Abstract
The anticoagulant factor protein S (PS) has direct cellular activities. Lack of PS in mice causes lethal coagulopathy, ischemic/thrombotic injuries, vascular dysgenesis, and blood-brain barrier (BBB) disruption with intracerebral hemorrhages. Thus, we hypothesized that PS maintains and/or enhances the BBB integrity. Using a BBB model with human brain endothelial cells, we show PS inhibits time- and dose-dependently (half maximal effective concentration [EC50] = 27 ± 3 nM) oxygen/glucose deprivation-induced BBB breakdown, as demonstrated by measurements of the transmonolayer electrical resistance, permeability of endothelial monolayers to dextran (40 kDa), and rearrangement of F-actin toward the cortical cytoskeletal ring. Using Tyro-3, Axl, and Mer (TAM) receptor, tyrosine kinase silencing through RNA interference, specific N-terminus–blocking antibodies, Tyro3 phosphorylation, and Tyro3-, Axl- and Mer-deficient mouse brain endothelial cells, we show that Tyro3 mediates PS vasculoprotection. After Tyro3 ligation, PS activated sphingosine 1-phosphate receptor (S1P1), resulting in Rac1-dependent BBB protection. Using 2-photon in vivo imaging, we show that PS blocks postischemic BBB disruption in Tyro3+/+, Axl−/−, and Mer−/− mice, but not in Tyro3−/− mice or Tyro3+/+ mice receiving low-dose W146, a S1P1-specific antagonist. