cultured atop type I collagen gels demonstrate that MT1-MMP is found in direct association with F-actin- and cortactin-rich regions reminiscent of invadopodia, specialized proteolytically active plasma membrane protrusions commonly found in invasive cancer cells (Figure 2E; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).23 When MT1-MMP is silenced, however, F-actin- and cortactin-rich protrusions are nonetheless formed but without MT1-MMP colocalization or associated subjacent collagen degradation (Figure 2E; supplemental Figure 1). Although MT1-MMP is able to process either MMP-2 or MMP-13 zymogens to enzymically active forms that could potentially participate in subjacent degradation,24,25 silencing expression of either of the secreted enzymes did not affect collagenolytic potential (Figure 2B-C). Although MMP-2 mRNA levels are not silenced completely by the specific siRNA (see Figure 2A), MMP-2 protein expression is nevertheless repressed efficiently (supplemental Figure 2), a finding consistent with the inability of TIMP-1 to block collagenolytic activity (see Figure 1B-C). As expected, the MT1-MMP siRNA-specific effect on hMSC collagenolysis is reversed after transfection with a mouse MT1-MMP expression vector that escapes siRNA-dependent targeting (Figure 2B-C).
MT1-MMP mediates hMSC-dependent collagenolytic activity. (A) siRNA-dependent silencing of MMP expression in hMSCs as assessed by RT-PCR. hMSCs were electroporated with 40nM scrambled (Scr siRNA) or MMP-1–, MMP-2–, MMP-13–, MT1-MMP–, or MT3-MMP–specific siRNAs and cultured in growth medium for 2 days followed by RT-PCR analysis of MMP expression with GAPDH as loading control. (B-C) hMSCs electroporated with each of the respective siRNAs or cotransfected with a control expression vector (MT1siRNA + Vec) or mouse MT1-MMP expression vector (MT1siRNA + mMT1) were seeded atop Alexa Fluor 594–labeled type I collagen film (red) for 4 days and collagenolytic activity quantified by hydroxyproline release (mean ± SEM; n = 3. *P < .05) as well as visualized by confocal laser microscopy. Bar, 50 μm. (D) Cells electroporated with Scr or MT1 siRNA were incubated on Alexa Fluor 647–labeled collagen (blue) for 2 days, and cell surface was stained with anti–MT1-MMP (red). Colocalizations of MT1-MMP “dots” on the basal cell surface with areas of collagen degradation were found in Scr siRNA–treated cells. Neither MT1-MMP nor zones of degraded collagen were found in MT1 siRNA–treated cells. The position of a cell body overlying the collagen substratum is outlined by the dotted white lines. Bar, 10 μm. (E) 3D reconstructions of collagen (Alexa Fluor 647 labeling, blue), phalloidin (green), and cell surface-localized MT1-MMP (red). Black arrows mark the direction from top to bottom. White lines mark either the yellow dots (comprised of MT1-MMP–positive areas that colocalize with phalloidin), which appear in the zones of collagen degradation in Scr siRNA-cells (left), or green dots (ie, MT1-MMP–negative and phalloidin-positive), which appear atop the intact collagen surface in MT1-MMP siRNA-silenced cells (right). Bar, 10 μm.
MT1-MMP mediates hMSC-dependent collagenolytic activity. (A) siRNA-dependent silencing of MMP expression in hMSCs as assessed by RT-PCR. hMSCs were electroporated with 40nM scrambled (Scr siRNA) or MMP-1–, MMP-2–, MMP-13–, MT1-MMP–, or MT3-MMP–specific siRNAs and cultured in growth medium for 2 days followed by RT-PCR analysis of MMP expression with GAPDH as loading control. (B-C) hMSCs electroporated with each of the respective siRNAs or cotransfected with a control expression vector (MT1siRNA + Vec) or mouse MT1-MMP expression vector (MT1siRNA + mMT1) were seeded atop Alexa Fluor 594–labeled type I collagen film (red) for 4 days and collagenolytic activity quantified by hydroxyproline release (mean ± SEM; n = 3. *P < .05) as well as visualized by confocal laser microscopy. Bar, 50 μm. (D) Cells electroporated with Scr or MT1 siRNA were incubated on Alexa Fluor 647–labeled collagen (blue) for 2 days, and cell surface was stained with anti–MT1-MMP (red). Colocalizations of MT1-MMP “dots” on the basal cell surface with areas of collagen degradation were found in Scr siRNA–treated cells. Neither MT1-MMP nor zones of degraded collagen were found in MT1 siRNA–treated cells. The position of a cell body overlying the collagen substratum is outlined by the dotted white lines. Bar, 10 μm. (E) 3D reconstructions of collagen (Alexa Fluor 647 labeling, blue), phalloidin (green), and cell surface-localized MT1-MMP (red). Black arrows mark the direction from top to bottom. White lines mark either the yellow dots (comprised of MT1-MMP–positive areas that colocalize with phalloidin), which appear in the zones of collagen degradation in Scr siRNA-cells (left), or green dots (ie, MT1-MMP–negative and phalloidin-positive), which appear atop the intact collagen surface in MT1-MMP siRNA-silenced cells (right). Bar, 10 μm.
MT1-MMP regulates the hMSC 3D invasion program
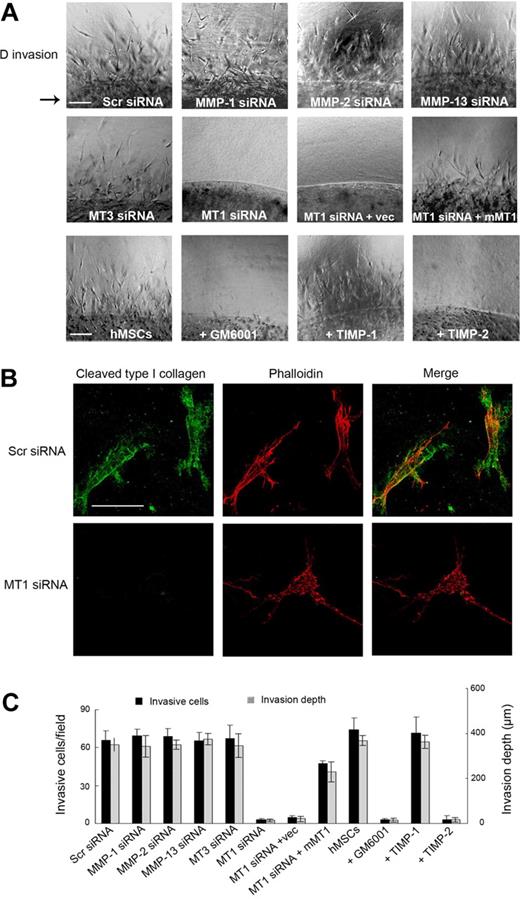
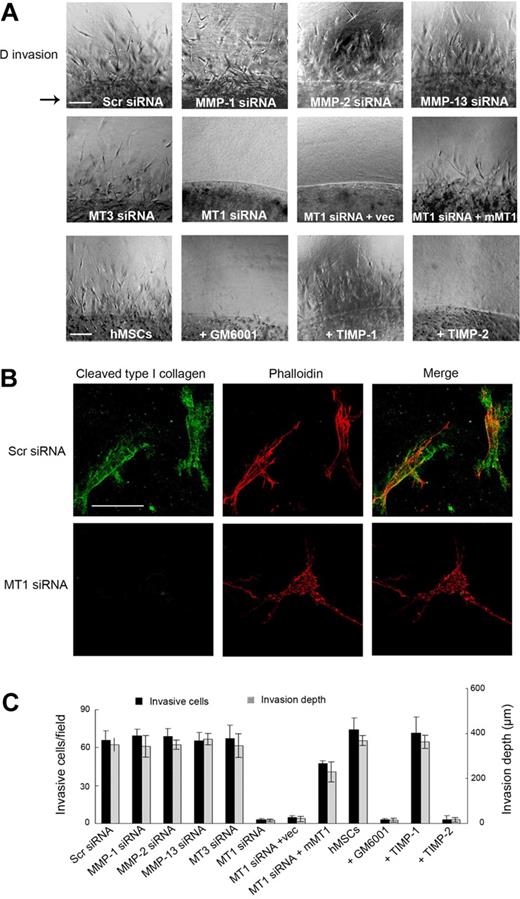
To recapitulate hMSC migration through an interstitial compartment, stem cells were enmeshed within a 3D matrix of type I collagen fibrils (Figure 3). Under these conditions, hMSCs initiate an invasion program that proceeds in a manner coincident with pericellular collagenolysis, leaving the cells surrounded by collagen degradation products as detected by immunofluorescence microscopy with a monoclonal antibody that reacts specifically with collagenase-degraded collagen (Figure 3A-B).17 As observed for hMSC-dependent subjacent collagenolysis activity, cell invasion is unaffected by the addition of supraphysiologic concentrations of TIMP-1 or after silencing of MMP-1, MMP-2, MMP-13, or MT3-MMP (Figure 3A,C). By contrast, hMSC invasion is blocked completely by either recombinant TIMP-2 or after MT1-MMP silencing, an effect that occurs in tandem with the loss of pericellular collagenolytic activity (Figure 3A-C). Although the authors of recent studies14,26 have suggested that MT1-MMP can directly regulate cell signaling or motility as well as exert proapoptotic effects, neither hMSC migration atop collagen gels, 3D proliferation, nor apoptosis are affected by MT1-MMP silencing (supplemental Figures 3-4). Furthermore, a required role for MT1-MMP in hMSC invasion is not confined to 3D culture conditions per se because stem cells cultured atop (rather than embedded within) 3D collagen gels likewise infiltrate collagenous barriers by a process dependent on MT1-MMP alone (supplemental Figure 5). As such, hMSCs are reliant on MT1-MMP alone to support proinvasive activity in vitro and are unable to traverse collagenous barriers by proteinase-independent schemes.
MT1-MMP directs the 3D collagen-invasive activity of hMSCs. (A) hMSCs (2 × 105) electroporated with Scr siRNA, MMP-directed siRNAs, or cotransfected with MT1-MMP siRNA and either a control expression vector (MT1siRNA + Vec) or mouse MT1-MMP expression vector (MT1siRNA + mMT1) were cultured within 3D type I collagen gels (2.2 mg/mL) in growth