In chronic lymphocytic leukemia (B-CLL), aberrations along the p53 axis lead to decreased overall survival and therapy resistance. Recent studies identified microRNA-34a (miR-34a) as a major downstream target of p53. We monitored the expression of miR-34a during disease development in a murine B-CLL model. miR-34a was up-regulated more than 20-fold during the leukemic but not during the preleukemic phase. In the human system, B-CLL cells also had 4.6-fold higher miR-34a expression compared with B cells of healthy controls. In B-CLL cells of patients with p53 aberrations, miR-34a expression was consistently low. The broad distribution of miR-34a levels in p53 wild-type patients prompted us to study the correlation between single nucleotide polymorphism 309 (SNP309) in the intronic promoter of MDM2 and miR-34a expression. B-CLL cells of patients with the SNP309 GG genotype had significantly lower miR-34a expression levels compared with patients with the TT genotype (P = .002). Low miR-34a levels were able to predict shorter time to treatment (P = .003) and were associated with an abbreviated lymphocyte doubling time. Further, overexpression of miR-34a in primary B-CLL cells induced apoptosis. These findings suggest miR-34a as a possible therapeutic avenue and a sensitive indicator of the activity of the p53 axis in B-CLL.
Introduction
microRNAs (miRNAs) are small noncoding RNAs that regulate gene expression by near-perfect base pairing with target mRNAs resulting in either degradation or silencing.1,2 They play important roles in leukemias3,–5 and solid malignancies6,7 and offer elegant possibilities for future therapeutic intervention.8,,–11 Recently, the miR-34 family, consisting of miR-34a, b, and c, has been described as a downstream target of the tumor suppressor p53.12,,,,–17 Overexpression of miR-34 can evoke p53-like effects, including senescence, apoptosis, or cell cycle arrest depending on the cell type analyzed.12,,,,–17 In normal unstressed cells, p53 functions are tightly controlled mainly by the ubiquitin ligase murine double minute zprotein (MDM2). It has been demonstrated that single nucleotide polymorphism 309 (SNP309) resulting in a T to G alteration within the first intron of the intronic MDM2 gene promoter, henceforth termed SNP309, can enhance MDM2 expression and therefore lead to a reduced tumor suppressor function of p53.18 SNP309 has been shown to affect onset of disease or tumor progression in various cancer types including gastric carcinoma,19 colorectal cancer,20 and also chronic lymphocytic leukemia (B-CLL).21 B-CLL is the most prevalent lymphoma in the western world, and p53 deletion has dramatic effects such as therapy resistance and reduced median overall survival from 111 to 32 months.22 Two independent recent studies suggest that p53 mutations, which go undetected in routine sample workup, are equivalent to p53 deletions in their frequency and prognostic impact.23,24 In B-CLL miR-34b and c are not expressed, and several studies have confirmed the dependence of miR-34a expression on p53 status in this disease.25,–27 However, the reasons for low miR-34a expression in many B-CLL cases without p53 deletions or mutations and its role in the development of B-CLL have not been addressed.
In this study, the expression of 7 microRNAs including miR-34a was assessed over the course of disease in the TCL1a mouse, an established B-CLL model.28 miR-34a levels were monitored in a cohort of 75 well-characterized B-CLL patients. Moreover, effects of modulating miR-34a levels in primary B-CLL cells were examined.
Methods
Patient samples and cell lines
Peripheral blood samples from 75 consecutive B-CLL patients from our outpatient care facility at the IIIrd Medical Department were collected after obtaining informed patient consent in accordance with the Declaration of Helsinki (Table 1). Of 75 patients, 74 received no chemotherapy within 6 months prior to the date of analysis. Of 75 patients, 42 had received no chemotherapy at all. Independent from this initial cohort of B-CLL, samples of 5 additional patients bearing del11q were analyzed to clarify the role of this deletion on miR-34a expression. The immunoglobulin heavy chain variable region (IgVH) mutational status, CD38 expression, p53 mutations, and SNP309 genotype were determined as previously described.21,29 For detection of genomic aberrations, interphase fluorescent in situ hybridization (FISH) analysis using the multicolor probe set (Abbott/Vysis) was performed. Peripheral blood mononuclear cells (PBMCs) were separated by density centrifugation using Biocoll (Biochrom AG). CD19+ B cells were isolated by magnetic-activated cell sorting, and purity of each sample was assessed by CD19/CD5+/+ staining using flow cytometry. The mean purity of the samples was 98.9% (± 1.06%) and more than 96% in all cases (Miltenyi Biotec). The prolymphocytic MEC1 cell line (DSMZ) was cultured in Iscove modified Dulbecco medium (Gibco) supplemented with 10% fetal calf serum, 100 μg/mL streptomycin, and 100 U/mL penicillin. The prolymphocytic MEC1 cells were used for testing the effect of miR-34a in a proliferating B-cell line. It does not provide an ideal model for B-CLL because of genotypic and phenotypic differences. Lymphocyte doubling time (LDT) was determined according to the guidelines of the international workshop on chronic lymphocytic leukemia.30
Mice
The original Eμ-TCL1a transgenic mice that were generously provided by C. Croce (Columbus, OH) have been backcrossed for more than 9 generations to C57BL/6 mice. Mice were killed at stated ages, or when signs of illness developed, and samples for histology, flow cytometry, and miRNA isolation were collected. The tumor load in all lymphoid organs was assessed by flow cytometry after staining for the surface markers CD19, CD5, and IgM. Approval from the Austrian Animal Ethics committee was obtained prior to performing the experiments.
microRNA quantification
RNA from CD19+ purified B cells was isolated using the MiRNeasy Mini Kit (QIAGEN). For each miRNA, the specific reverse transcription and real-time polymerase chain reaction (PCR) Assay Kit (Applied Biosystems) was used following the manufacturer's protocol. Real-time PCR was performed using the Applied Biosystems 7500 machine and endogenous snRNU-48 levels as standard.
To test possible effects of the positive selection process on microRNA expression, direct selection with CD19 antibody-coupled magnetic beads (Miltenyi Biotec) or negative selection with antibodies against all non–B cells (EasySep; StemCell Technologies) was compared. In 5 patients whose B cells were purified using both systems, no difference associated with positive or negative selection in miR-34a expression could be observed (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
microRNA transfection
Transfection experiments with prolymphocytic MEC1 cells were performed with a total number of 5 × 105 cells using miR-34a microRNA-mimics, miR-34 antisense oligonucleotides (Ambion), and negative control oligonucleotides for mock transfection (QIAGEN). For transfection of primary CLL cells, 2 × 106 freshly prepared PBMCs were used in a total volume of 0.5 mL of RPMI supplemented with 10% fetal calf serum and 2mM l-glutamine. The transfection reagent Hiperfect (QIAGEN) was used according to the manufacturer's instructions.
Cell viability
Apoptosis was assessed by staining with annexin V–fluorescein isothiocyanate (Alexis Pharmaceuticals), 7-amino-actinomycin D (Beckman Coulter), and fluorochrome-labeled monoclonal antibodies to CD5 and CD19 (all from Beckman Coulter), and analysis was carried out by flow cytometry (FC500; Beckman Coulter).
Statistics
Statistical analysis was performed with SPSS 14.0 for Windows. Unpaired and paired Student t tests and the log-rank test for Kaplan-Meier analysis were used to determine significance if not indicated otherwise. The cutoff value for definition of miR-34a subgroups was determined by receiver operating characteristic analysis and subsequent Youden index calculation.31 Using this approach, we established a cutoff value of 2.4 relative expression level of miR-34a to best distinguish between 2 groups of B-CLL patients with (p53 mutations, del17p, del11q, MDM2 SNP309 GG) or without DNA alterations along the p53 axis. In the box plots outliers are marked as dots. Outliers are cases that are more than 1.5-fold the box length away from the upper or lower edge of the box. One box length equals the interquartile range.
