Abstract
Prolonged inhibition of the kinase, mammalian target of rapamycin (mTOR), during myeloid dendritic cell (DC) generation confers resistance to maturation. Recently, however, mTOR inhibition immediately before Toll-like receptor ligation has been found to exert proinflammatory effects on myeloid cells, notably enhanced IL-12p40/p70 production. We show, for the first time, that mouse or human DCs generated under mTOR inhibition exhibit markedly enhanced IL-12p70 production after lipopolysaccharide (LPS) stimulation, despite impaired costimulatory molecule expression and poor T-cell stimulatory ability. Consistent with this finding, we reveal that increased IL-12p40 production occurs predominantly in CD86lo immature DCs. High IL-12p40/p70 production by CD86lo DC resulted from failed down-regulation of glycogen synthase kinase-3 (GSK-3) activity and could not be ascribed to enhanced Akt function. Despite high IL-12p70 secretion, rapamycin-conditioned, LPS-stimulated DCs remained poor T-cell stimulators, failing to enhance allogeneic Th1 cell responses. We also report that inhibition of GSK-3 impedes the ability of LPS-stimulated DCs to induce forkhead box p3 in CD4+CD25− T cells, as does the absence of IL-12p40/p70. Thus, GSK-3 activity in DC is regulated via signaling linked to mTOR and modulates their capacity both to produce IL-12p40/p70 and induce forkhead box p3 in CD4+ T cells under inflammatory conditions.
Introduction
Mammalian target of rapamycin (mTOR) is an integrative kinase that coordinates environmental signals, especially those activating phosphoinositide 3-kinase (PI3K) and its effector, the Akt kinase.1,2 The relationship between the 2 identified mTOR-containing complexes (mTORC1 and mTORC2) and PI3K/Akt is under intensive investigation, but it is understood that mTORC1 is located downstream of PI3K and activated by Akt.1 Akt, however, lies both upstream and downstream of mTOR and must be phosphorylated on S473 by mTORC2 to be fully activated.1 Although the immunosuppressant rapamycin (RAPA) potently targets mTORC1 activity to limit cell growth and proliferation, mTORC2 is RAPA-resistant, although prolonged RAPA exposure can limit its activity in some cells and tissues.3
Consistent with ubiquitous leukocyte mTOR expression, RAPA exerts significant immunomodulatory effects.4 At clinically relevant concentrations, it inhibits cytokine-induced proliferation of effector T cells while sparing the proliferation and function of regulatory T cells (Treg).4-6 Both in vitro and in vivo, continued exposure to RAPA suppresses myeloid (m) dendritic cell (DC) generation, maturation, and T-cell stimulatory function.7-13 More precisely, propagation of murine bone marrow (BM)–derived mDCs in RAPA (RAPA-conditioned mDCs; RAPA-DCs) generates mDCs with low surface major histocompatibility complex and costimulatory molecules, even after exposure to potent inflammatory stimuli, such as Toll-like receptor (TLR) ligands, and CD40 ligation.8,10-12 Although in vitro-generated RAPA-DCs are weak stimulators of T cells8,10-12 and induce T-cell anergy10 and apoptosis,11 they enrich for Treg.11 Experimentally, RAPA-DCs inhibit graft-versus-host disease (GVHD)13 and promote organ allograft survival without immunosuppressive therapy.10
In apparent discord with these findings, mTOR inhibition has recently been implicated in promotion of proinflammatory cytokine production by myeloid cells. Specifically, short-term (ie, 20-180 minutes) exposure to RAPA immediately before TLR ligation reduces interleukin-10 (IL-10) secretion by these cells while promoting IL-12 production.14-16 Monocytes or mDCs activated in this way are potent inducers of strong T helper type-1 (Th1) and Th17 cell responses.15 Given our previous finding that generation of mDCs in RAPA markedly inhibits their maturation in response to inflammatory stimuli, our initial goal was to elucidate the impact of mTOR inhibition under these conditions, on cytokine production after TLR4 ligation. In addition, we sought to ascertain how disruption of signaling through mTOR and related pathways shapes the capacity of mDCs to induce differentiation of alloreactive CD4+ T cells. Our results show that poorly stimulatory RAPA-DCs, when exposed to bacterial lipopolysaccharide (LPS), paradoxically exhibit enhanced IL-12p40/p70 production, resulting from failure to inhibit glycogen synthase kinase-3 (GSK-3). Notably, increased IL-12p40 was observed predominantly in CD86lo cells, which failed to enhance Th1 cell differentiation. We also reveal that GSK-3 activity and IL-12p40/p70 are crucial for the ability of LPS-stimulated mDCs to induce forkhead box p3 (Foxp3) expression in CD4+ T cells.
Methods
Animals
Male C57BL/6J (B6; H2Kb), B6.129S1-IL-12btm1Jm/J (IL-12p40−/−; H2Kb), and BALB/c (H2Kd) mice were from The Jackson Laboratory. Thy1.1+ 1H3.1 T-cell receptor (TCR) transgenic (tg) mice were kindly provided by Dr Adrian Morelli (University of Pittsburgh).17 Animals were maintained in specific pathogen-free facility at the University of Pittsburgh School of Medicine until use at 8 to 12 weeks of age. Experiments were conducted under an institutional animal care and use committee-approved protocol in accordance with National Institutes of Health guidelines.
DC generation and treatment
Murine mDCs were propagated from BM cells in the presence or absence of RAPA for 7 days in murine recombinant (r) granulocyte-macrophage colony-stimulating factor (GM-CSF) and rIL-4 (R&D Systems), as described.12 The mTORC1/2 inhibitor Torin-1 was graciously provided by Dr Nathanael Gray (Harvard Medical School, Boston, MA) and used identically to RAPA during DC generation.12 Nonadherent cells were harvested on day 7 and CD11c+ DCs positively selected (purity > 90%) using anti-CD11c immunomagnetic beads (Miltenyi Biotec). Human monocyte-derived DCs were generated from peripheral blood mononuclear cells (PBMCs) isolated from normal leukopacks (Central Blood Bank) by Ficoll-Hypaque density gradient centrifugation, then CD14 immunomagnetic bead isolation (Miltenyi Biotec). Human control (CTR)–DCs were differentiated in 10% fetal calf serum AIM-V media (Invitrogen) supplemented with 1000 U/mL rhGM-CSF (Berlex) and 1000 U/mL rhIL-4 (R&D Systems). For RAPA-DCs, RAPA (10 ng/mL; LC Laboratories) was added to cultures on day 2 and day 5 after plating. At the time of harvest, more than 94% of nonadherent cells were phenotypically DCs (CD11c+, HLA-DR+). After harvest on day 7, where indicated, human and mouse DCs were incubated (2 hours) with lithium chloride (LiCl; 10mM; Sigma-Aldrich) or 10-(4′-(N-diethylamino)butyl)-2-chlorophenoxazine, HCl (AktX; 10μM; Calbiochem) before exposure to LPS (0.1 μg/mL; Escherichia coli; Invivogen) or rmIL-33 (0.1μg/mL; PeproTech).
Flow cytometry
DC surface antigen (Ag) expression was analyzed as described.12 Fluorophore-conjugated monoclonal antibodies (mAbs) were from BD Biosciences or eBioscience. For assessment of intracellular IL-12p40, Golgi Plug (BD Biosciences) was added for the final 4 to 5 hours of DC stimulation with LPS, followed by surface staining for CD11c and CD86, treatment with fixation/permeabilization buffer (eBioscience), and intracellular staining with anti–IL-12p40. Murine T cells were examined by surface staining with mAbs to CD4 and CD25 and intracellular staining for Foxp3, as described.11 For intracellular cytokine detection, T cells were assessed after 4- to 5-hour restimulation with phorbol myristate acetate (PMA) and ionomycin (Sigma-Aldrich) in the presence of Golgi Plug. After surface staining and fixation/permeabilization, intracellular staining with anti–IL-4 and –interferon-γ (IFN-γ) was performed. For some experiments, T cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Vibrant CFDA SE Cell Tracer Kit; Invitrogen), according to the manufacturer's instructions. Appropriately conjugated, isotype-matched IgGs served as controls. Data were acquired with a LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo, Version 8.8.3 (TreeStar).
Immunoblots and electrophoretic mobility shift assays
DCs (2-4 × 106) were lysed in CelLytic M (Sigma-Aldrich) containing a complete, ethylenediaminetetraacetic acid-free protease inhibitor cocktail (Mini-Tablets; Roche Diagnostics), supplemented with phenylmethanesulfonyl fluoride (1mM) and sodium orthovanadate (1mM). Debris was removed by centrifugation (13 100g) and 10- to 20-μg protein separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels before membrane transfer. Immunoblotting was performed following the protocol recommended by Cell Signaling Technology with primary Abs from Cell Signaling Technology, including rabbit antiphosphorylated c-Jun NH2-terminal kinase (JNK; T183/Y185), p38 (T180/Y182), mTOR (S2448), mTOR (S2481), p70S6K (T389), Akt (T308), Akt (S473), GSK-3α/β (S21/S9), or GSK-3α/β (Y279/Y216; ECM Biosciences). In addition, primary rabbit Abs to total JNK (Santa Cruz Biotechnology), mTOR, p70S6K, or Akt (Cell Signaling Technology), as well as mAbs to GAPDH (Novus Biologicals) or β-catenin (Sigma-Aldrich) were used. After washing, subsequent incubation was with appropriate horseradish peroxidase-conjugated secondary Abs (Cell Signaling Technology). Band visualization was achieved with SuperSignal West Pico or Femto Chemiluminescent Substrate (Pierce Chemical) and exposure to film. Nuclear factor-κB (NF-κB) DNA-binding activity was measured in nuclear extracts by electrophoretic mobility shift assay, as described.12
MLR
BALB/c CD4+ T cells were obtained from lymph nodes and spleens, as described,11 and mixed leukocyte reaction (MLR) performed8 using graded numbers of γ-irradiated (20 Gy) B6 DCs to stimulate 2 × 105 T cells for 72 hours. For human MLR, responder PBMCs were isolated from heparinized peripheral blood, as described in “DC generation and treatment,” and then incubated (0.1 × 106) in RPMI 1640 containing 5% vol/vol heat-inactivated normal human serum at a 20:1 ratio with irradiated (20 Gy) allogeneic DCs in 96-well, round-bottom plates for 6 days. The number of HLA mismatches between responder and stimulator was constant between experiments. Over the last 18 hours of culture, 1 μCi [3H] thymidine (NEN Life Science Products) was added. After cell harvesting, radioisotope incorporation was determined using a β-scintillation counter.
DC induction of Th1, Th2, Th17, and Treg cells
Normal CD4+ T cells were obtained from BALB/c lymph nodes and spleens as described in “MLR.” In some instances, CD4+CD25− cells were isolated using phycoerythrin-conjugated anti-CD25 mAb and depletion of bound cells via antiphycoerythrin microbeads (Miltenyi Biotec). T cells (2 × 105) were cocultured with 2 × 104 B6 DCs for 3 to 5 days in 96-well, round-bottom plates. Where indicated, murine rIL-12p70 (5 ng/mL; PeproTech) was added at the start of cocultures. CD4+ T cells stimulated by CTR-DCs under Th1- (10 μg/mL anti–IL-4 and 2.5 ng/mL rIL-12p70) or Th2-polarizing conditions (10 μg/mL anti–IFN-γ and 10 ng/mL rIL-4) were included controls. Normal human PBMCs were incubated with irradiated (20 Gy) allogeneic DCs at a ratio of 10:1 for 6 days. Supernatants were then collected for assessment of cytokine concentrations, and cells were harvested for flow cytometric analysis, as described in “Flow cytometry.”
Adoptive 1H3.1 TCR tg CD4+ T-cell transfer and assessment
CD4+ 1H3.1 Tg T cells (CD90.1+Vβ6+) were purified from lymph nodes and spleens of 1H3.1 mice by negative depletion, as described in “MLR” and 5 × 106 cells administered intravenously to naive B6 mice (CD90.2+). Two days later, animals received intravenously 2 × 106 B6 DCs pulsed with the BALB/c I-Eα-derived allopeptide (ASFEAQGALANIAVDKA; IEα52-68). Five days after DC infusion, isolated splenocytes were restimulated (4-5 hours) with PMA/ionomycin in the presence of Golgi Plug. This was followed by surface labeling for CD3, CD4, CD90.1, and Vβ6 TCR, and intracellular staining for IL-4 and IFN-γ, before assessment by flow cytometry for expansion and cytokine expression by the transferred cells.
ELISA and Luminex assays
Murine IL-12p70 (p35/p40) and IL-23 (p19/p40) were quantified using Ready-SET-Go! enzyme-linked immunosorbent assay (ELISA) kits (eBioscience), following the manufacturer's protocol. Human IL-12p70 was also determined by ELISA (R&D Systems). Murine IL-2, -4, -10, -17, and IFN-γ were quantified via Luminex assay (Luminex), conducted by the University of Pittsburgh Cancer Biomarkers Facility.
Statistical analysis
Results are expressed as mean (± SD) or SEM. Significances of differences between means were determined using the Student t test and the JMP IN 4.04 Statistical Package (SAS Institute Inc) with P values less than .05 considered significant.
Results
Differentiation of mDCs in RAPA limits their allostimulatory capacity after exposure to LPS while increasing IL-12p70 production
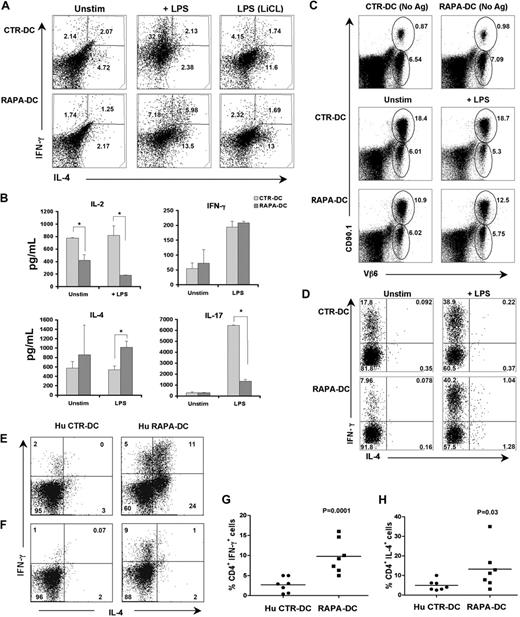
As reported,10-13 murine (B6) BM-derived mDCs differentiated in RAPA (RAPA-DCs) displayed markedly reduced CD86 expression compared with CTR-DCs (data not shown) and were weak stimulators of allogeneic (BALB/c) T cells, even after LPS stimulation (Figure 1A). However, like mDCs or macrophages exposed to RAPA only briefly (20-90 minutes) before TLR ligation,14-16 RAPA-DCs showed enhanced IL-12p40 expression after LPS stimulation (Figure 1B). Thus, after LPS exposure, 27.5% (± 8.8%) of CTR-DCs compared with 57.0% (± 6.0%) of RAPA-DCs were IL-12p40+ (Figure 1C).
Although they display poor CD4+ T-cell allostimulatory capacity, mouse and human RAPA-DCs produce increased IL-12p70 when exposed to LPS. BM-derived mDCs were generated from B6 mice with GM-CSF and IL-4, in the presence (RAPA-DCs) or absence of RAPA (CTR-DCs) and then isolated and purified on day 7 of culture. These DCs were incubated for 18 hours in media alone (Unstim) or with 0.1 μg/mL LPS. (A) RAPA-DCs, compared with similarly stimulated CTR-DCs, showed inferior capacity to induce proliferation of alloreactive CD4+ BALB/c T cells, even after exposure to LPS. The data are mean ± SD from sample replicates of 1 experiment representative of more than 4 performed. (B) Intracellular staining for IL-12p40 revealed that DC generation in RAPA promoted an increase in IL-12p40 expression after exposure to LPS. Numbers on dot plots indicate percentage of CD11c+ cells. (C) Comparison of the percentages (mean ± SD) of IL-12p40+CD11c+ cells from 2 experiments representative of more than 6 performed. Enzyme-linked immunosorbent assay (D-E) or Luminex (F) analysis of culture supernatants revealed significantly increased secretion of IL-12p70 by LPS-stimulated RAPA-DCs, whereas the release of IL-23 (E) and IL-10 (F) was reduced. (A-F) *P < .05. The data shown are representative of more than 3 experiments performed. (G-H) Hu DCs were generated from CD14+ monocytes in the presence (Hu RAPA-DCs) or absence (Hu CTR-DCs) of RAPA. (G) Like their murine counterparts, unstimulated Hu RAPA-DCs and those stimulated for 18 hours with LPS showed a reduced capacity to stimulate allogeneic T cells in MLR. [3H]-thymidine (TdR) incorporation for each condition was normalized to that obtained with unstimulated Hu CTR-DCs for each experiment. Data represent mean percentage ± SD across multiple experiments (Unstim, n = 10; LPS, n = 4). *P < .05. (H) Hu RAPA-DCs also displayed significantly increased IL-12p70 secretion after exposure to LPS (0.1 μg/mL) Data are mean ± SD from multiple experiments (Unstim, n = 9; LPS, n = 5). *P < .05.
Although they display poor CD4+ T-cell allostimulatory capacity, mouse and human RAPA-DCs produce increased IL-12p70 when exposed to LPS. BM-derived mDCs were generated from B6 mice with GM-CSF and IL-4, in the presence (RAPA-DCs) or absence of RAPA (CTR-DCs) and then isolated and purified on day 7 of culture. These DCs were incubated for 18 hours in media alone (Unstim) or with 0.1 μg/mL LPS. (A) RAPA-DCs, compared with similarly stimulated CTR-DCs, showed inferior capacity to induce proliferation of alloreactive CD4+ BALB/c T cells, even after exposure to LPS. The data are mean ± SD from sample replicates of 1 experiment representative of more than 4 performed. (B) Intracellular staining for IL-12p40 revealed that DC generation in RAPA promoted an increase in IL-12p40 expression after exposure to LPS. Numbers on dot plots indicate percentage of CD11c+ cells. (C) Comparison of the percentages (mean ± SD) of IL-12p40+CD11c+ cells from 2 experiments representative of more than 6 performed. Enzyme-linked immunosorbent assay (D-E) or Luminex (F) analysis of culture supernatants revealed significantly increased secretion of IL-12p70 by LPS-stimulated RAPA-DCs, whereas the release of IL-23 (E) and IL-10 (F) was reduced. (A-F) *P < .05. The data shown are representative of more than 3 experiments performed. (G-H) Hu DCs were generated from CD14+ monocytes in the presence (Hu RAPA-DCs) or absence (Hu CTR-DCs) of RAPA. (G) Like their murine counterparts, unstimulated Hu RAPA-DCs and those stimulated for 18 hours with LPS showed a reduced capacity to stimulate allogeneic T cells in MLR. [3H]-thymidine (TdR) incorporation for each condition was normalized to that obtained with unstimulated Hu CTR-DCs for each experiment. Data represent mean percentage ± SD across multiple experiments (Unstim, n = 10; LPS, n = 4). *P < .05. (H) Hu RAPA-DCs also displayed significantly increased IL-12p70 secretion after exposure to LPS (0.1 μg/mL) Data are mean ± SD from multiple experiments (Unstim, n = 9; LPS, n = 5). *P < .05.
Because IL-12p40 is a shared component of IL-12p70 and IL-23, supernatants were harvested after exposure of DCs to LPS and IL-12p70 and IL-23 quantified. IL-12p70, but not IL-23, secretion by LPS-stimulated RAPA-DCs was increased (Figure 1D-E). As reported for short-term inhibition of mTOR in mDCs and macrophages,14-16 IL-10 secretion by LPS-stimulated RAPA-DCs was reduced significantly (Figure 1F). Human (Hu) monocyte-derived DCs generated in RAPA (Hu RAPA-DCs) also displayed both impaired ability to stimulate alloreactive T cells (especially after exposure to LPS; Figure 1G) and increased LPS-induced IL-12p70 secretion (Figure 1H).
Differentiation of mDCs in RAPA inhibits TLR-induced activation of MAPK and NF-κB
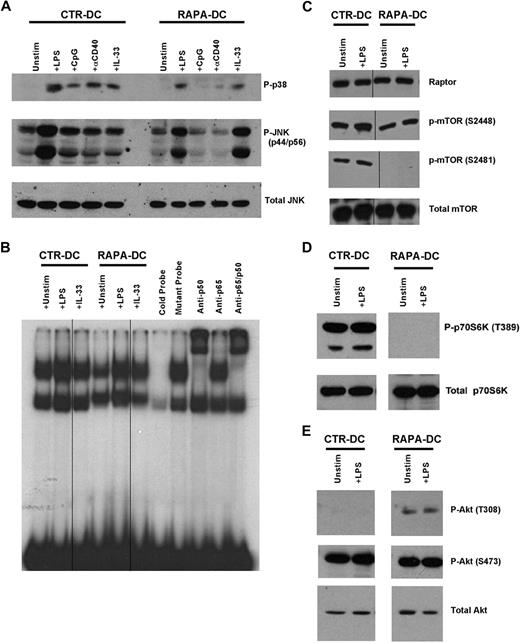
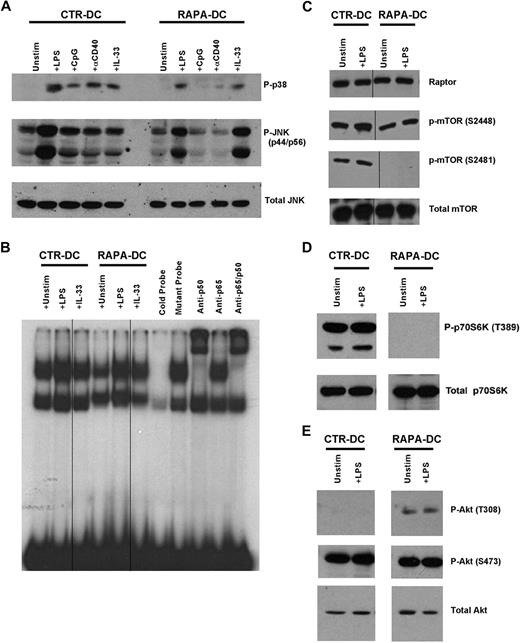
We have shown that RAPA-DCs overexpress the IL-1 receptor (R) family member, ST2L,12 which acts both as the IL-33R18 and as a negative regulator of TLR4 that sequesters MyD88.19 Consistent with this, RAPA-DCs displayed inhibited activation of NF-κB, p38, and ERK after exposure to LPS, CpG, or CD40 ligation.12 Western blot analysis of RAPA-DCs demonstrated that, although activation of JNK and p38 downstream of TLR4, TLR9, and CD40 was also strongly inhibited, IL-33–induced signaling through ST2L was not (Figure 2A). In the case of IL-33–induced JNK activation, RAPA-DCs displayed enhanced phosphorylation (Figure 2A). Analysis of nuclear proteins revealed reduced NF-κB DNA-binding activity in unstimulated and LPS-stimulated RAPA-DCs (Figure 2B). IL-33 failed to activate NF-κB in CTR-DCs, whereas in RAPA-DCs IL-33 promoted NF-κB activation (Figure 2B). Thus, augmented NF-κB or mitogen-activated protein kinase (MAPK) activity does not appear to underlie enhanced IL-12p70 production by RAPA-DCs, which display reduced responsiveness to TLR4 but not to ST2L ligation.
Modulation of MAPK, NF-κB, mTOR, and Akt signaling in DCs generated in RAPA. Murine CTR- and RAPA-DCs were generated, positively selected using immunomagnetic beads, and cytoplasmic and nuclear proteins isolated after 20 minutes of exposure to 0.1 μg/mL LPS, 1 μg/mL CpG, 5 μg/mL agonistic CD40 mAb, or 0.1 μg/mL IL-33 for for 20 minutes. (A) Western blot analysis revealed that generation of DCs in RAPA limited activation of p38 and JNK after ligation of TLR4, TLR9, and CD40 (IL-33 receptor), but not ST2L. (B) Nuclear extracts were assessed for DNA-binding activity in electrophoretic mobility shift assay using 32P-labeled NF-κB probes. The data show inhibited NF-κB activation downstream of TLR4 in RAPA-DCs. Vertical lines indicate repositioned gel lanes within the same gel and experiment. (C-D) Generation of DCs in RAPA did not modulate expression of the mTOR kinase or the mTORC1 component Raptor. In addition, generation of mDCs in RAPA blocked mTOR autophosphorylation on S2481 and p70S6K phosphorylation on T389. Vertical lines indicate repositioned gel lanes within the same gel and experiment. (E) Phosphorylation of Akt on T308 was increased in RAPA-DCs and the mTORC2-mediated phosphorylation of Akt on S473 was maintained. Data are representative of at least 2 independent experiments.
Modulation of MAPK, NF-κB, mTOR, and Akt signaling in DCs generated in RAPA. Murine CTR- and RAPA-DCs were generated, positively selected using immunomagnetic beads, and cytoplasmic and nuclear proteins isolated after 20 minutes of exposure to 0.1 μg/mL LPS, 1 μg/mL CpG, 5 μg/mL agonistic CD40 mAb, or 0.1 μg/mL IL-33 for for 20 minutes. (A) Western blot analysis revealed that generation of DCs in RAPA limited activation of p38 and JNK after ligation of TLR4, TLR9, and CD40 (IL-33 receptor), but not ST2L. (B) Nuclear extracts were assessed for DNA-binding activity in electrophoretic mobility shift assay using 32P-labeled NF-κB probes. The data show inhibited NF-κB activation downstream of TLR4 in RAPA-DCs. Vertical lines indicate repositioned gel lanes within the same gel and experiment. (C-D) Generation of DCs in RAPA did not modulate expression of the mTOR kinase or the mTORC1 component Raptor. In addition, generation of mDCs in RAPA blocked mTOR autophosphorylation on S2481 and p70S6K phosphorylation on T389. Vertical lines indicate repositioned gel lanes within the same gel and experiment. (E) Phosphorylation of Akt on T308 was increased in RAPA-DCs and the mTORC2-mediated phosphorylation of Akt on S473 was maintained. Data are representative of at least 2 independent experiments.
RAPA-DCs display inhibited mTOR and p70S6K phosphorylation
Within a few minutes of exposure to LPS or Listeria monocytogenes, mTOR is phosphorylated in myeloid cells, as is the mTORC1 target, p70S6K.14,15 Accordingly, phosphorylation of S2448 on mTOR was increased after exposure of CTR-DCs to LPS (Figure 2C), as was also observed in RAPA-DCs, although to a lesser extent (Figure 2C). mTOR can also be phosphorylated on S2481, a proposed site of autophosphorylation.20 Whereas CTR-DCs displayed constitutive S2481 phosphorylation (Figure 2C), generation of mDCs in RAPA ablated S2481 phosphorylation, both before and after LPS stimulation (Figure 2C). RAPA-DCs did not display phosphorylation of p70S6K, even after LPS exposure (Figure 2D), consistent with inhibition of mTORC1 by RAPA. Expression of the mTOR kinase, or regulatory associated protein of mTOR (raptor), a defining binding partner with mTOR in mTORC1,21 was unmodified in RAPA-DCs (Figure 2C). Thus, generation of mDCs in RAPA results in the loss of mTOR S2481 and p70S6K phosphorylation after TLR4 ligation.
Akt phosphorylation is maintained in RAPA-DCs
TLR ligation stimulates PI3K22 activity needed for negative regulation of IL-12 production.23 Akt is a critical kinase in the PI3K pathway and is phosphorylated on T308 after PI3K activation.24 However, to be fully active, Akt must also be phosphorylated on S473 by mTORC2,25 a step susceptible to long-term mTOR inhibition.3 TLR4 ligation promotes phosphorylation of S473,14 although whether mDC generation in RAPA prevents Akt phosphorylation on S473, potentially removing Akt negative regulation of IL-12 production, is unknown. As shown in Figure 2E, generation of mDCs in RAPA did not ablate phosphorylation of S473 on Akt, as has been observed in some cell lines and mouse tissues.3 Interestingly, whereas CTR-DCs lacked Akt phosphorylation on T308 at the time of assessment, Akt in RAPA-DCs displayed constitutive T308 phosphorylation (Figure 2E). These data suggest that generation of mDCs in RAPA does not result in failure to phosphorylate the requisite sites for Akt activation.
Failure to phosphorylate inhibitory serines on GSK-3 promotes IL-12 production by RAPA-DCs
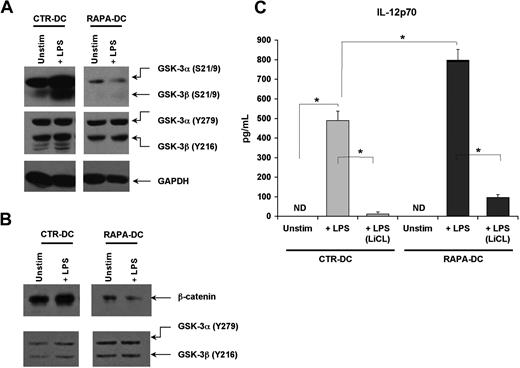
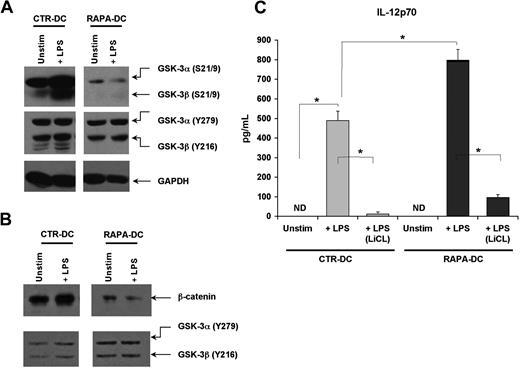
GSK-3, like mTOR, is an integrator of activating and inhibitory signals in DCs26 and is involved in regulating cytokine secretion.27 The activity of the 2 GSK-3 isoforms (α and β) is inhibited by phosphorylation of inhibitory N-terminal serines (S21 for GSK-3α; S9 for GSK-3β), and facilitated through tyrosine phosphorylation of Y279 in GSK-3α or Y216 in GSK-3β.26 Thus, the activity of GSK-3α/β is determined by their collective phosphorylation. The aggregate phosphorylation of GSK-3α/β before and after TLR4 ligation was assessed in CTR- and RAPA-DCs. Compared with CTR-DCs, and especially after LPS exposure, RAPA-DCs displayed very little phosphorylation of S21/9 but comparatively intact phosphorylation of Y279/216 (Figure 3A). GSK-3 activity inversely correlates with the level of nuclear β-catenin, as active GSK-3 phosphorylates and directs β-catenin degradation.28 Compared with CTR-DCs, RAPA-DCs exhibited little nuclear β-catenin, especially after exposure to LPS (Figure 3B). Also evident in RAPA-DCs was an increase in nuclear GSK-3α/β (Figure 3B), which is associated with negative regulation of β-catenin signaling.29 Thus, generation of mDCs in RAPA results in a profound failure to phosphorylate GSK-3α/β on inhibitory S9/21 after TLR4 ligation.