medium for 4 days. In the bottom row of panels, hMSCs in 3D collagen gels were cultured with GM6001 (25μM), TIMP-1 (7.5 μg/mL), or TIMP-2 (2.5 μg/mL). For reference, the arrow to the left of the panels in the top row marks the edge of the embedded island of hMSCs. Arrowheads mark cells migrating into surrounding collagen from the central collagen island. Images shown are representatives of 3 experiments performed. Bar, 100 μm. (B) hMSCs transfected with Scr or MT1-MMP siRNAs were cultured in 3D collagen for 4 days and stained with phalloidin (red) as well as monoclonal antibody 9A4 directed against a type I collagen cleavage neoepitope (green) and visualized by fluorescence microscopy. Bar, 50 μm. (C) The number of invading cells and invasion depth in the 3D collagen gels are expressed as the mean ± SD in 5 randomly selected fields in a single representative experiment of 3 or more performed.
MT1-MMP directs the 3D collagen-invasive activity of hMSCs. (A) hMSCs (2 × 105) electroporated with Scr siRNA, MMP-directed siRNAs, or cotransfected with MT1-MMP siRNA and either a control expression vector (MT1siRNA + Vec) or mouse MT1-MMP expression vector (MT1siRNA + mMT1) were cultured within 3D type I collagen gels (2.2 mg/mL) in growth medium for 4 days. In the bottom row of panels, hMSCs in 3D collagen gels were cultured with GM6001 (25μM), TIMP-1 (7.5 μg/mL), or TIMP-2 (2.5 μg/mL). For reference, the arrow to the left of the panels in the top row marks the edge of the embedded island of hMSCs. Arrowheads mark cells migrating into surrounding collagen from the central collagen island. Images shown are representatives of 3 experiments performed. Bar, 100 μm. (B) hMSCs transfected with Scr or MT1-MMP siRNAs were cultured in 3D collagen for 4 days and stained with phalloidin (red) as well as monoclonal antibody 9A4 directed against a type I collagen cleavage neoepitope (green) and visualized by fluorescence microscopy. Bar, 50 μm. (C) The number of invading cells and invasion depth in the 3D collagen gels are expressed as the mean ± SD in 5 randomly selected fields in a single representative experiment of 3 or more performed.
hMSC tissue invasion and intravasation in vivo requires MT1-MMP
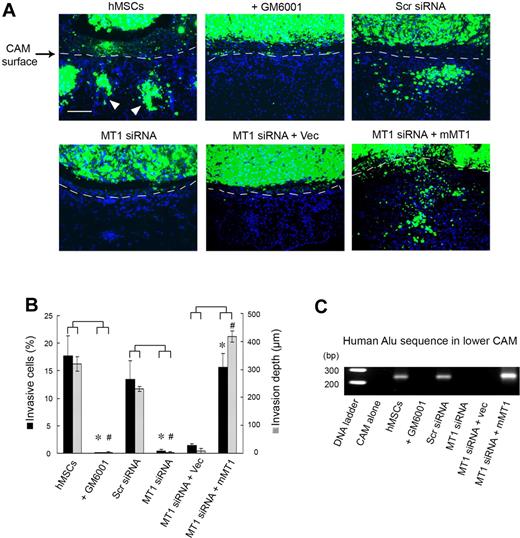
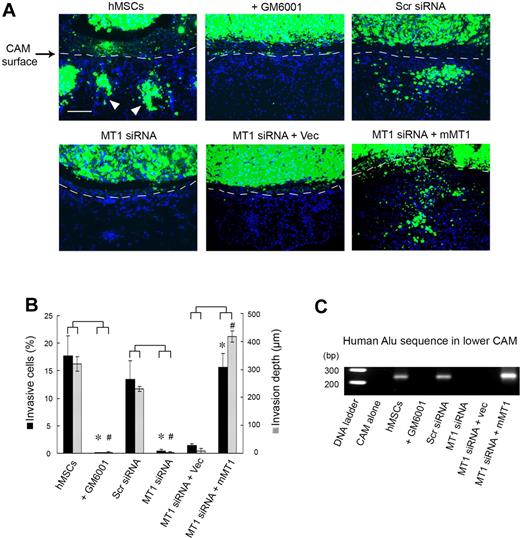
Although type I collagen gels recapitulate the bulk of the interstitial matrix found in most sites in vivo, the ECM is a complex composite of macromolecular constituents that are bathed in high concentrations of potent antiproteinases.7,27,28 To assess the role of MT1-MMP in regulating hMSC trafficking in an in vivo setting, the migratory patterns of mammalian stem cells in vivo were monitored directly in live chick embryo tissues.13,29 In this model system, hMSCs are fluorescently labeled and cultured atop the chick CAM, a tissue whose stromal compartment is rich in interstitial collagens (Figure 4).16 After a 2-day culture period, control hMSCs rapidly cross the CAM surface and infiltrate the underlying stromal tissues (Figure 4A-B). Because tissue-invasive hMSCs are frequently observed in association with the chick vasculature (data not shown), stem cell intravasation was evaluated by monitoring the lower CAM for the appearance of human Alu sequence-positive cells.13,29 Under these conditions, hMSCs not only infiltrate the upper CAM surface but also access the chick vascular bed to travel to distant sites in the embryo (Figure 4C). Consistent with our in vitro findings, both hMSC invasion and intravasation are blocked completely in either the presence of the synthetic MMP inhibitor, GM6001, or after MT1-MMP silencing (Figure 4A-C). The inhibitory effects exerted on hMSC invasion and intravasation by the MT1-MMP siRNA in vivo are reversed completely after MT1-MMP re-expression as the siRNA-resistant mouse orthologue (Figure 4A-C).
MT1-MMP regulates hMSC invasion and intravasation in vivo. (A) hMSCs were labeled with fluorescent nanobeads (green) and seeded atop the CAM of 11-day-old chick embryos for 2 days. CAM cross-sections were stained with DAPI and visualized by fluorescence microscopy. Dashed lines mark the outline of the upper CAM surface (ie, cells below the dashed line demarcate invading hMSCs). White arrowheads mark the invading cells. Bar, 100 μm. (B) CAM invasion is quantified as the number of hMSCs that cross the CAM surface (mean ± SEM; n = 3) and average depth of the leading front of invading cells (mean ± SEM; n = 3), * and # both represent P < .05, for invasive cells numbers and invasion depth, respectively. (C) hMSCs intravasation/extravasation was detected as Alu-sequences by PCR on DNA extracted from the lower CAM after a 2-day incubation period. Micrographs shown are representative of 3 experiments performed.
MT1-MMP regulates hMSC invasion and intravasation in vivo. (A) hMSCs were labeled with fluorescent nanobeads (green) and seeded atop the CAM of 11-day-old chick embryos for 2 days. CAM cross-sections were stained with DAPI and visualized by fluorescence microscopy. Dashed lines mark the outline of the upper CAM surface (ie, cells below the dashed line demarcate invading hMSCs). White arrowheads mark the invading cells. Bar, 100 μm. (B) CAM invasion is quantified as the number of hMSCs that cross the CAM surface (mean ± SEM; n = 3) and average depth of the leading front of invading cells (mean ± SEM; n = 3), * and # both represent P < .05, for invasive cells numbers and invasion depth, respectively. (C) hMSCs intravasation/extravasation was detected as Alu-sequences by PCR on DNA extracted from the lower CAM after a 2-day incubation period. Micrographs shown are representative of 3 experiments performed.
MT1-MMP controls hMSC differentiation
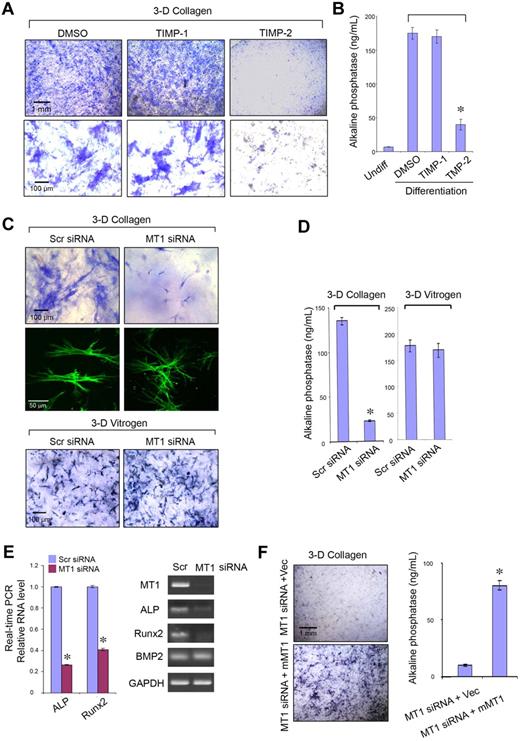
In 2D culture systems, hMSC differentiation is modulated by ECM composition and mechanical rigidity, along with the attendant effects these variables exert on stem cell shape.11,12 Because (1) hMSC osteogenic differentiation normally occurs within type I collagen-rich regions and (2) MT1-MMP is able to modulate both cell shape and pericellular ECM rigidity by proteolyzing the surrounding matrix,30,31 the role of the protease during osteoblastogenesis was assessed initially in 3D culture. Under pro-osteogenic conditions, 3D-embedded hMSCs express ALP activity as assessed by histochemical staining via a process that is inhibited significantly by TIMP-2 but not TIMP-1, indirectly supporting an additional role for MT1-MMP in this differentiation program (Figure 5A-B). Indeed, after MT1-MMP silencing for 7 days in 3D culture, hMSC shape and size is perturbed with the cells assuming a markedly contracted and dendritic phenotype that couples with a failure to undergo osteogenesis as assessed by ALP activity (Figure 5C-D). Furthermore, although cell number is unchanged by MT1-MMP silencing under these conditions (data not shown), decreases in ALP expression are linked with the suppression of the early osteogenic marker, Runx2, whereas BMP2 expression is left unaffected (Figure 5C-D). By contrast, when hMSCs are cultured atop type I collagen gels under 2D conditions, MT1-MMP knockdown does not affect cell shape (data not shown) or osteogenic potential (supplemental Figure 6). Likewise, if hMSCs are cultured within in 3D matrices comprised of pepsin-extracted type I collagen, a noncovalently cross-linked gel wherein embedded cells are able to mechanically displace collagen fibers independently of a requirement for MT1-MMP–mediated proteolysis,7 osteogenesis is unaffected after MT1-MMP knockdown (Figure 5C,E). Finally, as expected, the inability of MT1-MMP–silenced hMSCs to undergo osteoblast differentiation is recovered after rescue with mouse MT1-MMP (Figure 5F-G). Hence, MT1-MMP not only regulates hMSC collagenolytic and invasive activity but osteogenic potential as well within the confines of the type I collagen-rich 3D ECM.
MT1-MMP controls hMSC osteogenesis. ALP staining (A) or activity (B) after osteogenesis induction in hMSCs embedded within 3D gels of type I collagen in the presence of dimethyl sulfoxide (DMSO) control, TIMP-1 (7.5 μg/mL), or TIMP-2 (2.5 μg/mL) for 9 days. ALP staining (C) or activity (D) after osteogenesis induction in hMSCs electroporated with Scr siRNA or MT1 siRNA and cultured within 3D type I collagen gels (2.2 mg/mL) or pepsin-extracted type I collagen gels (Vitrogen; 2.2 mg/mL) in osteogenesis differentiation medium for 7 days. In 3D collagen gels, phalloidin-stained hMSCs (green) display a dendritic phenotype after MT1-MMP silencing. (E) Real-time PCR quantification analysis (left panel) of ALP, Runx2, and BMP2 mRNA levels in Scr siRNA or MT1 siRNA-treated hMSCs induced for osteogenesis in collagen gels for 7 days. RT-PCR confirms MT1-MMP mRNA silencing and verifies down-regulation of ALP and Runx2 mRNA levels without affecting BMP2 expression (GAPDH used as a reference; right panel). (F) ALP staining and activity of hMSCs cotransfected with MT1-MMP siRNA and either a control expression vector (MT1siRNA + Vec) or mouse MT1-MMP expression vector (MT1siRNA + mMT1) cultured in 3D collagen with osteogenesis differentiation medium for 7 days. Pictures shown are representative of 3 or more experiments performed. Bars, 1 mm and 100 μm, respectively, as shown in each figure. Relative enzyme quantification was expressed as ng/mL ± SD of 3 experiments. *P < .05.
MT1-MMP controls hMSC osteogenesis. ALP staining (A) or activity (B) after osteogenesis induction in hMSCs embedded within 3D gels of type I collagen in the presence of dimethyl sulfoxide (DMSO) control, TIMP-1 (7.5 μg/mL), or TIMP-2 (2.5 μg/mL) for 9 days. ALP staining (C) or activity (D) after osteogenesis induction in hMSCs electroporated with Scr siRNA or MT1 siRNA and cultured within 3D type I collagen gels (2.2 mg/mL) or pepsin-extracted type I collagen gels (Vitrogen; 2.2 mg/mL) in osteogenesis differentiation medium for 7 days. In 3D collagen gels, phalloidin-stained hMSCs (green) display a dendritic phenotype after MT1-MMP silencing. (E) Real-time PCR quantification analysis (left panel) of ALP, Runx2, and BMP2 mRNA levels in Scr siRNA or MT1 siRNA-treated hMSCs induced for osteogenesis in collagen gels for 7 days. RT-PCR confirms MT1-MMP mRNA silencing and verifies down-regulation of ALP and Runx2 mRNA levels without affecting BMP2 expression (GAPDH used as a reference; right panel). (F) ALP staining and activity of hMSCs cotransfected with MT1-MMP siRNA and either a control expression vector (MT1siRNA + Vec) or mouse MT1-MMP expression vector (MT1siRNA + mMT1) cultured in 3D collagen with osteogenesis differentiation medium for 7 days. Pictures shown are representative of 3 or more experiments performed. Bars, 1 mm and 100 μm, respectively, as shown in each figure. Relative enzyme quantification was expressed as ng/mL ± SD of 3 experiments. *P < .05.
Discussion
For marrow- or nonmarrow-derived hMSCs to home, engraft, or differentiate in nonhematopoietic tissues, these cells must negotiate networks of type I collagen fibrils.13,32 Herein, we have demonstrated that MT1-MMP, acting independently of all secreted collagenases, confers hMSCs with type I collagenolytic and 3D invasive activity. Although these results are in agreement with recent studies demonstrating a requirement for MT1-MMP in supporting the tissue-invasive activity of mouse mesenchymal cell populations as well as human carcinoma cell lines,13,15,31,33 earlier conclusions regarding a dominant role for MT1-MMP in (patho)physiologically relevant human cell populations have been questioned on the basis of the fact that (1) mice do not express MMP-1, a major secreted collagenase found in human cells, and (2) human carcinoma cell lines adapted for rapid in vitro growth may not recapitulate faithfully the behavior of primary neoplastic cells in the in vivo setting.34,35 To our knowledge, the demonstrated role for MT1-MMP in hMSC invasion not only defines the singular importance of this protease in trafficking but also provides the first evidence that MT1-MMP plays a dominant and direct role in regulating the type I collagenolytic phenotype associated with invasion or differentiation in a primary human cell population.
Presently, the unique structure/function properties of MT1-MMP that lend the proteinase its tissue-invasive activity remain the subject of debate.14 Aside from its type I collagenolytic potential, MT1-MMP has been reported to initiate complex signal transduction cascades via only partially defined processes.14,26,36,37 More recent studies, however, indicate that MT1-MMP can drive tissue-invasive activity by acting directly as a membrane-tethered collagenase, that is, even secreted collagenases can confer proinvasive activity if expressed as chimeric mutants that are tethered to the cell surface.7,9,14 These results should not, however, be construed to suggest that secreted MMPs such as MMP-1, MMP-2, or MMP-13 are without function in hMSCs. Although secreted collagenases do not support tissue-invasive activity, each of these proteinases are able to act as powerful collagenolytic enzymes that could participate in bulk ECM resorption such as that found at sites of tissue damage or fibrosis.9,10 Furthermore, increasing evidence suggests that the substrate repertoire of secreted MMPs, such as MMP-1 or MMP-2, can be extended to include hundreds of non–ECM-related substrates.38 In this regard, although recent studies, such as that by Ho et al,39 have focused on the ability of MMP-1 to modulate cell function, including that of hMSCs, by cleaving protease-activated receptor-1, MMP-1 silencing did not affect the hMSC invasion program in our studies. Regardless, although only MT1-MMP plays a required role in supporting hMSC invasion programs through interstitial matrix barriers, the secreted MMPs likely modulate stem cell function in as yet uncharacterized scenarios.
Normal stem cells and cancer stem cells have been proposed to display several similarities with regard to their ability to traffic through host tissues.40 In this regard, carcinoma cells have been reported to adopt a myeloid-like amoeboid phenotype that allows the cells to negotiate collagenous barriers independently of proteolytic activity.7,10 In our studies, however, wherein type I collagen gels have been assembled that recapitulate collagen architecture in vivo,7 hMSCs are unable to invade collagenous barriers in vitro or in vivo in the absence of MT1-MMP activity. Nevertheless, like neoplastic cells, we demonstrate that hMSCs assemble MT1-MMP–dependent, subjacent proteolytic activity into actin- and cortactin-rich puncta. Although invadopodia-like structures more frequently are associated with the ECM-degradative activities displayed by transformed cell populations,23 our findings indicate that hMSC may similarly assemble their proteolytic machinery into discrete zones at the basal cell surface. MT1-MMP is not, however, required for the formation of cortactin- and F-actin–rich invadosomes in hMSCs. This finding contrasts with other reports wherein MMP activity plays a required role in invadosome assembly,23,41 but our results corroborate earlier studies in which MT1-MMP silencing did not uniformly suppress the formation of invadopodia in normal or neoplastic cell populations.42
Although our studies have emphasized the role of hMSC-derived MT1-MMP in regulating cell trafficking through stromal tissues, MT1-MMP activity need not be restricted to the dissolution of type I collagen barriers. Indeed, MT1-MMP likely lends hMSCs the ability to traverse basement membranes, a specialized form of ECM that underlies both epithelial and endothelial cell layers.20,27 Although others have concluded that hMSCs traverse basement membranes by activating the secreted MMP zymogen, MMP-2, these studies were restricted to the use of an artificial ECM construct that does not recapitulate the structural characteristics of basement membranes in vivo.20,27,43,44 Indeed, by using authentic basement membranes recovered from in vivo tissues, we have demonstrated that MT1-MMP directly supports basement membrane transmigration by human cancer cells, and neither MMP-2 nor MMP-9 plays a required role in this progress.20,27 Given the ability of hMSCs to express MT1-MMP, preliminary studies indicate that this protease may also direct stem cell trafficking through intact basement membrane barriers as well (supplemental Figure 7). Nevertheless, it should be stressed that collagenous matrices do not define all in vivo settings. At wound sites, for example, cross-linked networks of fibrin are deposited as provisional matrices.45,46 Because the serine proteinase, plasmin, and multiple MMP family members can mediate fibrinolysis, the repertoire of proteases that control hMSC function in fibrin-rich environments is likely distinct from that specific to collagen-rich sites.14,45,46 Similarly, although hMSCs are able to migrate through brain tissues, this ECM environment is distinguished from all others by the virtual absence of interstitial collagens.47 Nevertheless, the broad distribution of type I collagen in virtually all other tissues outside of the central nervous system emphasizes the probable importance of MT1-MMP in controlling mesenchymal cell trafficking in vivo.