Results
miR-34a expression during disease development
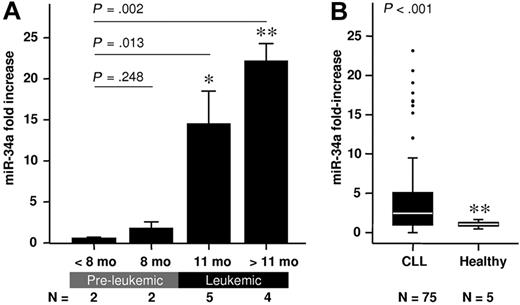
TCL1a transgenic mice currently represent the best-described animal model for the study of B-CLL and have been used for the validation of novel therapeutic targets,32 and as a tool for the preclinical assessment of anti–B-CLL therapies.33,34 Importantly, B-CLL development in these mice can be divided into 2 distinct phases: an asymptomatic preleukemic phase and the development of overt disease earliest seen at 10 months, characterized by splenomegaly, hepatomegaly, enlarged lymph nodes, and the presence of leukemic cells in all lymphoid organs. This clear distinction between disease phases allows the assessment of miRNAs in both the preleukemic and the leukemic stage of the disease. Seven miRNAs were selected for analysis (miR-16, miR-21, miR-29b, miR-150, miR-155, miR-331, and miR-34a) that have been shown to be deregulated in B-CLL or are involved in pathways known to affect the course of disease. Six of those microRNAs were unchanged when preleukemic and leukemic phases were compared. In the preleukemic phase, miR-34a expression was low and comparable with that observed in age-matched wild-type littermate controls. Strikingly, miR-34a levels increased more than 20-fold in the leukemic phase (Figure 1A). This suggests that a significant oncogenic event, which triggers the p53 pathway, occurs at the transition from premalignant disease to frank leukemia. To confirm this finding, expression of the p53 downstream effectors p21 and PUMA was monitored. As expected p21 and PUMA mRNA levels were increased parallel to miR-34a in leukemic mice (supplemental Figure 2).
miR-34a expression in CD19+ B cells of TCL1 transgenic mice and B-CLL patients both normalized to the corresponding healthy controls. (A) miR-34a expression levels during the course of disease in TCL1 transgenic mice (mean value ± SD; *P < .05; **P < .01). (B) miR-34a expression in B-CLL patients is higher compared with healthy controls. Box length equals interquartile range.
miR-34a expression in CD19+ B cells of TCL1 transgenic mice and B-CLL patients both normalized to the corresponding healthy controls. (A) miR-34a expression levels during the course of disease in TCL1 transgenic mice (mean value ± SD; *P < .05; **P < .01). (B) miR-34a expression in B-CLL patients is higher compared with healthy controls. Box length equals interquartile range.
miR-34a expression in human B-CLL
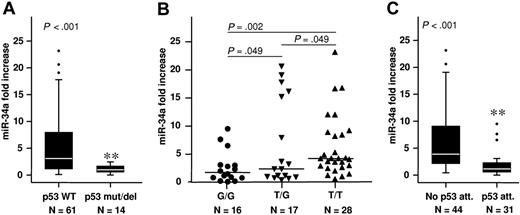
To corroborate our findings in a human B-CLL setting, the expression of miR-34a was assessed in B-CLL cells purified from 75 B-CLL patients, and in CD19+ B cells from 5 healthy donors. B-CLL cells exhibited a mean 4.6-fold higher overall expression of miR-34a compared with healthy controls (Figure 1B). However, the miR-34a levels were highly variable among patients, ranging from not detectable to a 23-fold increase over controls (Figure 1B). Stratification according to the p53 status revealed that this high variability arose from samples within the wild-type p53 cohort, whereas those B-CLL cells from patients bearing p53 mutations (n = 4), or del17p13 (n = 10), consistently showed low miR-34a expression (Figure 2A). This is congruent with recent reports25,–27 showing that B-CLL patients with p53 aberrations demonstrated significantly lower expression of miR-34a.
miR-34a expression in CD19+ B-CLL cells normalized to CD19+ B cells of healthy controls. Significantly lower expression was found in B-CLL patients with (A) p53 deletions or mutations, (B) the SNP309 G/G genotype, and (C) attenuated p53 (p53 del/mut, SNP309 G/G, del 11q). Box length equals interquartile range (**P < .01).
miR-34a expression in CD19+ B-CLL cells normalized to CD19+ B cells of healthy controls. Significantly lower expression was found in B-CLL patients with (A) p53 deletions or mutations, (B) the SNP309 G/G genotype, and (C) attenuated p53 (p53 del/mut, SNP309 G/G, del 11q). Box length equals interquartile range (**P < .01).
miR-34a expression is associated with MDM2 SNP309
However, in 24 of 61 p53 wild-types cases miR-34a expression was low despite the absence of p53 deletions or mutations. Low miR-34a expression was defined as being below the cutoff value of 2.4 relative expression level calculated by receiver operating characteristic analyses and Youden index calculation (“Statistics”). We therefore evaluated the impact of other p53 regulators on miR-34a levels in p53 wild-type patients. We recently demonstrated the association of the MDM2 polymorphism SNP309 with overall and treatment-free survival in B-CLL.21 SNP309 represents a T to G change within the MDM2 gene promoter resulting in increased MDM2 protein expression and attenuated p53 function.18 We grouped B-CLL cases according to the prognostically unfavorable G/G (n = 18), the heterozygous T/G (n = 25), and the wild-type T/T (n = 32) genotypes. Interestingly, samples within the G/G group had significantly lower miR-34a expression (P = .011) compared with those in the T/T group, whereas differences were not significant between the G/G and T/G groups (P = .125). Excluding patients with p53 deletions or mutations enhanced the significance of the findings (G/G vs T/T, P = .002; T/G vs G/G, P = .049; Figure 2B). Thus, in 38 of 75 B-CLL patients showing a low miR-34a expression, only 4 cases could not be linked to genetic alterations along the p53 axis (p53 mutation or deletion, n = 14; SNP309 G/G, without p53 aberration, n = 10; SNP309 T/G, without p53 aberration, n = 9; del11q, n = 1).
In our unselected patient cohort, only 1 patient with del11q was included. This patient had low miR-34a levels. To clarify the role of del11q on miR-34a expression, we analyzed 5 additional patients with del11q. Among those, 1 was above the threshold, 1 was at the threshold, and 3 were below the threshold (data not shown). In summary, 5 of 6 analyzed del11q patients were at or below the miR-34a threshold of 2.4. The IgVH mutational status, CD38 expression, or the presence of the chromosomal aberrations del13q14 and trisomy 12 often observed in B-CLL had no impact on miR-34a expression (supplemental Figure 3).
miR-34a expression as a surrogate marker for attenuated p53 function
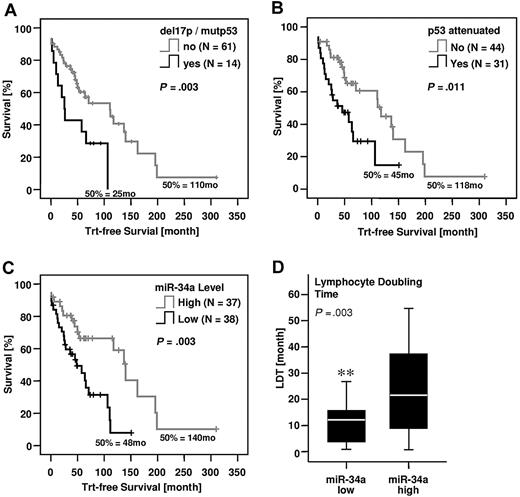
Our findings suggest that reduced miR-34a levels can serve as a biomarker for any dysfunction along the p53 axis. We defined a “p53 attenuated” group including patients with del11q, del17p, or p53 mutations, and the MDM2 SNP309 G/G genotype. A comparison between miR-34a expression levels in this newly defined cohort and patients without such alterations showed a highly significant miR-34a reduction in the p53-attenuated group (Figure 2C). Kaplan-Meier analysis revealed a significantly shorter time to treatment for patients with p53del/mut (Figure 3A) but also in the larger p53-attenuated group (P < .011, Figure 3B). To evaluate the prognostic value of miR-34a expression, we stratified patients into low (< 2.4) and high (≥ 2.4) miR-34a groups. Remarkably, the parameter of miR-34a expression alone was able to distinguish between low- and high-risk B-CLL, with significantly shorter treatment-free survival times observed within the miR-34a low group (P = .003, Figure 3C). Finally, we analyzed the lymphocyte doubling time (LDT) for 49 patients who were also characterized for miR-34a expression. LDT represents another readout for disease progression, with lower LDTs corresponding to shorter time to treatment. As expected, we observed a significantly lower LDT for B-CLL patients with low miR-34a expression (P = .003, Figure 3D).
Treatment-free survival of B-CLL patients with or without alterations in the p53 axis. (A) p53 del/mut, (B) p53-attenuated (p53 del/mut, SNP309 G/G, del11q), (C) low or high miR-34a expression, and (D) correlation of miR-34a expression with lymphocyte doubling time. Box length equals interquartile range (**P < .01).
Treatment-free survival of B-CLL patients with or without alterations in the p53 axis. (A) p53 del/mut, (B) p53-attenuated (p53 del/mut, SNP309 G/G, del11q), (C) low or high miR-34a expression, and (D) correlation of miR-34a expression with lymphocyte doubling time. Box length equals interquartile range (**P < .01).
miR-34a induces apoptosis in primary B-CLL cells
As miR-34a has been reported to mediate some of the well-known effects of p53, we investigated the downstream events after miR-34a overexpression in both primary B-CLL cells (n = 14) and a B-CLL–derived cell line, using synthetic oligonucleotides that mimic the mature miRNA molecule or nontargeting control oligonucleotides (NTCs). Primary B-CLL samples were grouped into “p53 attenuated” or not (see “Statistics”). In 10 patients without p53 attenuation, transfection of miR-34a microRNA-mimics led to a median apoptosis increase of 15% within 48 hours in comparison with transfection with the NTCs (Figure 4A). When miR-34a was overexpressed, apoptosis induction was also seen in the cell line MEC1 derived from B-CLL in prolymphocytoid transformation.35 In contrast, treatment with miR-34a antisense oligonucleotides, which reduced endogenous miR-34a levels to 50% of basal levels, had no significant effect on cell viability in prolymphocytic MEC1 cells (Figure 4B). Interestingly, overexpression of miR-34a had no effect in B-CLL cells from 4 p53 attenuated patients (n = 4: 2 SNP309 GG genotype, 1 del17q, and 1 del11q; Figure 4A). These findings indicate that the apoptosis-inducing effects of miR-34a require p53 activation, and suggest a positive feedback loop between miR-34a and p53.
Physiologic functions of miR-34a in primary B-CLL cells and the prolymphocytic MEC1 cell line. (A) In vitro miR-34a overexpression induces apoptosis in B-CLL cells in a p53-dependent manner and (B) in cell line MEC1. Antisense suppression of miR-34a has no effect. NTC indicates nontargeting control oligonucleotide. Box length equals interquartile range (*P < .05; **P < .01).
Physiologic functions of miR-34a in primary B-CLL cells and the prolymphocytic MEC1 cell line. (A) In vitro miR-34a overexpression induces apoptosis in B-CLL cells in a p53-dependent manner and (B) in cell line MEC1. Antisense suppression of miR-34a has no effect. NTC indicates nontargeting control oligonucleotide. Box length equals interquartile range (*P < .05; **P < .01).
Discussion
microRNAs have recently come into focus as key regulators in the process of tumorigenesis. In B-CLL, loss of miR-15a and miR-16-1, whose coding regions are deleted in more than half of all B-CLL patients,3 is thought to contribute to constitutive overexpression of Bcl2, a common feature of B-CLL.36 Moreover, a 9-microRNA signature that is associated with several important prognostic factors and able to predict time to treatment in B-CLL has been described.4 Not included in this signature is miR-34a, which is a highly p53-dependent microRNA12,,,,–17 and whose low expression is linked to chemotherapy resistance in B-CLL.27
We observed a deregulated expression of miR-34a in the TCL1a murine model for B-CLL. Specifically, we saw a massive increase in miR-34a expression at the transition step from a premalignant to a transformed B-CLL phenotype (Figure 1A), which was accompanied by an increase in the expression of canonical p53 targets, p21 and PUMA (supplemental Figure 2). p53 activation was also reflected in human B-CLL cells, where miR-34a expression was increased in a p53 wild-type situation (Figures 1B, 2A). As p53 is activated under conditions of oncogenic stress, we hypothesize that the pathogenesis of B-CLL includes an oncogenic signal that triggers p53. The resulting p53 activation can counteract tumor progression, which is reflected in the longer treatment-free survival periods observed in patients with an intact p53 pathway. This oncogenic signal also applies a selective pressure to eliminate p53 function, thus resulting in the incidence of several different aberrations all designed, at least in part, to disable p53. Importantly, because p53 input represents an important feature of B-CLL pathobiology, prognostic markers that are linked to p53 function (del17p, del11q, MDM2 SNP309) are able to discriminate between low- and high-risk B-CLL groups.