Profound failure to inhibit GSK-3 in RAPA-DC promotes increased IL-12p70 secretion after LPS stimulation. The activity of GSK-3α/β in murine CTR- and RAPA-DCs, determined by the extent of phosphorylation of inhibitory N-terminal serines (S21 for GSK-3α; S9 for GSK-3β) versus facilitatory tyrosines (Y279 in GSK-3α; Y216 in GSK-3β), was assessed by Western blot. (A) CTR-DCs demonstrated a profound increase in the inhibitory phosphorylation of S21/9 after TLR4 ligation. However, RAPA-DCs, either unstimulated or especially after 20 minutes of exposure to LPS, exhibited very little phosphorylation of S21/9 but comparable phosphorylation of the activating Y279/216. (B) CTR-DCs displayed increased β-catenin and little GSK in the nucleus compared with RAPA-DCs. (C) Treatment of murine RAPA-DCs with the GSK-3 inhibitor LiCl (10mM) before LPS stimulation profoundly inhibited IL-12p70 secretion after TLR4 ligation in both CTR- and RAPA-DCs (mean ± SD). *P < .05. The data shown are representative of more than 3 experiments performed.
Profound failure to inhibit GSK-3 in RAPA-DC promotes increased IL-12p70 secretion after LPS stimulation. The activity of GSK-3α/β in murine CTR- and RAPA-DCs, determined by the extent of phosphorylation of inhibitory N-terminal serines (S21 for GSK-3α; S9 for GSK-3β) versus facilitatory tyrosines (Y279 in GSK-3α; Y216 in GSK-3β), was assessed by Western blot. (A) CTR-DCs demonstrated a profound increase in the inhibitory phosphorylation of S21/9 after TLR4 ligation. However, RAPA-DCs, either unstimulated or especially after 20 minutes of exposure to LPS, exhibited very little phosphorylation of S21/9 but comparable phosphorylation of the activating Y279/216. (B) CTR-DCs displayed increased β-catenin and little GSK in the nucleus compared with RAPA-DCs. (C) Treatment of murine RAPA-DCs with the GSK-3 inhibitor LiCl (10mM) before LPS stimulation profoundly inhibited IL-12p70 secretion after TLR4 ligation in both CTR- and RAPA-DCs (mean ± SD). *P < .05. The data shown are representative of more than 3 experiments performed.
To assess whether GSK-3 activity was responsible for the significant increase in IL-12p70 production by LPS-stimulated RAPA-DCs, DCs were treated with the GSK-3 inhibitor LiCl before LPS exposure. In support of a lack of GSK-3 down-regulation driving increased IL-12p70 secretion by LPS-treated murine RAPA-DCs, exposure to LiCl markedly suppressed their secretion of IL-12p70 after LPS stimulation (Figure 3C). LiCl pretreatment of Hu RAPA-DCs also significantly reduced LPS-stimulated IL-12p70 secretion (RAPA-DCs + LPS: 777.4 ± 471.7 ng/mL vs RAPA-DC + LPS [LiCL]: 234.8 ± 76.1 ng/mL; P < .05).
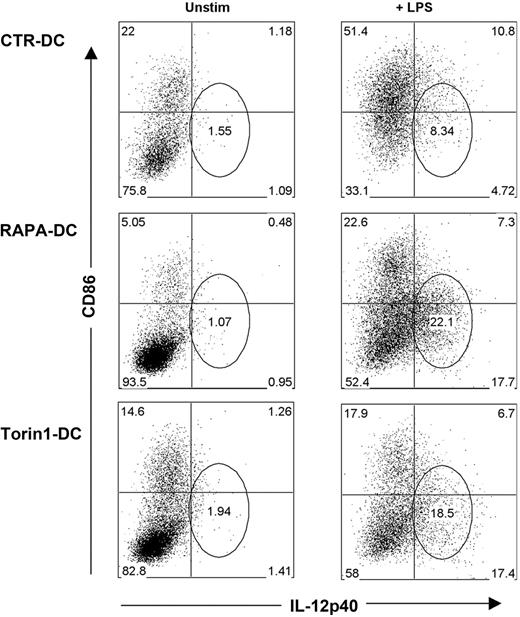
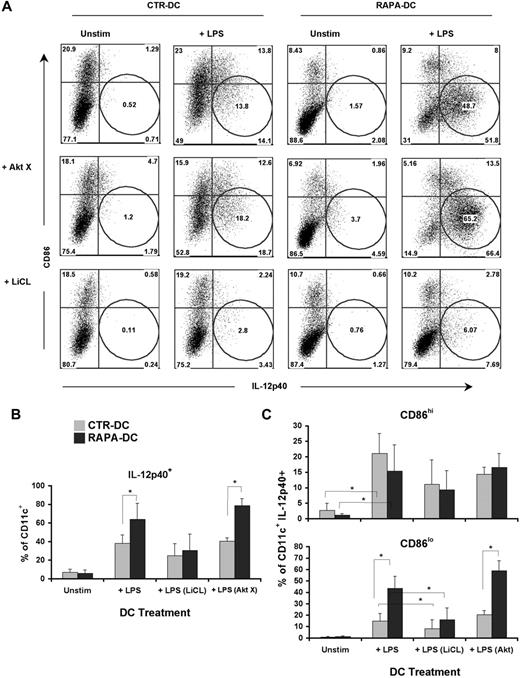
GSK-3 activity promotes overexpression of IL-12 in CD86lo RAPA-DCs on LPS stimulation
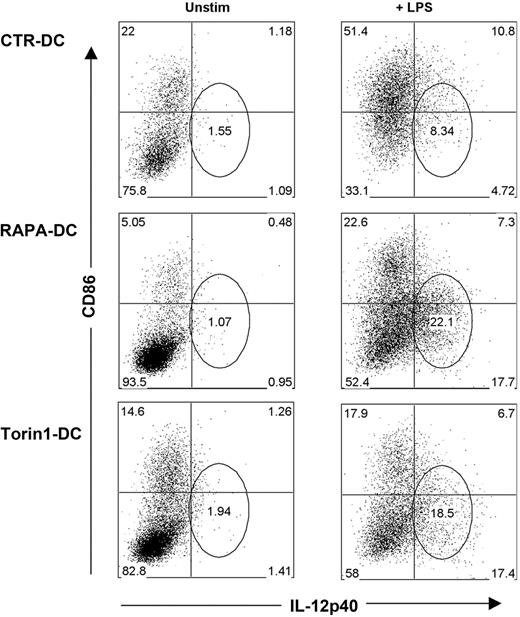
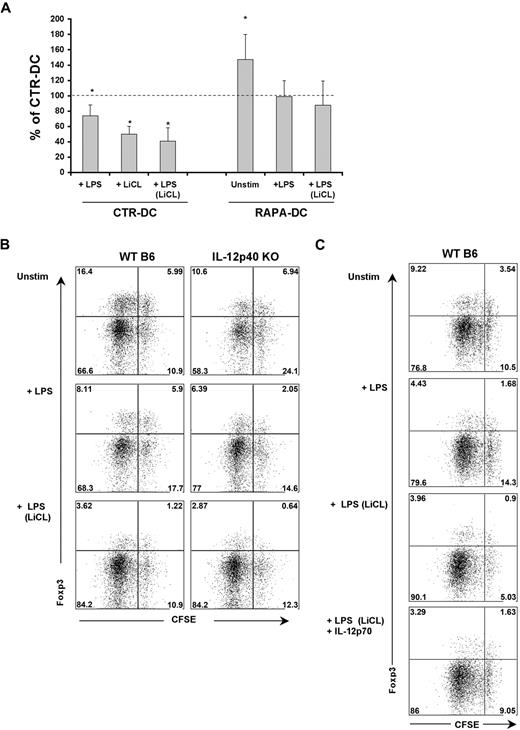
The foregoing findings constitute an apparent paradox, whereby exposure of RAPA-DCs to LPS culminates in weakly stimulatory Ag-presenting cells (APCs), producing high levels of IL-12p40/p70. A defining characteristic of murine RAPA-DCs is a profound reduction in CD86 expression that is maintained on exposure to IL-4,8 CpG,12 LPS,8,10,12 or CD40 ligation.11 Thus, we assessed the relationship between CD86 and IL-12p40 expression by CTR-DCs and RAPA-DCs. After LPS stimulation, the majority of CTR-DCs (> 60%) were CD86hi, whereas RAPA-DCs remained predominantly low expressors of CD86 (∼ 70% CD86lo; Figure 4). Interestingly, a prominent population of CD86lo/IL-12p40hi RAPA-DC emerged after LPS stimulation (Figure 4). This population was further examined by generating mDCs in the presence of a novel adenosine triphosphate-competitive mTOR inhibitor, Torin1, that unlike RAPA, targets both mTORC1 and mTORC2.30 There was little difference between RAPA-DCs and those generated in Torin1 (Torin1-DC; Figure 4). Consistent with our finding (Figure 4) that failure to inhibit GSK-3 activity after LPS stimulation increased IL-12p40 production by RAPA-DCs, as shown in Figure 5, inhibition of GSK-3 with LiCl, but not treatment with AktX, suppressed IL-12p40 expression in the prominent CD86lo RAPA-DC subset. These data establish, for the first time, that mTOR-dependent down-regulation of GSK-3 activity is required after LPS stimulation of mDCs to limit IL-12 production in CD86lo cells. As Torin1 did not modulate the level of IL-12p40 mDCs differently from RAPA, it can be concluded that increased IL-12 is the result of inhibited mTOR activity, not exaggerated mTORC2 activity.
Generation of DCs under conditions of mTORC1 and mTORC2 inhibition results in DCs that remain CD86lo but express IL-12 on LPS stimulation. RAPA- and CTR-DCs were generated as described in “DC generation and treatment.” BM-derived Torin1-DCs were generated from B6 mice with GM-CSF and IL-4 in the presence 50nM Torin1, a dual inhibitor of mTORC1 and mTORC2. After purification on day 7 of culture, these DCs were incubated for 18 hours in media alone (Unstim) or with 0.1 μg/mL LPS. Intracellular staining for IL-12p40, combined with surface staining for CD86, reveals that DCs generated under mTORC1 and mTORC2 inhibition have increased IL-12p40 production in CD86loCD11c+ cells. Numbers on dot plots indicate percentage CD11c+ cells, and data are representative of 3 experiments performed.
Generation of DCs under conditions of mTORC1 and mTORC2 inhibition results in DCs that remain CD86lo but express IL-12 on LPS stimulation. RAPA- and CTR-DCs were generated as described in “DC generation and treatment.” BM-derived Torin1-DCs were generated from B6 mice with GM-CSF and IL-4 in the presence 50nM Torin1, a dual inhibitor of mTORC1 and mTORC2. After purification on day 7 of culture, these DCs were incubated for 18 hours in media alone (Unstim) or with 0.1 μg/mL LPS. Intracellular staining for IL-12p40, combined with surface staining for CD86, reveals that DCs generated under mTORC1 and mTORC2 inhibition have increased IL-12p40 production in CD86loCD11c+ cells. Numbers on dot plots indicate percentage CD11c+ cells, and data are representative of 3 experiments performed.
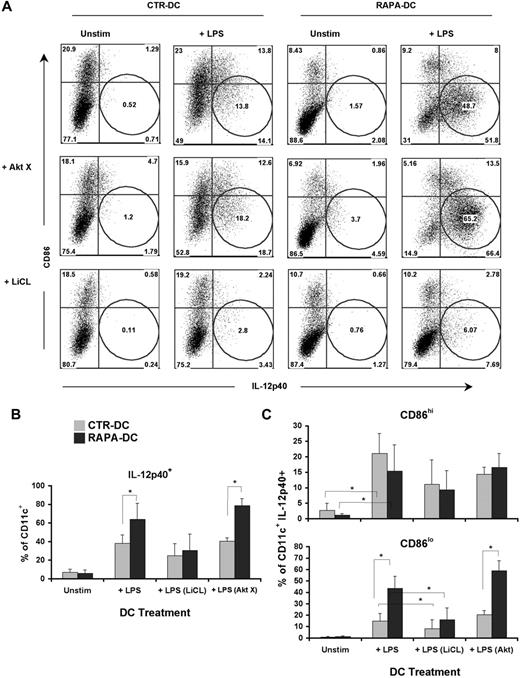
GSK-3 inhibition after TLR4 ligation regulates expression of IL-12 in CD86lo DCs. Murine CTR- and RAPA-DCs were treated for 2 hours with an Akt (AktX; 5μM) or GSK-3 (LiCl; 10mM) inhibitor before 18-hour exposure to 0.1 μg/mL LPS. (A) Staining for CD86 and IL-12 confirmed that increased IL-12 could be ascribed to a population of CD86lo RAPA-DCs. These analyses also demonstrated that inhibition of GSK-3, but not AKT, limited IL-12p40 expression in CD86lo DCs, especially RAPA-DCs. (B-C) Comparison of the percentages (mean ± SD) of IL-12p40+CD11c+ cells (B) between groups demonstrated a significant decrease in LiCl-treated CTR and RAPA-DCs. (C) However, on more detailed analysis, a significant difference between RAPA- and CTR-DCs was only observed in CD86lo, not CD86hi, mDCs. CD86lo DCs were the only subset that displayed a significant decrease in IL-12p40 after inhibition of GSK (n = 2-4). *P < .05.
GSK-3 inhibition after TLR4 ligation regulates expression of IL-12 in CD86lo DCs. Murine CTR- and RAPA-DCs were treated for 2 hours with an Akt (AktX; 5μM) or GSK-3 (LiCl; 10mM) inhibitor before 18-hour exposure to 0.1 μg/mL LPS. (A) Staining for CD86 and IL-12 confirmed that increased IL-12 could be ascribed to a population of CD86lo RAPA-DCs. These analyses also demonstrated that inhibition of GSK-3, but not AKT, limited IL-12p40 expression in CD86lo DCs, especially RAPA-DCs. (B-C) Comparison of the percentages (mean ± SD) of IL-12p40+CD11c+ cells (B) between groups demonstrated a significant decrease in LiCl-treated CTR and RAPA-DCs. (C) However, on more detailed analysis, a significant difference between RAPA- and CTR-DCs was only observed in CD86lo, not CD86hi, mDCs. CD86lo DCs were the only subset that displayed a significant decrease in IL-12p40 after inhibition of GSK (n = 2-4). *P < .05.
Despite increased IL-12 production, murine RAPA-DCs do not increase Th1 polarization
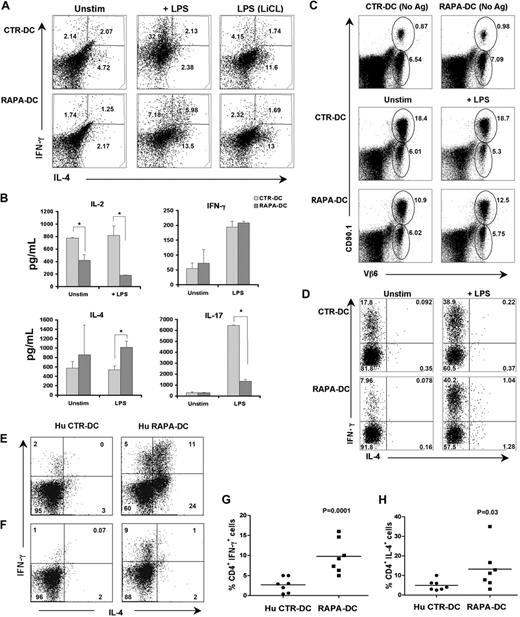
In addition to increasing IL-12 secretion, short-term (≤ 3 hours) inhibition of mTOR in APCs before their exposure to bacteria or LPS increases CD86 expression and their capacity to promote Th1 and Th17 responses.14,15 Given these reports and our finding that mDCs generated in RAPA produce increased IL-12p40/p70 but remain CD86lo and weak allostimulators after LPS stimulation, we examined the potential of RAPA-DCs to polarize Th cells. When used to activate freshly isolated allogeneic CD4+ T cells, LPS-stimulated RAPA-DCs, although exhibiting enhanced IL-12p40/p70 production, did not increase the incidence of IFN-γ+ cells (Figure 6A) or the level of secreted IFN-γ (Figure 6B) above that promoted by LPS-treated CTR-DCs. However, CD4+ T cells stimulated with LPS-treated RAPA-DCs displayed increased production of the Th2-type cytokine, IL-4 (Figure 6A-B). CD4+ T cells stimulated by CTR-DCs under Th1- or Th2-polarizing conditions exhibited a signature cytokine secretion profile for Th1- or Th2-polarized cells, respectively (n = 2; mean concentration ± SEM in ng/mL: IL-4: 0.0 Th1 vs 3889.8 ± 179.3 Th2; IL-5: 167.3 ± 103.2 Th1 vs 1311.9 ± 220.4 Th2; IL-10: 77.6 ± 109.8 Th1 vs 4063.0 ± 466.0 Th2; IL-13: 455.7 ± 349.3 vs 35 191.9 ± 8786.0 Th2; IFN-γ: 274.9 ± 213 vs 0.0 Th2). Consistent with their poor allostimulatory capacity, RAPA-DCs induced less IL-2 secretion than CTR-DCs by responder T cells (Figure 6B). LPS-stimulated RAPA-DCs also induced significantly less IL-17 production compared with LPS-exposed CTR-DCs (Figure 6B). LiCl treatment of CTR-DCs before LPS stimulation limited CD4+ T-cell IFN-γ production and increased IL-4 production, whereas LiCl treatment of RAPA-DCs exerted little impact on T-cell cytokine production. Unique to RAPA-DCs stimulated with LPS was an increase in the incidence of IL-4+/IFN-γ+CD4+ cells (Th0).31 The incidence of Th0 cells detected in LPS-stimulated CTR-DC cultures did not differ from that in unstimulated CTR-DC MLR (Figure 6A).
