Abstract
Activating transcription factor 3 (ATF3) is a basic leucine zipper transcription factor that plays a regulatory role in inflammation, cell division, and apoptosis. Mast cells (MCs) initiate many inflammatory responses and have a central role in allergy and allergic diseases. We report here that ATF3 has a central role in MC development and function. Bone marrow–derived MC populations from ATF3-deficient mice are unresponsive to interleukin-3 (IL-3)–induced maturation signals, and this correlates with increased apoptosis, diminished activation of the Akt kinase, and decreased phosphorylation of the proapoptotic protein Bad. Furthermore, ATF3-null mice lacked MCs in the peritoneum and dermis, showing that the in vitro results are recapitulated in vivo. ATF3-null MCs also showed functional defects; high-affinity immunoglobulin E receptor–mediated degranulation was significantly inhibited, whereas IL-4 and IL-6 expression was enhanced. This dual role of ATF3 provides insight into the complex interplay between MC development and its subsequent physiologic role.
Introduction
Mast cells (MCs) are tissue-resident immune effector cells that arise from immature bone marrow (BM) precursors.1 They produce and secrete numerous bioactive agents and have been implicated in diverse homeostatic functions such as angiogenesis, wound healing, and tissue remodeling.2 Because of their armamentarium of mediators and strategic localization, MCs also play central roles in various disease states, in particular T helper cells type 2–driven inflammatory conditions such as asthma.3 Therefore, it is critical that MC activation be tightly controlled.
Recent studies have begun to define the transcription factors (TFs) involved in the regulation of MCs; these include nuclear factor–activated T cell, globin transcription factor, Jun/Fos (AP-1), Egr, and members of the signal transducer and activator of transcription family.4-8 Most of these TFs are positive regulators of MC phenotype. We have identified the TF activating transcription factor 3 (ATF3) as a negative regulator of the inflammatory response in macrophages.9 ATF3 is a member of the cyclic adenosine monophosphate response element binding (CREB) family of basic leucine zipper TFs and has been reported to function in the stress response, the regulation of the cell cycle, and apoptosis.10 In the present study we characterize the function of ATF3 in MC development and mediator release.
Methods
Animals
C57/Bl6 mice were purchased from Charles River Breeding Laboratories. ATF3−/− mice were a kind gift from Dr Tsonwin Hai (Ohio State University). All animal use procedures were approved by the University of Washington Animal Care Committee.
Mast cells
BM-derived MCs (BMMCs) were derived from BM cells cultured in RPMI 1640 with 15% fetal bovine serum (FBS), 30 ng/mL murine interleukin-3 (IL-3; PeproTech) and/or 50 ng/mL stem cell factor (SCF; PeproTech). Maturity of BMMC cultures was determined by toluidine blue or safranin staining of cytospun cells as previously described. BMMCs were also characterized by staining with fluorescein isothiocyanate–labeled anti–high-affinity immunoglobulin E receptor (FcϵRI; Becton Dickinson) and anti–phycoerythrin-labeled anti–c-kit antibody (eBioscience) and analyzed by flow cytometry (Becton Dickinson).
MC activation
For all activation experiments, MCs were washed in phosphate-buffered saline (PBS) after which time the cells were starved for 6 hours in RPMI 1640 (2% FBS) containing no growth factors. MCs were then replated in RPMI 1640 with 15% FBS with IL-3 and/or SCF at the indicated concentrations. MCs collected at this point were considered as the 0-hour time point in all experiments.
Sensitization of MCs
After 6 hours of starvation, BMMCs were sensitized with immunoglobulin E (IgE; anti–2,4 dinitrophenol [DNP]; 2 μg/mL; Serotec) for 2 hours at 37°C. Cells were washed and resuspended in HEPES-buffered Tyrode solution, then stimulated with DNP (100 ng/mL; Sigma-Aldrich).
Histologic analysis
Ear, back skin, and stomach were removed, fixed in 4% paraformaldehyde, and embedded in paraffin. Sections (4-μm thick) were cut and stained with 0.05% acidic toluidine blue (pH 1.0). MCs were counted under an optical microscope.
Passive cutaneous anaphylaxis
Mice were sensitized by intradermal injection of 25 ng of anti-DNP IgE monoclonal antibody (Sigma-Aldrich) in 20 μL of PBS. After 18 hours, mice were challenged by intravenous injection of 100 μg of DNP–bovine serum albumin in 200 μL of Evan blue dye (0.5% wt/vol; Sigma-Aldrich). Forty-five minutes later, ear tissue was collected in 800 μL of formamide and incubated at 80°C for 2 hours. The absorbance was determined at 620 nm.
Apoptosis assay
Five-week BMMC cultures from wild-type (WT) and ATF3-null mice were washed and incubated 6 hours in low serum (1% FBS) containing RPMI. Cells were then placed in RPMI containing various concentration of IL-3 or SCF. Apoptosis was determined by flow cytometry after annexin V/propidium iodide staining as recommended by the manufacturer (Invitrogen).
Cell proliferation analysis
BMMCs were washed and seeded as for the apoptosis assays. Cell proliferation was determined 48 hours after activation with the use of the 4,5-dimethylthiazol-2-yl-3,5-diphenylformazan, thiazolyl blue formazan assay according to the ATCC protocol.
Real-time polymerase chain reaction analysis
Total RNA was prepared from MCs with TRIzol reagent with the use off the manufacturer's protocols. RNA was treated with heparinase I (Sigma-Aldrich) as previously described.11 Synthesis of cDNA was completed with Moloney murine leukemia virus reverse transcriptase (Promega) according to the manufacturer's recommendations and primed with oligo d(T) (Promega). Quantitative real-time polymerase chain reaction (PCR) was performed on an ABI 7700 (Applied Biosystems) with the use of TaqMan Universal PCR Master Mix (Applied Biosystems). Commercially available primer/probe pairs (Applied Biosystems) were used at a final concentration of 0.9μM. All data were normalized to elongation factor-1 α expression in the same cDNA set.
Western blot
MCs were incubated in 24-well plates at 106 cells/well. Cells were dissociated in RIPA buffer (PBS, 1% NP-40). The total protein content was determined by the Bradford technique (Bio-Rad Laboratories). Protein from each sample (10 μg) was mixed with Laemmli loading buffer containing sodium dodecyl sulphate and β-mercaptoethanol. Samples were electrophoretically separated on a 4% to 20% gradient sodium dodecyl sulphate–polyacrylamide gel electrophoresis gel and transferred onto a polyvinylidene fluoride membrane (Bio-Rad Laboratories). The membrane was then incubated with primary antibodies for 1 hour at room temperature. Antibodies against ATF3 (Santa Cruz Biotechnology), phosphor-Akt (Cell Signaling Technology), Akt, and Bad were used. The secondary antibody, 1/5000 horseradish peroxidase–conjugated goat anti–rabbit IgG (AbD Serotec) was added to the membrane and incubated for 1 hour at room temperature. Labeling was detected by chemiluminescence by the addition of SuperSignal substrate solution (Pierce Chemical).
Confocal microscopy
Localization of ATF3 in MCs was performed on BMMCs fixed in 4% paraformaldehyde for 20 minutes, then permeabilized with 0.1% Triton X-100 in PBS. Slides were blocked for 30 minutes with 10% FBS and 3% bovine serum albumin in PBS. All slides were incubated overnight at 4°C with primary antibody. Specific antibody binding was detected with Alexa-labeled mouse anti–rabbit secondary antibodies (Invitrogen). Negative controls with rabbit serum were run concurrently.
All cell images were obtained using a 40×/1.3 oil Plan Neofluar objective on a Zeiss confocal microscope with laser-scanning microscope (LSM) 510 software. Images were obtained using a Zeiss Axiocam HR camera.
Chromatin immunoprecipitation
After stimulation, BMMCs were fixed in formaldehyde and chromatin immunoprecipitation assay was performed as previously described.9 The purified DNA was analyzed by PCR with the use of primers specific for the IL-4 and IL-6 promoter regions.9,12 The PCR products were visualized on an ethidium bromide gel.
Enzyme-linked immunoabsorbent assay
IL-4 and IL-6 (R&D Systems) and cysteinyl leukotriene (Cayman Chemical) levels were measured with the use of specific commercially available enzyme-linked immunoabsorbent assay according to the manufacturer's protocols.
Degranulation assay
Degranulation was determined by the measure of β-hexosaminidase as previously described.13
Statistical analysis
All experiments were performed at least 3 times. Data were analyzed with the use of analysis of variance. P values less than .05 were considered significant.
Results
ATF3 expression in mast cells
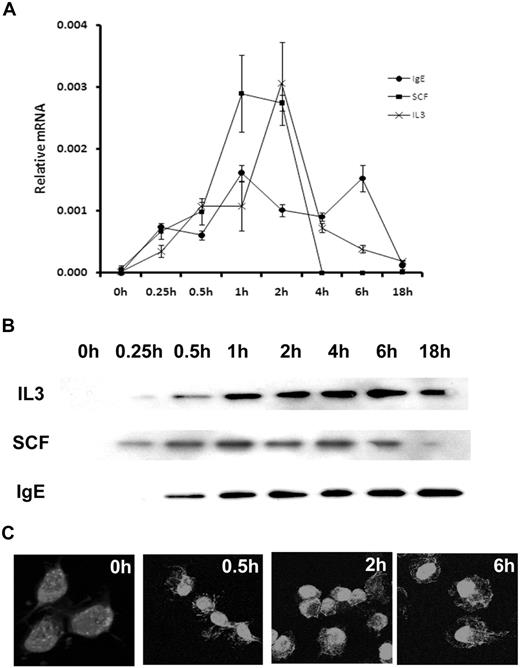
ATF3 mRNA was negligible in starved (unstimulated) BMMCs. Furthermore, BMMCs removed directly from culture, before starvation, also showed minimal ATF3 expression (data not shown). IgE cross-linking with antigen resulted in an immediate induction of ATF3 expression within 15 minutes. The mRNA levels remained relatively high at 1, 2, 4, and 6 hours after activation, with a return to baseline levels after 18 hours (Figure 1A). Similar results were seen after SCF activation, although levels had returned to baseline at 4 hours (Figure 1A). Results were comparable with IL-3 activation; peak expression occurred at 2 hours. This induction of ATF3 mRNA expression correlated with increases in protein levels (Figure 1B). Furthermore, IgE cross-linking stimulated the translocation of ATF3 to the nucleus at 0.5 to 6 hours (Figure 1C).
ATF3 is expressed in MCs after growth factor or FcϵRI activation. (A) Mouse BMMCs were starved of growth factors for 6 hours, then sensitized with anti-DNP IgE and stimulated with DNP–bovine serum albumin (BSA; 100 ng/mL), IL-3 (30 ng/mL), or SCF (50 ng/mL) for 0, 0.25, 0.5, 1, 2, 4, 6, or 18 hours. RNA isolated from these cells was analyzed by real-time reverse transcription–PCR. ATF3 expression was normalized to elongation factor-1 α levels. Error bars represent SE from 3 independent experiments. (B) Mouse BMMCs were sensitized with DNP-BSA (100 ng/mL), IL-3 (30 ng/mL), or SCF (50 ng/mL) for 0, 0.25, 0.5, 1, 2, 4, 6, or 18 hours. Protein was extracted from whole cells and separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis, and ATF3 was detected with specific antibody. (C) Mouse BMMCs were sensitized with anti-DNP IgE and stimulated with DNP-BSA (100 ng/mL) for 0, 0.5, 2, or 6 hours. Cells were fixed in paraformaldehyde and incubated with rabbit anti-ATF3 antibody. Antibody labeling was detected with boron-dipyrromethene–conjugated goat anti–rabbit antibody. Original magnification ×600.
ATF3 is expressed in MCs after growth factor or FcϵRI activation. (A) Mouse BMMCs were starved of growth factors for 6 hours, then sensitized with anti-DNP IgE and stimulated with DNP–bovine serum albumin (BSA; 100 ng/mL), IL-3 (30 ng/mL), or SCF (50 ng/mL) for 0, 0.25, 0.5, 1, 2, 4, 6, or 18 hours. RNA isolated from these cells was analyzed by real-time reverse transcription–PCR. ATF3 expression was normalized to elongation factor-1 α levels. Error bars represent SE from 3 independent experiments. (B) Mouse BMMCs were sensitized with DNP-BSA (100 ng/mL), IL-3 (30 ng/mL), or SCF (50 ng/mL) for 0, 0.25, 0.5, 1, 2, 4, 6, or 18 hours. Protein was extracted from whole cells and separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis, and ATF3 was detected with specific antibody. (C) Mouse BMMCs were sensitized with anti-DNP IgE and stimulated with DNP-BSA (100 ng/mL) for 0, 0.5, 2, or 6 hours. Cells were fixed in paraformaldehyde and incubated with rabbit anti-ATF3 antibody. Antibody labeling was detected with boron-dipyrromethene–conjugated goat anti–rabbit antibody. Original magnification ×600.
ATF3 is required for in vitro MC maturation
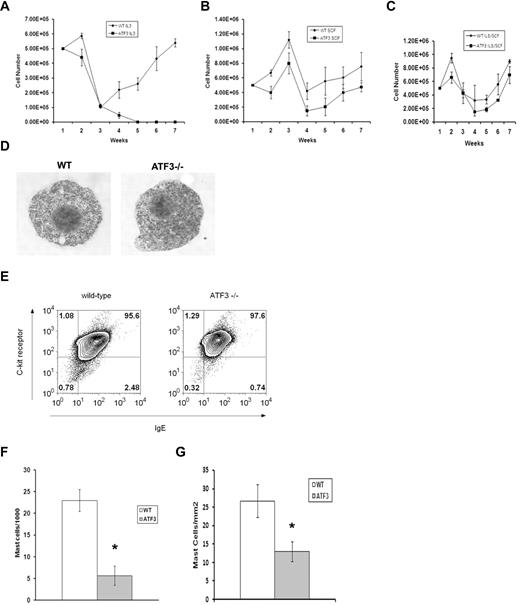
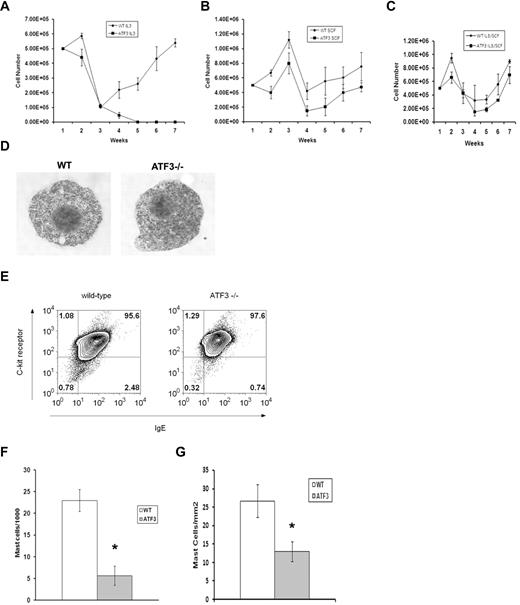
CREB/ATF family TFs have defined roles in cell-cycle regulation and apoptosis; thus, we examined the growth of MCs derived from WT or ATF3-null mice in liquid culture.10 In the presence of IL-3, WT BM cells produced MC populations that were greater than 95% pure by 4 weeks (Figure 2A), whereas ATF3-null BM cells did not generate viable MC cultures. MCs were derived from ATF3-null BM cultured in SCF or a combination of IL-3/SCF, although culture expansion was delayed (Figure 2B-C). MC populations from WT and ATF3-null mice grown in a combination of IL-3 and SCF (IL3/SCF) showed similar patterns of granulation and expressed FcϵRI and C-kit (CD117) at similar levels (Figure 2D-E). Mac1+ macrophages may be acting as feeder cells during the early time points of MC culture.14 We attempted mixing experiments to determine whether the growth defect was intrinsic to the MCs or other cells types present early in culture. MC cultures did not develop when ATF3-null MCs were plated onto adherent WT feeder cells. By contrast, WT MCs developed well when plated on feeder cells derived from ATF3-null mice (data not shown).
ATF3 deficiency reduces MC numbers in vitro and in vivo. (A) BM cells were cultured at a starting density of 5 × 105 cells/mL in the presence of IL-3 (30 ng/mL). Total number of cells was counted every 7 days. (B) BM cells were cultured at a starting density of 5 × 105 cells/mL in the presence of SCF (50 ng/mL). Total number of cells was counted every 7 days. (C) BM cells were cultured at a starting density of 5 × 105 cells/mL in the presence of IL-3 (30 ng/mL) and SCF (50 ng/mL). (D) BM cells from WT and ATF3-null mice were cultured in IL-3/SCF for 5 weeks and examined for morphology by toluidine blue staining or (E) 2-color analysis of flow cytometry for IgE receptor (horizontal axis) and c-kit expression (vertical axis), percentages of positive cells are indicated. (F) Peritoneal lavage from WT or ATF3-null mice were spun onto cytospin slides and stained with Wright-Giemsa. Results are expressed as mean ± SE from counts of 3 separate experiments. (G) Sections from skin were stained with toluidine blue for MCs. Results are expressed as mean ± SE from counts of 3 separate experiments. *P < .05 by comparison to WT.
ATF3 deficiency reduces MC numbers in vitro and in vivo. (A) BM cells were cultured at a starting density of 5 × 105 cells/mL in the presence of IL-3 (30 ng/mL). Total number of cells was counted every 7 days. (B) BM cells were cultured at a starting density of 5 × 105 cells/mL in the presence of SCF (50 ng/mL). Total number of cells was counted every 7 days. (C) BM cells were cultured at a starting density of 5 × 105 cells/mL in the presence of IL-3 (30 ng/mL) and SCF (50 ng/mL). (D) BM cells from WT and ATF3-null mice were cultured in IL-3/SCF for 5 weeks and examined for morphology by toluidine blue staining or (E) 2-color analysis of flow cytometry for IgE receptor (horizontal axis) and c-kit expression (vertical axis), percentages of positive cells are indicated. (F) Peritoneal lavage from WT or ATF3-null mice were spun onto cytospin slides and stained with Wright-Giemsa. Results are expressed as mean ± SE from counts of 3 separate experiments. (G) Sections from skin were stained with toluidine blue for MCs. Results are expressed as mean ± SE from counts of 3 separate experiments. *P < .05 by comparison to WT.
ATF3 is required for in vivo MC maturation
To determine the effect of ATF3 on in vivo–derived MC populations, the frequency of mature MCs in peritoneal lavage and skin sections of WT and ATF3-null mice was determined. MC numbers were decreased approximately 60% in the peritoneum of ATF3-null mice (Figure 2F), and decreased MC numbers were also seen in the dermis of ATF3-null animals (Figure 2G).
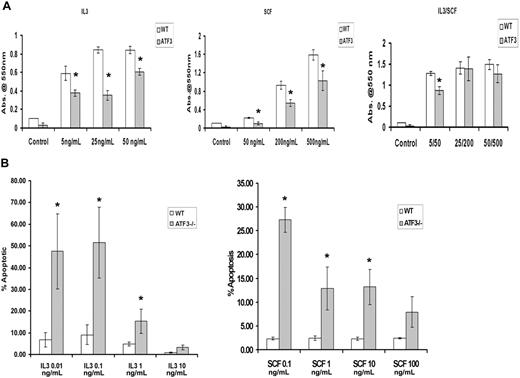
Proliferation defect in ATF3-null MCs
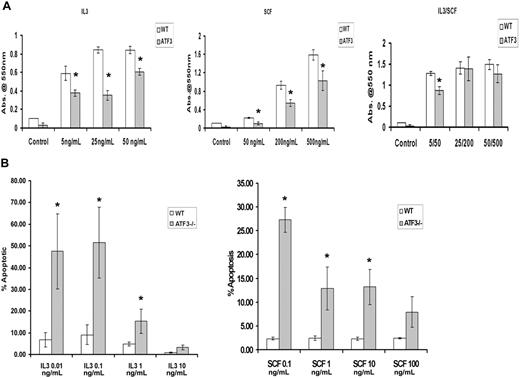
Both IL-3 and SCF are known to induce proliferation of MC populations,1 and this was also seen in our WT MC cultures with the use of an 4,5-dimethylthiazol-2-yl-3,5-diphenylformazan, thiazolyl blue formazan assay (Figure 3A). By contrast, ATF3-null MCs showed significant inhibition of proliferation when cells were stimulated with either IL-3 or SCF, even at high doses (Figure 3A).
ATF3-null MCs have diminished proliferation and enhanced apoptosis after growth factor withdrawal. WT or ATF3-null MCs were cultured in IL-3/SCF for 5 weeks. Mature MCs were starved of growth factors for 6 hours, washed, and reseeded in the indicated concentrations of IL-3 (in ng/mL) or SCF (in ng/mL). (A) Proliferation of MCs. Cells were incubated with growth factors for 48 hours then stained with 4,5-dimethylthiazol-2-yl-3,5-diphenylformazan, thiazolyl blue formazan to evaluate proliferation. (B) MC apoptosis. Cells were stained with annexin V/PI, and apoptosis was determined by flow cytometry. Results are expressed as mean ± SEM for 3 independent experiments. *P < .05 by comparison to WT.
ATF3-null MCs have diminished proliferation and enhanced apoptosis after growth factor withdrawal. WT or ATF3-null MCs were cultured in IL-3/SCF for 5 weeks. Mature MCs were starved of growth factors for 6 hours, washed, and reseeded in the indicated concentrations of IL-3 (in ng/mL) or SCF (in ng/mL). (A) Proliferation of MCs. Cells were incubated with growth factors for 48 hours then stained with 4,5-dimethylthiazol-2-yl-3,5-diphenylformazan, thiazolyl blue formazan to evaluate proliferation. (B) MC apoptosis. Cells were stained with annexin V/PI, and apoptosis was determined by flow cytometry. Results are expressed as mean ± SEM for 3 independent experiments. *P < .05 by comparison to WT.
ATF3-null MCs undergo apoptosis
IL-3 and SCF are critical factors for MC survival, and removal of these factors induces rapid apoptosis.15 We determined MC apoptosis from WT and ATF3-null mice cultured in IL-3 or SCF for 48 hours by flow cytometry of annexin V and propidium iodide–stained MCs. Although IL-3/SCF allowed survival of both MC populations, culture in individual cytokines showed differing concentration-dependent apoptotic responses. There was a 10-fold difference in the concentration of IL-3 required for half-maximal survival in WT compared with ATF3-null MCs (Figure 3B). Increased apoptosis was seen in ATF3-null MCs at all concentrations of SCF used.
ATF3 deficiency leads to enhanced apoptosis and diminished proliferation at early time points in MC culture
MCs were grown for 1 to 4 weeks in IL-3, SCF, or both cytokines, and the levels of apoptosis or proliferation were determined. As shown in Table 1, ATF3-null MCs cultured in IL-3 showed similar levels of apoptosis and proliferation during the first week of culture. By week 3, there were negligible numbers of cells present in ATF3/IL-3 cultures, and those that remained were highly apoptotic. By contrast, WT MCs cultured in IL-3 showed less apoptosis and more proliferation, accounting for the increased numbers of MCs. ATF3-null MC cultures in IL-3/SCF showed a slightly increased level of apoptosis at week 3, yet proliferation levels were similar to WT.
The ATF3 MC defect was not associated with changes in c-Kit and FcϵRI levels during maturation
Diminished Akt signaling in ATF3-null MCs
We examined Akt activation in ATF3-null MCs because this kinase has been shown to have an important role in MC function and development.16 IL-3 stimulated Akt phosphorylation in WT MCs, whereas this activation was decreased in ATF3-null MCs (Figure 4A). Similar results were also seen after SCF or FcϵRI-mediated activation (Figure 4A-B). Concurrent stimulation with both IL-3 and SCF resulted in partial restoration of Akt phosphorylation in ATF3-null MCs (Figure 4A).
Impaired Akt pathways in ATF3-null MCs. (A) WT or ATF3-null MCs were cultured in IL-3/SCF for 5 weeks, starved of growth factor for 6 hours, washed, then cultured in IL-3 (30 ng/mL) or SCF (50 ng/mL) or both for the indicated times (in minutes). Whole-cell lysates were analyzed by Western blot with antibodies against phosphorylated Akt, total AKT. (B) After sensitization with anti-DNP IgE, MCs were stimulated with KLH-DNP for the indicated times. Whole-cell lysates were analyzed by Western blot with antibodies against phosphorylated Akt, total AKT. (C) WT or ATF3-null MCs were starved for 6 hours, washed, and then cultured in IL-3 (30 ng/mL) or SCF (50 ng/mL) or both for the indicated times. Whole-cell lysates were analyzed by Western blot with antibodies against phospho-Bad and total Bad.
Impaired Akt pathways in ATF3-null MCs. (A) WT or ATF3-null MCs were cultured in IL-3/SCF for 5 weeks, starved of growth factor for 6 hours, washed, then cultured in IL-3 (30 ng/mL) or SCF (50 ng/mL) or both for the indicated times (in minutes). Whole-cell lysates were analyzed by Western blot with antibodies against phosphorylated Akt, total AKT. (B) After sensitization with anti-DNP IgE, MCs were stimulated with KLH-DNP for the indicated times. Whole-cell lysates were analyzed by Western blot with antibodies against phosphorylated Akt, total AKT. (C) WT or ATF3-null MCs were starved for 6 hours, washed, and then cultured in IL-3 (30 ng/mL) or SCF (50 ng/mL) or both for the indicated times. Whole-cell lysates were analyzed by Western blot with antibodies against phospho-Bad and total Bad.
The Bcl2 family promotes cell survival by inhibiting apoptosis.17 Akt is known to inhibit apoptosis18 by phosphorylating Bad, a Bcl2 family member, which, in turn, results in the sequestration of Bad by the 14-3-3 protein. This prevents Bad dimerizing with Bcl2 or BCL-XL, which is required for apoptosis. In WT MCs, phosphorylation of Bad increased after SCF or IL-3 treatment in a pattern concordant with Akt activation. Conversely, Bad phosphorylation was diminished in ATF3-null MCs, with peak activation at 5 minutes (Figure 4C). Costimulation with both IL-3 and SCF resulted in Bad phosphorylation that approached WT levels, particularly at earlier time points. Collectively, these data indicate that signaling in the Akt pathway is impaired by ATF3 deficiency.
Granule mediator and leukotriene production
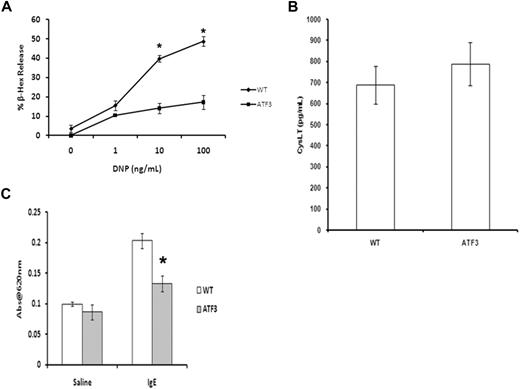
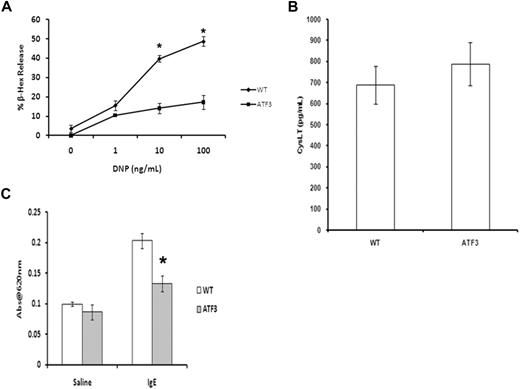
Activation of MCs by cross-linking of the FcϵRI initiates a signaling cascade that leads to the rapid release of both preformed granule mediators and newly synthesized arachidonic acid metabolites.19 IgE-induced release of β-hexosaminidase (a marker of granule release) in ATF3-null MCs was significantly lower than that seen in WT MCs across the entire dose response. Interestingly, there was no difference in the levels of cysteinyl-leukotriene produced under these conditions (Figure 5B). Decreased vascular permeability (passive cutaneous anaphylaxis) was observed in sensitized ATF3-null mice, compared with WT, showing that the ATF3 deficiency in granule release observed in vitro also occurs in vivo (Figure 5C). Taken together, these data show that ATF3 has a role in IgE-mediated MC degranulation but not in leukotriene production.
ATF3 deficiency inhibits MC granule mediator release. (A) WT or ATF3-null MCs were cultured in IL-3/SCF for 5 weeks, starved of growth factor for 6 hours, washed, and then sensitized with anti-DNP IgE and stimulated with various concentrations of DNP–bovine serum albumin (BSA) for 30 minutes. Degranulation was measured as β-hexosaminidase (β-Hex) release. (B) Cysteinyl leukotrienes from culture medium was determined by specific enzyme-linked immunoabsorbent assay. Results are expressed as mean ± SEM for 3 independent experiments. *P < .05 by comparison to WT. (C) Passive cutaneous anaphylaxis. Anti-DNP IgE (20 ng) was injected intradermally in the left ear, whereas the right ear received saline as a control. After 24 hours, mice received 100 μg of DNP-BSA containing Evan blue dye (0.5% wt/vol) by tail vein injection. Tissue from both ears was collected 45 minutes later and was used for extraction of the Evan blue dye. The optical density was measured at 620 nm. Data are means ± SEM (n = 4 mice in each group). *P < .05 compared with the IgE group of the WT mice.