Independent of the postulated ability of hMSCs to actively home to injured or neoplastic tissues, increased attention—and debate—has focused on their in vivo potential to differentiate into cell types of mesodermal, ectodermal, or endodermal lineage.48 Although the propensity of hMSCs to differentiate in vivo remains controversial (indeed, hMSCs are alternatively termed “marrow stromal cells”),48 significant efforts are directed toward the in vitro generation of 3D tissue equivalents for tissue engineering.49 Under 2D culture conditions, the authors of recent studies11,12 have demonstrated that hMSC differentiation can be controlled by modulating either cell shape, ECM ligand density, or matrix rigidity. Because MT1-MMP–dependent pericellular collagenolysis would be expected to control each of these parameters in 3D culture,30,31 we examined the role of MT1-MMP in hMSC osteoblastogenesis. In 2D culture, osteogenesis is dependent on the ability of hMSCs to adopt a spread cell shape, a program that proceeds independently of MT1-MMP activity as the collagen substratum does not act as a physical barrier to lateral cell movement.21,30,31 By contrast, within the confines of the 3D ECM, MT1-MMP plays a required role in osteogenesis, most likely by controlling hMSC shape and matrix rigidity (ie, as a function of proteolyzing the surrounding collagen fibrils).30,31 Indeed, when hMSCs are embedded in pepsin-extracted collagen gels where embedded cells can mechanically displace the noncovalently associated fibrils and matrix rigidity is decreased as a function of the absence of aldimine cross-links,7 MT1-MMP is no longer required during the osteogenic process (similarly, MT1-MMP is not required for hMSC invasion through pepsin-extracted collagen gels; supplemental Figure 8). Although the apparent plasticity of hMSC differentiation programs in vitro relative to the in vivo setting may be questioned,48 we note with interest that osteoprogenitor cells isolated from MT1-MMP−/− mice likewise display an inability to generate bone when transplanted into wild-type mice. Apparently, hMSC cells embedded in 3D collagen gels in vitro rely on MT1-MMP activity in a fashion similar, if not identical, to that observed in osteoprogenitor cells in vivo.
Regardless of the roles played by endogenous or exogenously introduced hMSCs in vivo, our data demonstrate that primary human cell populations use MT1-MMP as a pericellular collagenase to regulate a diverse set of cellular functions, ranging from ECM remodeling and invasion to differentiation. Regardless of lineage origin, virtually all human mesenchymal cell populations find themselves embedded in type I collagen-rich tissues,2,3,48 as do normal or neoplastic epithelia undergoing epithelial–mesenchymal cell transition.50 As such, we posit that the functions outlined herein for hMSC-derived MT1-MMP may well serve as a general paradigm for the biologic activity subserved by this uniquely endowed protease in diverse populations of human cells in both health and disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Julie Ann Semon and Darwin Prockop for providing access to human mesenchymal stem cells and S. Filippov (Pfizer Inc) for 9A4.
This work was supported by the National Institutes of Health (CA88308 and CA071699) and the Breast Cancer Research Foundation.
National Institutes of Health
Authorship
Contribution: C.L., X.Y.L., R.G.R., and S.J.W. designed research; C.L., X.Y.L., Y.H., and R.G.R. performed research; C.L., X.Y.L. R.G.R., and S.J.W. analyzed data; and C.L., X.Y.L., and S.J.W. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen J. Weiss, MD, University of Michigan, Life Sciences Institute, 5000 LSI, 210 Washtenaw, Ann Arbor, MI 48109-2216; e-mail: SJWEISS@umich.edu.