Murine and human LPS-stimulated RAPA-DCs promote increased IFN-γ production associated with increased IL-4 in alloreactive CD4+ T cells. Murine CTR- and RAPA-DCs (B6; H-2b), treated as indicated, were used to stimulate normal BALB/c CD4+ T cells (H-2d) for 5 days. (A) After T-cell restimulation with PMA and ionomycin in the presence of Golgi plug, intracellular IL-4 and IFN-γ were determined by flow cytometry. Compared with LPS-stimulated CTR-DCs, which greatly increased the incidence of IFN-γ+CD4+ cells, LPS-stimulated RAPA-DCs promoted less IFN-γ expression and increased the incidence of IL-4+ CD4+ T cells. Data are representative of 4 independent experiments. Numbers are percentage CD4+ cells. (B) Cytokines secreted during BALB/c CD4+ T-cell stimulation were assessed by Luminex on day 5 culture supernatants. The mean ± SEM from 2 independent experiments are shown. *P < .05. (C) In vivo comparison of the T-cell stimulatory and early skewing capacity of allopeptide-presenting CTR- and RAPA-DCs indicated that LPS-exposed RAPA-DCs (C) had reduced stimulatory capacity and (D) did not increase IFN-γ+ in allospecific CD4+ T cells. (D) Assessment of adoptively transferred CD4+CD90.1+Vβ6+ 1H3.1 T cells, specific for the IEα-derived allopeptide IEα52-68 in I-Ab, 5 days after intravenous injection of the indicated DCs presenting BALB/c allopeptides, revealed that RAPA-DCs, even when LPS-treated, induced less expansion than CTR-DCs (gated: CD3+CD4+). (D) Further examination of 1H1.3 cells (gated: CD90.1+Vβ6+) revealed that LPS-stimulated RAPA-DCs did not increase the incidence of IL-4+ cells over that induced by CTR-DCs. (E-F) Hu monocyte-derived CTR- and RAPA-DCs generated from CD14+ monocytes were stimulated for 24 hours with LPS (0.1 μg/mL). These DCs were incubated with allogeneic PBMCs (1:10 ratio) for 6 days, followed by 4 hours of restimulation with PMA and ionomycin in the presence of Golgi plug. (E-F) Two representative profiles showing IFN-γ and IL-4 production by alloreactive CD4+ T cells from 2 different individual responders to the same set of LPS-exposed CTR- and RAPA-DCs. (G-H) Overall IFN-γ (P < .001) and IL-4 (P = .03) production by alloreactive CD4+ T cells incubated with LPS-stimulated Hu CTR-DCs or RAPA-DCs. Data are from 7 independent experiments.
Murine and human LPS-stimulated RAPA-DCs promote increased IFN-γ production associated with increased IL-4 in alloreactive CD4+ T cells. Murine CTR- and RAPA-DCs (B6; H-2b), treated as indicated, were used to stimulate normal BALB/c CD4+ T cells (H-2d) for 5 days. (A) After T-cell restimulation with PMA and ionomycin in the presence of Golgi plug, intracellular IL-4 and IFN-γ were determined by flow cytometry. Compared with LPS-stimulated CTR-DCs, which greatly increased the incidence of IFN-γ+CD4+ cells, LPS-stimulated RAPA-DCs promoted less IFN-γ expression and increased the incidence of IL-4+ CD4+ T cells. Data are representative of 4 independent experiments. Numbers are percentage CD4+ cells. (B) Cytokines secreted during BALB/c CD4+ T-cell stimulation were assessed by Luminex on day 5 culture supernatants. The mean ± SEM from 2 independent experiments are shown. *P < .05. (C) In vivo comparison of the T-cell stimulatory and early skewing capacity of allopeptide-presenting CTR- and RAPA-DCs indicated that LPS-exposed RAPA-DCs (C) had reduced stimulatory capacity and (D) did not increase IFN-γ+ in allospecific CD4+ T cells. (D) Assessment of adoptively transferred CD4+CD90.1+Vβ6+ 1H3.1 T cells, specific for the IEα-derived allopeptide IEα52-68 in I-Ab, 5 days after intravenous injection of the indicated DCs presenting BALB/c allopeptides, revealed that RAPA-DCs, even when LPS-treated, induced less expansion than CTR-DCs (gated: CD3+CD4+). (D) Further examination of 1H1.3 cells (gated: CD90.1+Vβ6+) revealed that LPS-stimulated RAPA-DCs did not increase the incidence of IL-4+ cells over that induced by CTR-DCs. (E-F) Hu monocyte-derived CTR- and RAPA-DCs generated from CD14+ monocytes were stimulated for 24 hours with LPS (0.1 μg/mL). These DCs were incubated with allogeneic PBMCs (1:10 ratio) for 6 days, followed by 4 hours of restimulation with PMA and ionomycin in the presence of Golgi plug. (E-F) Two representative profiles showing IFN-γ and IL-4 production by alloreactive CD4+ T cells from 2 different individual responders to the same set of LPS-exposed CTR- and RAPA-DCs. (G-H) Overall IFN-γ (P < .001) and IL-4 (P = .03) production by alloreactive CD4+ T cells incubated with LPS-stimulated Hu CTR-DCs or RAPA-DCs. Data are from 7 independent experiments.
To gain insight into how RAPA-DCs differ from CTR-DCs in their in vivo CD4+ T-cell stimulating/polarizing capacity, we assessed the expansion and cytokine expression of adoptively transferred TCR tg 1H3.1 cells, which recognize a BALB/c allopeptide IEα52-68 on I-Ab.17 1H3.1 cells were transferred into B6 mice, which were infused 2 days later with syngeneic IEα52-68-pulsed CTR- or RAPA-DCs (either unstimulated or LPS-stimulated). When assessed 5 days thereafter, 1H1.3 cell expansion was much lower in animals given RAPA-DCs or LPS-stimulated RAPA-DCs, compared with their CTR-DC counterparts (Figure 6C). Assessment of 1H3.1 cytokine expression revealed that, in vivo, as in vitro, LPS-stimulated RAPA-DCs did not differ in the extent of IFN-γ expression induced compared with LPS-treated CTR-DCs (Figure 6D). Again, compared with CTR-DCs, RAPA-DCs increased IL-4 expression and the presence of Th0 1H3.1 cells in vivo (Figure 6D). Overall, in vivo administration of LPS-stimulated RAPA-DCs resulted in a similar immunomodulatory profile to that demonstrated in vitro, with less activation/proliferation of alloreactive T cells, and even though expressing high levels of IL-12p70, failure to increase the incidence of CD4+IFN-γ+ cells above that induced by LPS-stimulated CTR-DCs. Furthermore, IL-4+ and dual IL-4+/IFN-γ+ cells, which were absent under CTR-DC conditions, were observed in LPS-stimulated RAPA-DC–injected animals.
Human RAPA-DCs increase IFN-γ+ alloreactive CD4+ T cells and further skew their polarization toward Th1/Th2 (IFN-γ+/IL-4+) cells
The ability of LPS-stimulated human RAPA- and CTR-DCs to promote polarization of alloreactive CD4+ cells was also compared. LPS-stimulated monocyte-derived RAPA-DCs generated from 6 of 7 healthy subjects induced significantly higher incidences of CD4+IFN-γ+ alloreactive T cells (10% ± 6%) compared with those from the same persons generated in the absence of RAPA (Hu CTR-DCs; 3% ± 2%; P < .001; Figure 6F-G) Interestingly, RAPA-DCs generated from 4 of the 7 persons further promoted alloreactive CD4+ T cells to become IL-4+ (15% ± 10% for LPS-stimulated RAPA-DCs vs 4% ± 3% for LPS-stimulated CTR-DC; P = .03; Figure 6E,H). In RAPA-DC cultures, IL-4+/IFN-γ+CD4+ cells were also observed, in 3 of 7 persons used to generate the RAPA-DCs (Figure 6E). Overall, human RAPA-DCs, like their murine counterparts, displayed poor allostimulatory capacity, despite a high potential to secrete IL-12p70 on LPS stimulation. Distinct from murine RAPA-DCs, however, human LPS-stimulated RAPA-DCs displayed increased Th1-polarizing ability for alloreactive CD4+ T cells. Nevertheless, they also demonstrated the ability to promote CD4+ T-cell responses characterized by increased IL-4 and/or IL-4/IFN-γ polarization, similar to that observed for murine RAPA-DCs.
IL-12 and active GSK-3 promote the induction of Foxp3+ in CD4+CD25− T cells
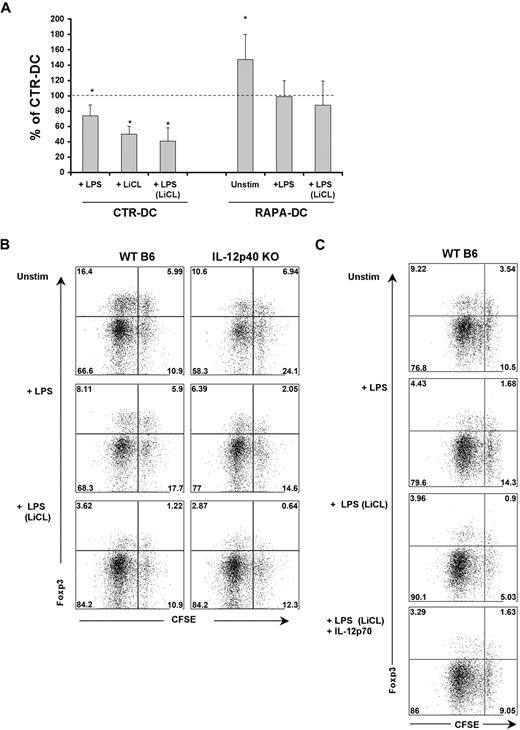
Compared with CTR-DCs, unstimulated murine RAPA-DCs enhance the incidence of Foxp3+ Treg in CD4+ T-cell populations, in vitro11 and in vivo.4 However, whether LPS stimulation influences the capacity of RAPA-DCs to modulate Treg in allogeneic CD4+ T-cell populations has not been defined. Consistent with past reports,11 bulk CD4+ BALB/c T cells cultured with unstimulated RAPA-DCs in MLR displayed an increased incidence of Foxp3+ cells (Figure 7A). However, a significant increase was not maintained when RAPA-DCs were exposed to LPS and compared with LPS-stimulated CTR-DCs (Figure 7A; P =.078). Thus, LPS exposure reduces the capacity of both RAPA-DCs and CTR-DCs to maintain Foxp3+ Treg in total CD4+ T cells (Figure 7A).
Active GSK-3 and IL-12 are necessary for Treg induction by LPS-stimulated DCs. (A) Murine (B6) CTR- and RAPA-DCs were generated, purified, and treated with LiCl where indicated and then exposed to LPS as before. These DCs were then used to stimulate normal BALB/c CD4+ T cells for 5 days and flow cytometry used to quantify the percentage CD4+Foxp3+ cells under each condition. These flow cytometric data were normalized by calculating the percentage CD4+ Foxp3+ cells for each condition compared with that for CTR-DCs alone (mean ± SEM); n = 3. *P < .05. Unstimulated RAPA-DCs promoted an increase in the incidence of Foxp3+ cells relative to CTR-DCs. However, exposure of RAPA-DCs to LPS, or treatment of CTR-DCs with LiCl before LPS stimulation markedly reduced the capacity of these DCs to maintain Foxp3+ cells. (B) WT or IL-12p40−/− mDCs were used to stimulate highly purified CD4+CD25− BALB/c T cells. (B) Both IL-12p40−/− mDCs and LiCl-treated DCs displayed a reduced capacity to support the induction of Foxp3 in CD25− T cells. Data indicate percentage CD4+CD25hi gated cells and are representative of 3 experiments performed. (C) The addition of exogenous IL-12p70 (5 ng/mL) at the start of cocultures of LiCL-treated, LPS-stimulated CTR-DCs and CD4+CD25− T cells reestablished the population of CFSEhiFoxp3hi cells. Data indicate percentage of CD4+CD25hi gated cells and are representative of 3 experiments performed.
Active GSK-3 and IL-12 are necessary for Treg induction by LPS-stimulated DCs. (A) Murine (B6) CTR- and RAPA-DCs were generated, purified, and treated with LiCl where indicated and then exposed to LPS as before. These DCs were then used to stimulate normal BALB/c CD4+ T cells for 5 days and flow cytometry used to quantify the percentage CD4+Foxp3+ cells under each condition. These flow cytometric data were normalized by calculating the percentage CD4+ Foxp3+ cells for each condition compared with that for CTR-DCs alone (mean ± SEM); n = 3. *P < .05. Unstimulated RAPA-DCs promoted an increase in the incidence of Foxp3+ cells relative to CTR-DCs. However, exposure of RAPA-DCs to LPS, or treatment of CTR-DCs with LiCl before LPS stimulation markedly reduced the capacity of these DCs to maintain Foxp3+ cells. (B) WT or IL-12p40−/− mDCs were used to stimulate highly purified CD4+CD25− BALB/c T cells. (B) Both IL-12p40−/− mDCs and LiCl-treated DCs displayed a reduced capacity to support the induction of Foxp3 in CD25− T cells. Data indicate percentage CD4+CD25hi gated cells and are representative of 3 experiments performed. (C) The addition of exogenous IL-12p70 (5 ng/mL) at the start of cocultures of LiCL-treated, LPS-stimulated CTR-DCs and CD4+CD25− T cells reestablished the population of CFSEhiFoxp3hi cells. Data indicate percentage of CD4+CD25hi gated cells and are representative of 3 experiments performed.
It was observed, however, that blocking CTR-DC GSK-3 activity, especially before LPS stimulation, profoundly reduced the incidence of Foxp3+ cells in CTR-DC–stimulated cultures (Figure 7A). IL-12Rβ2, a component of the heterodimeric IL-12 and IL-23 receptors, is necessary for induction of Foxp3 expression in CD25−CD4+ T cells, but not the generation of natural Treg (CD4+CD25+Foxp3+).32 Given our observation that GSK-3 inhibition potently blocks mDC IL-12p40/p70 production after exposure to LPS (Figure 3C) and reduces the capacity of CTR-DCs to maintain Foxp3+ cells in culture (Figure 7A), we examined the roles of IL-12 and GSK-3 in the capacity of mDCs to induce Foxp3 expression in CD4+CD25− T cells. When B6 wild-type (WT) or IL-12p40−/− mDCs were used to stimulate naive BALB/c CD4+CD25− T cells, there was a reduction in the incidence of Foxp3+CD4+CD25hi cells in cultures stimulated with LPS-exposed mDCs (Figure 7B). However, stimulation with IL-12p40−/− mDCs resulted in a further, pronounced reduction of induced Treg compared with WT mDCs (Figure 7B). Notably, IL-12p40−/− mDCs treated with LiCl before LPS stimulation displayed an additional reduction, especially in the incidence of nondividing, induced Treg (Figure 7B; CFSEhiFoxp3hi cells). Addition of rIL-12p70 to cocultures of LiCL-treated, LPS-stimulated mDCs and CD4+CD25− T cells reestablished the CFSEhiFoxp3hi population of induced Treg but did not increase the proliferating population (CFSEloFoxp3hi; Figure 7C). These data reveal that, after LPS stimulation, both mDC GSK-3 activity and IL-12p40/p70 production contribute to the induction of Foxp3 in CD4+CD25− T cells.
Discussion
The importance of mTOR in regulation of APC function is becoming evident.4 By targeting mTORC1, RAPA exerts suppressive effects on mDC differentiation and maturation in vitro and in vivo.4 It also limits plasmacytoid DC production of IFN-α, suppressing antiviral immunity.33 Weichhart et al15 and others14,16 have shown recently that mTORC1 in myeloid APC functions as an early regulator of IL-12 and promoter of IL-10 production. Our data are consistent with mTORC1 acting as a negative regulator of IL-12 and facilitator of IL-10 production after exposure to microbial products. However, we show herein that increased IL-12 production by RAPA-DCs is restricted largely to CD86lo cells, with poor inherent ability to stimulate allogeneic Th1 cell responses. Our data also suggest that augmented NF-κB or MAPK activity does not underlie increased IL-12p40/70 production by RAPA-DCs, which instead results from lost capacity to inhibit GSK-3. Previously, we demonstrated that induction of IL-1β during generation of RAPA-DCs promoted up-regulation of ST2L,12 a negative regulator of TLR4-triggered NF-κB activation.19 This is in agreement with the finding that caspase-1 is suppressed by active mTOR.16 In addition, RAPA-DCs express increased IL-1R-activating kinase-M (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), an additional negative regulator of NF-κB.19 Thus, our observations concerning mDCs generated in RAPA are distinct from those where myeloid cells are exposed to RAPA briefly, then stimulated with LPS.15 In the latter instance, increased activation of NF-κB was implicated in the increased inflammatory potential of myeloid APC, including augmented IL-12p70 production. Thus, duration of exposure to mTOR inhibition and the presence or absence of negative regulators of TLR signaling appear critical in determining the immunoregulatory function of the APC. In addition, it appears that 2 pathways contribute to increased IL-12p70 production by mDCs exposed to mTOR inhibition: one involving mTOR/NF-κB and the other involving mTOR/GSK-3.
The PI3K/Akt and mTOR pathways are intricately linked.34 Active Akt positively signals to mTORC1 by inhibiting the TSC1/2 complex, a negative regulator of mTORC1,1,24 whereas Akt is phosphorylated on S473 by mTORC2 to become fully active.1 Akt activity does not appear to be disrupted in RAPA-DCs, and it does not play a facilitating role in increased IL-12p40/p70 production. Indeed, inhibition of Akt increases IL-12p40, consistent with a negative regulatory role of Akt in IL-12 production.23 However, we cannot rule out the impaired capacity of Akt in RAPA-DCs to function as an inhibitor of GSK-3.35 In the case of T308 on Akt, we observed increased phosphorylation in RAPA-DCs, suggesting that generation of mDCs in RAPA may inhibit a negative regulator of the PI3K pathway, potentially p70S6K.24
In addition to mTOR and Akt, there is evidence that GSK-3 regulates mDC function.14,26 Active GSK-3 promotes IL-12p40/p70 and limits IL-10 production by E coli–stimulated mDCs.26 GSK-3 and mTOR appear to be linked in IL-12 regulation, as inhibition of GSK-3 limits RAPA-enhanced IL-12 production and blunts in vivo Th1 responses.14 Our current observations corroborate and significantly extend these recent findings. Our data are consistent with mTOR and GSK acting regulation of IL-10 and IL-12 after LPS stimulation of mDCs. Importantly, through the use of the mTORC1/TORC2 inhibitor Torin1, we now establish that IL-12 regulation by mTOR is not a RAPA-insensitive function of mTORC1 or mediated by mTORC2/Akt signaling.30 It will now be critical to indentify the mTOR-dependent GSK-3 inhibitor that regulates IL-12. Also of significance is our observation that failed inhibition of GSK-3 in mDCs differentiated in RAPA results in CD86loIL-12p40/p70hi mDCs that are poor promoters of Th1 responses. This resolves an apparent paradox in the literature regarding mTOR/GSK-3 regulation of mDC function. Our data are consistent with a model where active mTOR is needed to suppress GSK-3 and limit mDC IL-12 secretion subsequent to TLR4 ligation.14-16,26 However, we reaffirm that mDCs generated under conditions of mTORC1/2 inhibition display decreased CD86 and are poorly stimulatory. This is also consistent with reports that RAPA-DCs can function as immunoregulatory APCs that promote transplantation survival8,10-12 and inhibit GVHD.13
It is accepted that IL-12p70 facilitates Th1 polarization.36,37 Relatedly, DC production of IL-1, -6, and -23 promotes Th17 development.37,38 It is less clear what underlies Th2-type polarization as DCs produce little IL-4 and IL-4–deficient DCs can promote Th2 responses.39 Limited mDC/CD4+ T-cell interaction or poor TCR stimulation may facilitate Th2 polarization.40 Costimulatory molecule expression is critical for expansion of both Th1 and Th2 cells.41 Inhibition of mTOR immediately before LPS stimulation increases IL-12 and -23 secretion by APCs while augmenting CD86 expression.15 Moreover, these APCs enhance IFN-γ and IL-17 production by CD4+ T cells.14,15 We observed that LPS-stimulated RAPA-DCs exhibited reduced capacity to induce T-cell proliferation or Th17 responses, consistent with their low CD86 expression and reduced IL-23 secretion. RAPA-DCs instead facilitated a heterogeneous, Th0/Th1/Th2 profile. Given their increased IL-12p40/p70 production, it was surprising not to observe more vigorous Th1 polarization by LPS-stimulated RAPA-DCs. Thus, adequate costimulatory molecule expression probably is required, in addition to IL-12p70, for effective Th1 polarization by LPS-stimulated mDCs. Our data do not exclude the possibility that mDC generation in RAPA results in the expression/production of molecules, or alternatively, favors DC interactions with CD4+ T cells, that facilitate Th2 responses.