ATF3 deficiency inhibits MC granule mediator release. (A) WT or ATF3-null MCs were cultured in IL-3/SCF for 5 weeks, starved of growth factor for 6 hours, washed, and then sensitized with anti-DNP IgE and stimulated with various concentrations of DNP–bovine serum albumin (BSA) for 30 minutes. Degranulation was measured as β-hexosaminidase (β-Hex) release. (B) Cysteinyl leukotrienes from culture medium was determined by specific enzyme-linked immunoabsorbent assay. Results are expressed as mean ± SEM for 3 independent experiments. *P < .05 by comparison to WT. (C) Passive cutaneous anaphylaxis. Anti-DNP IgE (20 ng) was injected intradermally in the left ear, whereas the right ear received saline as a control. After 24 hours, mice received 100 μg of DNP-BSA containing Evan blue dye (0.5% wt/vol) by tail vein injection. Tissue from both ears was collected 45 minutes later and was used for extraction of the Evan blue dye. The optical density was measured at 620 nm. Data are means ± SEM (n = 4 mice in each group). *P < .05 compared with the IgE group of the WT mice.
Cytokine production
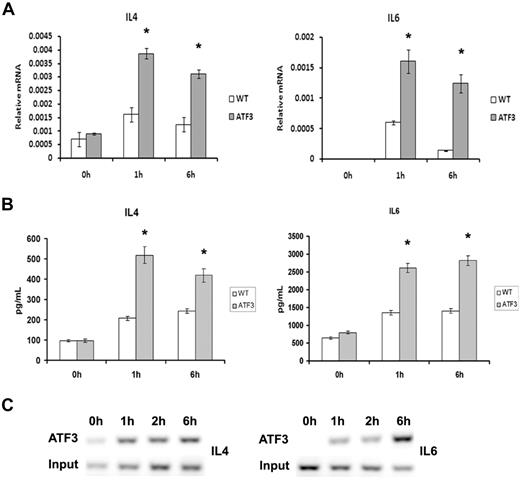
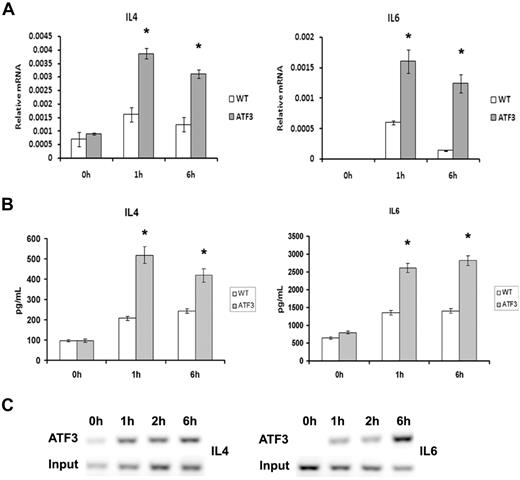
Activation of the IgE receptor after antigen cross-linking also induces the transcription and secretion of numerous cytokines and chemokines, and we found that ATF3-null MCs had increased levels of FcϵRI-induced transcription and secretion of IL-4 and IL-6 from MCs (Figure 6A-B). This observation is consistent with our previous data showing that ATF3 is a negative regulator of cytokine transcription.9,12 Chromatin immunoprecipitation analysis showed that ATF3 bound the IL-4 and IL-6 promoters after IgE cross-linking (Figure 6C). The kinetics of ATF3 binding was consistent with it having a role in transcriptional regulation of the 2 cytokines.
Enhanced cytokine production in ATF3-null MCs. WT or ATF3-null MCs were cultured in IL-3/SCF for 5 weeks, starved of growth factor for 6 hours, washed, and then sensitized with anti-DNP IgE and stimulated with DNP–bovine serum albumin (100 ng/mL) for 0, 1, and 6 hours. (A) Cell pellets were used for RNA isolation and real-time reverse transcription–PCR was performed to determine cytokine expression. (B) Cytokine release was determined in cell-free supernatants by enzyme-linked immunoabsorbent assay. (C) Chromatin immunoprecipitation analysis of ATF3 to predict binding sites in the IL-4 and IL-6 promoters was performed as outlined in “Chromatin immunoprecipitation” with the use of rabbit anti-ATF3 antibody. DNA from input or immunoprecipitated (ATF3) fractions was measured by PCR amplification of specific promoter sequences. Results are expressed as mean ± SEM for 3 independent experiments. *P < .05 by comparison to WT.
Enhanced cytokine production in ATF3-null MCs. WT or ATF3-null MCs were cultured in IL-3/SCF for 5 weeks, starved of growth factor for 6 hours, washed, and then sensitized with anti-DNP IgE and stimulated with DNP–bovine serum albumin (100 ng/mL) for 0, 1, and 6 hours. (A) Cell pellets were used for RNA isolation and real-time reverse transcription–PCR was performed to determine cytokine expression. (B) Cytokine release was determined in cell-free supernatants by enzyme-linked immunoabsorbent assay. (C) Chromatin immunoprecipitation analysis of ATF3 to predict binding sites in the IL-4 and IL-6 promoters was performed as outlined in “Chromatin immunoprecipitation” with the use of rabbit anti-ATF3 antibody. DNA from input or immunoprecipitated (ATF3) fractions was measured by PCR amplification of specific promoter sequences. Results are expressed as mean ± SEM for 3 independent experiments. *P < .05 by comparison to WT.
Discussion
SCF and IL-3 are required for MC growth and survival in vivo and in vitro.1 This process is associated with changes in transcriptional regulation mediated by Fos, Jun, and Egr1.6,7,20 We report here that ATF3, a member of the Creb/ATF family of TFs, also has a regulatory role in MC development and function. MCs from ATF3-deficient mice become apoptotic in the presence of SCF or IL-3. This is correlated with defects in the Akt signaling pathway, particularly in the context of Akt-dependent phosphorylation of the Bcl2 family member, Bad. This phosphorylation is known to inhibit apoptosis because it prevents Bad from dimerizing with Bcl2 or BCL-XL, an event that is required for MC survival.
A possible mechanism for the decreased survival in ATF3-null MCs could be due to a defect in a proximal multipotential progenitor. This defect is not found in macrophages, dendritic cells, and neutrophils derived from ATF3-null BM. In addition, levels of splenic B cells and T cells as well as percentages of blood leukocytes were similar to that seen in WT (data not shown). Given these findings, it appears that the survival defect in MCs from ATF3-null mice is cell-type specific.
Note that the defect in survival and proliferation seen in ATF3-null MCs could be partially overcome by the combined addition of IL-3 and SCF. This is probably due to the restored levels of Akt phosphorylation seen in the ATF3-null MCs concurrently stimulated with both cytokines. In addition, a recent study has shown that ATF3 regulates the expression of cyclin D1 and cyclin A in chondrocytes.21,22 Although we did not investigate this link, it is possible that ATF3 is a key regulator of cyclin expression and thus cell cycle progression in MCs, which would account for the diminished proliferation seen in ATF3-null MCs stimulated with IL-3 or SCF. Indeed, even stimulation with IL-3 and SCF in tandem still resulted in an observed lag in MC cell counts in vitro. Studies are currently under way to define cyclin gene expression in ATF3-null MCs and the resulting effect on cell-cycle progression.
The developmental phenotype of MCs in ATF3-null mice is reminiscent of that seen in phosphoinositol-3 kinase (PI3K)–null mice (which lack MCs in the gastrointestinal tract and have diminished MC numbers in the skin MCs).23 Furthermore, PI3K-null MCs also show deficient growth in vitro. Despite these similarities, ATF3-null mice show mature MCs in skin and gastrointestinal tract. This appearance of MCs in tissue sites is interesting. Although IL-3 and SCF have overlapping effects on MC development, there are also important differences, particularly in the context of other cytokines. A role for tumor necrosis factor (TNF) in regulating MC survival has been identified.14 TNF-null MCs show reduced (< 50%) MC numbers in the peritoneum compared with WT. This is similar to the numbers seen in ATF3-null peritoneum. However, TNF-null MCs also showed diminished FcϵRI levels, whereas our studies do not identify a similar decrease. Interestingly, we have previously showed that macrophages activated with lipopolysaccharide (LPS) showed increased TNF release from ATF3-null mice, and Mac1+ cells are significant contributors to TNF levels in MC culture.9 Nevertheless, our mixing studies showed that TNF levels measured in both WT and ATF3-null cultures were similar. However, a paracrine roll of other cytokines cannot be excluded.
In addition to its role in MC development, ATF3 also has a profound role in FcϵRI-mediated signaling. MCs from ATF3-null mice showed significantly reduced degranulation, although leukotriene release was unaffected, and IL-4 and IL-6 secretion was enhanced. Previous work has shown a defect in degranulation in MCs from PI3K-null mice.24 This observation is reinforced by the finding that the PI3K inhibitor, wortmannin, also inhibits MC degranulation.25 Thus, the reduced degranulation in ATF3-null MCs is probably due to the diminished Akt phosphorylation.
Previous studies have shown roles for nuclear factor–activated T cell, globin transcription factor, Jun/Fos (AP-1), Egr, and members of the signal transducer and activator of transcription family in MC development.4-8 However, the transcriptional program underlying this phenomenon is complicated and incomplete. One of the key transcription factors downstream of Erk and PI3K is c-Fos, which is involved in cell-cycle control. Recent work in MCs from c-Fos–deficient MCs shows a moderate decrease in MC proliferation with decreased cells in the G2 phase. Because members of the AP1 family of TFs have been shown to interact with ATF3 in other systems, further experiments with the use of combinations of TF-deficient knockout animals will help elucidate the spectrum of mechanisms that control MC proliferation.
It is not clear why the lack of Akt activation after FcϵRI stimulation does not result in attenuated cytokine production. We have shown that ATF3 regulates gene transcription by changing the acetylation status of histones.9 It is, therefore, possible that the epigenetic influence of ATF3 masks the Akt effect.
We report here that ATF3, a member of the Creb/ATF family, has a profound role in IL-3–dependent MC development, whereas SCF-mediated development is less impaired. This differential effect is mediated by the Akt pathway.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by grants from the National Institutes of Health (A.A., W.R.H., and K.D.S.).
Authorship
Contribution: M.G., A.M., C.D.J., A.N., F.S., and K.D.S. performed experiments; W.R.H. designed the research; and M.G. and A.A. designed the research, interpreted results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alan Aderem, Institute for Systems Biology, 1441 N 34th St, Seattle, WA 98103; e-mail aaderem@systemsbiology.org.