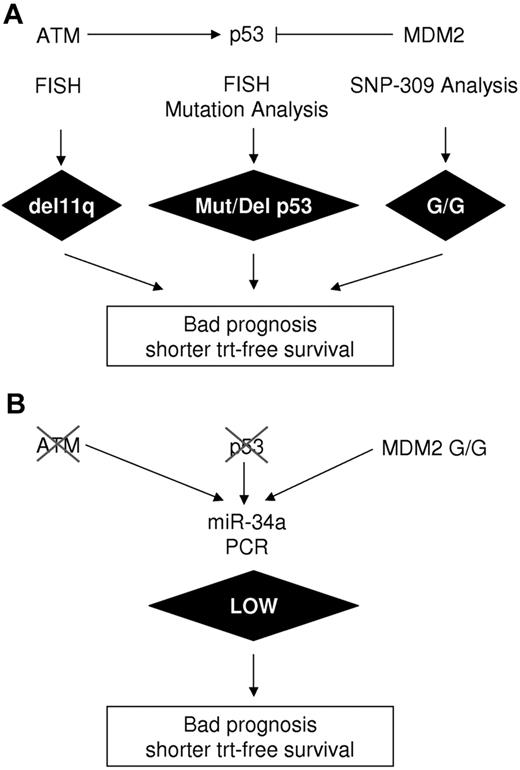
In this report, we present miR-34a as a simple and reliable surrogate marker for p53 function (Figure 5) and demonstrate that the level of miR-34a expression can predict treatment-free survival in B-CLL. In agreement with a previous report,27 low miR-34a expression was consistently observed in B-CLL patients who have deletions or mutations within the p53 gene (Figure 2A). In this study, we extend this observation to include those patients bearing the MDM2 SNP309 G/G polymorphism (Figure 2B). The MDM2 SNP309 G/G polymorphism influences age of onset in colorectal cancer,20 enhances the risk for gastric19 and non–small cell lung carcinoma,37 and was shown to be related to shorter overall and treatment-free survival in B-CLL.21 The role of SNP309 in B-CLL, however, is under debate as another study has found no correlation to overall and treatment-free survival.38
Experimental determination of aberrations along the p53 axis. (A) Classical methods including FISH, mutation analysis, and SNP309 analysis. (B) miR-34a as single parameter and readout for p53 activity.
Experimental determination of aberrations along the p53 axis. (A) Classical methods including FISH, mutation analysis, and SNP309 analysis. (B) miR-34a as single parameter and readout for p53 activity.
In our cohort, B-CLL cases carrying the SNP309 G/G polymorphism had significantly lower miR-34a levels even when no other mutations or deletions within the p53 gene were present, demonstrating that MDM2 modulation of p53 impacts miR-34a expression (Figure 2B). Another protein that influences p53 function is the p53 activating kinase ataxia telangiectasia mutated (ATM), which is lost on 1 allele in most del11q patients. In this study, we found low miR-34 expression in 5 of 6 del11q cases. However, Zenz et al report low miR-34 expression in only 5 of 12 analyzed del11q patients.27 One reason for this heterogeneity within the del11q group could be the presence or absence of mutations in the remaining ATM allele. Whether miR-34a expression can be related to the functionality of the ATM kinase is an interesting question. It is important to note that the detection of genetic aberrations along the p53 axis is laborious and time consuming because various techniques such as fluorescently labeled in vitro hybridization (FISH), single-strand conformation polymorphism analysis, and PCR with allele-specific fluorescently labeled probes are necessary (Figure 5). In contrast, determination of miR-34a expression levels requires a single reverse-transcription–PCR reaction (Figure 5), and as an independent variable is able to discriminate between low- and high-risk B-CLL groups equally well as the combination of all p53-related factors. In fact, in our analysis of 34 of 38 cases with miR-34a, low expression could be attributed to genetic lesions known to attenuate p53 function in B-CLL.
