In this issue of Blood, Zaslavsky and colleagues report the novel observation that megakaryocytes in tumor-bearing mice endocytose circulating thrombospondin-1 (TSP-1) and increase its synthesis to produce platelets with elevated levels of TSP-1.1 These TSP-1–enriched platelets are shown to adhere to tumors, where they act as potent inhibitors of angiogenesis and growth.
As platelets form by budding from protrusions in the megakaryocyte membrane, small organelles, designated α-granules, are encapsulated in them. These granules deliver proteins that are essential for wound healing to sites of vascular injury. Several of these proteins including TSP-1 regulate angiogenesis, and TSP-1 has been shown to dictate the course of wound healing.2 α-Granules have recently been shown to vary in their content, with some being enriched in proangiogenic proteins, while others preferentially contain antiangiogenic factors.3 In many ways, the stromal response in wounds is similar to that seen in the tumor microenvironment, where TSP-1 is expressed by various types of stromal cells.4 Multiple studies have demonstrated that TSP-1 is a potent inhibitor of tumor angiogenesis and growth.2,5,6 This activity results from direct effects on signaling pathways that mediate endothelial cell migration, survival and apoptosis, as well as indirect effects on vascular endothelial growth factor (VEGF) mobilization and clearance.2,5,6
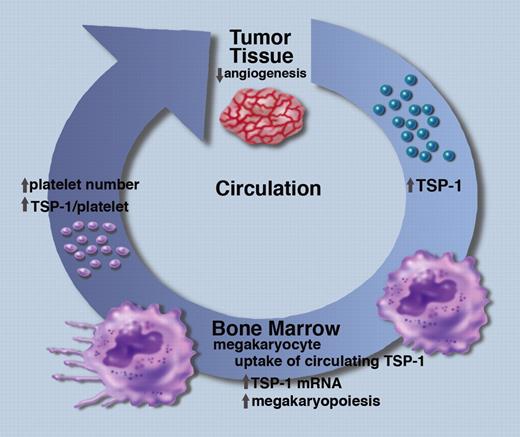
A schematic representation of the role of circulating TSP-1 and megakaryocytes in the inhibition of tumor angiogenesis. Professional illustration by Marie Dauenheimer.
A schematic representation of the role of circulating TSP-1 and megakaryocytes in the inhibition of tumor angiogenesis. Professional illustration by Marie Dauenheimer.
Zaslavsky et al have made the intriguing observation that the amount of TSP-1 in circulating platelets correlates with tumor size in several murine models of cancer1 (see figure). Furthermore, when the tumor is resected, the level of platelet TSP-1 returns to normal. Some of the increased TSP-1 in platelets is clearly derived from the tumor cells because the effect is seen in TSP-1–null mice where the implanted tumor cells are the only source of TSP-1. To explore the mechanism underlying these effects, the authors injected recombinant TSP-1 directly into the circulation of TSP-1–null mice and showed that TSP-1 can be detected in megakaryocytes in the bone marrow within 24 hours, and in circulating platelets 5 days later. These data indicate that megakaryocytes have the ability to take up circulating TSP-1 and incorporate it into newly synthesized platelets. This conclusion is supported by in vitro experiments, showing that isolated megakaryocytes can endocytose TSP-1, whereas isolated platelets do not.
Zaslavsky et al also show that megakaryocytes respond to the presence of tumors by increasing their number in the bone marrow and by increasing TSP-1 mRNA levels.1 These differences are accompanied by increases in the number of platelets in circulation and the quantity of TSP-1 in each platelet. Whether or not these changes in megakaryocytes are induced by TSP-1 itself or by other tumor-derived circulating factors remains to be determined. Further studies are also needed to determine how megakaryocytes direct the endocytosed TSP-1 to newly synthesized platelets. The increased number of platelets and the increased amount of TSP-1 per platelet is shown by Zaslavsky et al to have potent anti-angiogenic activity as measured by a significant decrease in microvascular density within tumors.1 In addition, platelets and platelet-derived TSP-1 are shown to significantly inhibit tumor growth in several model systems.
The studies of Zaslavsky et al suggest that TSP-1 levels in platelets may have diagnostic value for the detection and monitoring of cancer progression.1 However, to establish that platelet TSP-1 levels can be used as a marker of tumor burden in cancer patients, it will be important to establish that human and mouse megakaryocytes respond similarly to the presence of tumors. Since TSP-1 is a multifunctional protein that also affects thrombosis and metastasis, it will be important to determine all of the effects of increased circulating and platelet TSP-1 in the complex pathological context of cancer.2,5,6 The work of Zaslavsky et al has identified megakaryocytes as new players in the body's response to cancer,1 and future studies will elucidate the potential of these findings for the diagnosis and treatment of human cancer.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
Acknowledgment
This work is supported by a grant from the National Cancer Institute (CA130895).
National Institutes of Health