DCs can enrich, expand, and induce CD4+CD25+Foxp3+ Treg.42 We have found that RAPA-DCs, compared with CTR-DCs, especially after CD40 ligation, enrich for alloAg-specific, naturally occurring Treg (nTreg).11 Again, herein, we observed increased Foxp3+ T cells in cultures of unstimulated RAPA-DCs and bulk CD4+. However, when LPS-stimulated RAPA-DCs and CTR-DCs were compared, no significant difference was observed, with both displaying a reduced capacity to maintain nTreg. CD40 ligation does not increase IL-12p70 secretion by RAPA-DCs.11 Thus, it cannot be concluded that increased IL-12 production by RAPA-DCs plays a role in their enrichment of nTreg. Notably, it was apparent that LiCl treatment, which potently blocked IL-12p40/p70 production by both RAPA- and CTR-DCs, profoundly reduced the incidence of Foxp3+ cells in CTR-DC-stimulated cultures, especially those with LPS-treated CTR-DCs. IL-12p70 is a promoter of IFN-γ–producing Th1 responses.37 However, IFN-γ has also been shown to support the induction of Foxp3 in CD4+CD25− cells.43 Administration of IL-12, by promoting IFN-γ, can prevent GVHD44 and experimental autoimmune encephalomyelitis.45 Moreover, IL-12Rβ2−/− mice exhibit an accelerated onset of autoimmune diabetes, associated with severely impaired Treg induction.32 Our data support a crucial need for IL-12p40/p70 in the induction of Foxp3 in CD4+CD25− cells. We also have identified a requirement for GSK-3 activity in mDCs for Treg induction, beyond the support of IL-12 production. Thus, the capacities of mDCs to induce Th1 and Treg responses appear to be closely linked.
Our current findings are relevant to the chronic administration of RAPA in patients. mTOR inhibition in renal transplantation has been associated, in less than 20% of patients, with inflammatory disorders, including interstitial pneumonitis and glomerulonephritis.46,47 It remains unclear whether these pathologies are mediated by a particular subset of Th cells favored by endogenous DCs under the influence of RAPA. Decreased Th1 responses were observed in kidney transplantation patients receiving rapamycin versus cyclosporine in combination with either mycophenolate mofetil or methylprednisolone.48 However, RAPA monotherapy of type 1 diabetes has revealed no differential effect on Th1 versus Th2 cells but increased Treg function.6 Nonetheless, analysis of PBMCs from kidney or liver transplantation patients converted to RAPA from calcineurin inhibitors revealed that RAPA increased Th1 cells and decreased IL-10 gene expression.49 With growing clinical use of RAPA, it is imperative to gain a fuller understanding of the impact that increased IL-12 production by RAPA-exposed APCs may have on systemic Th and Treg responses in patients receiving mTOR inhibitors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lisa Mathews for excellent technical assistance.
This work was supported by the National Institutes of Health (NIH; grants R01AI67541 and P0181678; A.W.T.). H.R.T. was supported by the American Society of Transplantation (nonconcurrent fellowships) and the NIH (T32CA82084, F32AI72940, and K99HL097155). J.C. was supported by the NIH (training grant T32AI74490). T.L.S. was supported by NIH (training grant T32CA82084) and the American Society of Transplantation (nonconcurrent basic science fellowship).
National Institutes of Health
Authorship
Contribution: H.R.T. designed and performed research, collected and analyzed data, and wrote the paper; J.C., C.M., B.R.R., and T.L.S. performed research and collected data and analyzed data; D.A.G. analyzed data; D.M. designed research and analyzed data; and A.W.T. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angus W. Thomson, Departments of Surgery and Immunology, University of Pittsburgh School of Medicine, Thomas E. Starzl Transplantation Institute, 200 Lothrop St, Biomedical Science Tower, W1540, Pittsburgh, PA 15213; e-mail: thomsonaw@upmc.edu.

![Figure 1. Although they display poor CD4+ T-cell allostimulatory capacity, mouse and human RAPA-DCs produce increased IL-12p70 when exposed to LPS. BM-derived mDCs were generated from B6 mice with GM-CSF and IL-4, in the presence (RAPA-DCs) or absence of RAPA (CTR-DCs) and then isolated and purified on day 7 of culture. These DCs were incubated for 18 hours in media alone (Unstim) or with 0.1 μg/mL LPS. (A) RAPA-DCs, compared with similarly stimulated CTR-DCs, showed inferior capacity to induce proliferation of alloreactive CD4+ BALB/c T cells, even after exposure to LPS. The data are mean ± SD from sample replicates of 1 experiment representative of more than 4 performed. (B) Intracellular staining for IL-12p40 revealed that DC generation in RAPA promoted an increase in IL-12p40 expression after exposure to LPS. Numbers on dot plots indicate percentage of CD11c+ cells. (C) Comparison of the percentages (mean ± SD) of IL-12p40+CD11c+ cells from 2 experiments representative of more than 6 performed. Enzyme-linked immunosorbent assay (D-E) or Luminex (F) analysis of culture supernatants revealed significantly increased secretion of IL-12p70 by LPS-stimulated RAPA-DCs, whereas the release of IL-23 (E) and IL-10 (F) was reduced. (A-F) *P < .05. The data shown are representative of more than 3 experiments performed. (G-H) Hu DCs were generated from CD14+ monocytes in the presence (Hu RAPA-DCs) or absence (Hu CTR-DCs) of RAPA. (G) Like their murine counterparts, unstimulated Hu RAPA-DCs and those stimulated for 18 hours with LPS showed a reduced capacity to stimulate allogeneic T cells in MLR. [3H]-thymidine (TdR) incorporation for each condition was normalized to that obtained with unstimulated Hu CTR-DCs for each experiment. Data represent mean percentage ± SD across multiple experiments (Unstim, n = 10; LPS, n = 4). *P < .05. (H) Hu RAPA-DCs also displayed significantly increased IL-12p70 secretion after exposure to LPS (0.1 μg/mL) Data are mean ± SD from multiple experiments (Unstim, n = 9; LPS, n = 5). *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/23/10.1182_blood-2009-10-251488/4/m_zh89991053340001.jpeg?Expires=1770600116&Signature=sF~vOC~X2zc9Xj7RbLj9QTbKGVigyVqqt5GkEwA8wEtJzrkeLIx0ie-5HOgzWh0AcViTD5dO0OK0-iZDOMK-uiNR0HowVeyHGm~Y7E2wdjHfsXsDjlZE3ZnduG9DS2AXCFQt0hK4XnaZRAFjyBZjYGn3yp9e9qQkVi0VhGGSjPzKsem1mD-QG0y~Syf-3KiC3yUTQl0955cWYB3wnwwaKJDH-sXJGeiJabngzBrpi3pYMakUIZ4A-PWCO9F6ObGHdh94mcrLWMWI1fzLFrq091uDOr9HC4rMZ3ZEGZN-ZKRw7cWZUN0zA9nzzqU4lW9PuynGgd3CGon7u5~2wrF6Tg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Although they display poor CD4+ T-cell allostimulatory capacity, mouse and human RAPA-DCs produce increased IL-12p70 when exposed to LPS. BM-derived mDCs were generated from B6 mice with GM-CSF and IL-4, in the presence (RAPA-DCs) or absence of RAPA (CTR-DCs) and then isolated and purified on day 7 of culture. These DCs were incubated for 18 hours in media alone (Unstim) or with 0.1 μg/mL LPS. (A) RAPA-DCs, compared with similarly stimulated CTR-DCs, showed inferior capacity to induce proliferation of alloreactive CD4+ BALB/c T cells, even after exposure to LPS. The data are mean ± SD from sample replicates of 1 experiment representative of more than 4 performed. (B) Intracellular staining for IL-12p40 revealed that DC generation in RAPA promoted an increase in IL-12p40 expression after exposure to LPS. Numbers on dot plots indicate percentage of CD11c+ cells. (C) Comparison of the percentages (mean ± SD) of IL-12p40+CD11c+ cells from 2 experiments representative of more than 6 performed. Enzyme-linked immunosorbent assay (D-E) or Luminex (F) analysis of culture supernatants revealed significantly increased secretion of IL-12p70 by LPS-stimulated RAPA-DCs, whereas the release of IL-23 (E) and IL-10 (F) was reduced. (A-F) *P < .05. The data shown are representative of more than 3 experiments performed. (G-H) Hu DCs were generated from CD14+ monocytes in the presence (Hu RAPA-DCs) or absence (Hu CTR-DCs) of RAPA. (G) Like their murine counterparts, unstimulated Hu RAPA-DCs and those stimulated for 18 hours with LPS showed a reduced capacity to stimulate allogeneic T cells in MLR. [3H]-thymidine (TdR) incorporation for each condition was normalized to that obtained with unstimulated Hu CTR-DCs for each experiment. Data represent mean percentage ± SD across multiple experiments (Unstim, n = 10; LPS, n = 4). *P < .05. (H) Hu RAPA-DCs also displayed significantly increased IL-12p70 secretion after exposure to LPS (0.1 μg/mL) Data are mean ± SD from multiple experiments (Unstim, n = 9; LPS, n = 5). *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/23/10.1182_blood-2009-10-251488/4/m_zh89991053340001.jpeg?Expires=1770600117&Signature=DQaby6lXOJGuWyfEUU5osi1JaslEM2F1t-W6NGi8JcFlJSbZvqgIStZxgvyqZLBZaUZ17ApyQ1IjsgkZESevYfreMywrgY2V6TflNGd5VT4YC9oCW-ZJq11vCwYJvo75Ny7SqTfiOmsiTIzopkxi1bvgBy2o845yHgxfbuJQaDjcajxkOfrlnJLUKUtyrmzj81mxKhzeBulUUBGX1NZeYiMqjLcVPpzMMYSnMqtX0NEfCq1ZMzhnuAzJjc8AteF-nOT3wYapkd62ZaC2HqimyMh2WDnFZoulwIWM3i871xIJR6qkkYC9TwDvpik5EOZYH0LY6nt78g9rKiLy7GYWrg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)