Previous studies suggest possible lines of explanation for the remaining 4 cases. The abnormalities may arise within miR-34a itself, in the form of del1p36, which is described in more than 30% of advanced-stage neuroblastomas and abrogates the coding region of miR-34a.17 This aberration has not been described in B-CLL but is also not routinely assayed for. In addition, the promoter region of miR-34a contains a CpG island whose methylation leads to reduced miR-34a expression in 79% of prostate cancer tissues.39 Finally, ATM mutations have been described in CLL.40,41 Thus, although the ATM gene itself may not harbor deletions, deleterious ATM mutations could contribute to p53 down-modulation and indirectly influence miR-34a expression.
Our studies concerning in vivo regulation of miR-34a are complemented by functional studies demonstrating the role of miR-34a in primary B-CLL cells and the prolymphocytic cell line MEC1 (Figure 4). In both systems, overexpression of miR-34a led to apoptosis induction. However, in 4 of 14 B-CLL patient samples, no response to miR-34a overexpression was detected. The nonresponders all belonged to the p53-attenuated group, suggesting p53 dependence of miR-34a–induced apoptosis.
This is interesting in the context of a recent publication showing that miR-34a depends on the intactness of the p53 pathway to exert its apoptosis-inducing effects.
Therefore, despite its role as a p53 downstream target, miR-34a seems to influence factors upstream of p53 that again lead to p53 activation in a positive feedback loop.13,42 Specifically, it has been demonstrated in the colon cancer cell line HCT-116 that miR-34a suppresses the p53 suppressor SIRT1, a nicotinamide adenine dinucleotide–dependent deacetylase, which in turn leads to increased p53 acetylation and activation.42 However, there are other reports describing apoptosis induction by miR-34 in p53 aberrant cells in gastric cancer43 and pancreatic cancer.44 Because of the small number of p53 attenuated patients tested in our study, we believe that it is too early to decide whether miR-34a represents a therapeutic option for p53-attenuated B-CLL.
Taken together, we demonstrated in this work that miR-34a expressions levels (1) are a functional marker for p53 activity including MDM2 SNP309 status, (2) are related to the lymphocyte doubling time, (3) are a predictor for time to treatment, and (4) can induce apoptosis in B-CLL cells. These data suggest an important role for miR-34a in the pathophysiology of CLL and identify it, if confirmed by other studies, as a surrogate marker for p53 functionality. Potential therapeutic applications of miR-34a overexpression are a challenge for the future, and the question of a miR-34a/p53 feedback loop will have to be carefully considered.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge Lucia Haginger and Dr Ursula Denk for excellent technical assistance in the preparation of PBMCs, and mouse handling and cell preparation, respectively. We are grateful to all patients and their clinicians for their participation in this study.
This work was supported by Klinische Malignom und Zytokinforschung Salzburg-Innsbruck GmbH, the Jubiläumsfond der Österreichischen Nationalbank (no. 12170 [R.G.] and no. 10990 [A.E.]), grants from the Austrian Science Foundation (FWF no. P19481-B12 [A.E.], L488-B13 [A.E.], and SFB program P021 to [R.G.]), and grants from the Paracelsus Medical University (nos. 05/02/014 and 06/04/025 [I.T. and I.S.]).
Authorship
Contribution: D.A., J.D.P., A.E., and O.M. designed the research; D.A., J.D.P., M.S., and P.D. performed the experimental work; I.S. performed SNP-309 genotyping; D.A. performed statistical analyses; D.A., J.D.P., and O.M. wrote the paper; R.G., I.T., A.E., and O.M. contributed to project development and obtained funding; and all authors were involved in critical discussion of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address for I.T. is Translational Radiobiology and Radiooncology Research Laboratory, Clinical Department for Radiotherapy (CCM/CVK), Charité University Hospital, Berlin, Germany.
Correspondence: Olaf Merkel or Richard Greil, Laboratory for Immunological and Molecular Cancer Research, 3rd Medical Department, Paracelsus Medical University Salzburg, Müllner Hauptstrasse 48, 5020 Salzburg, Austria; e-mail: o.merkel@salk.at; r.greil@mac.com.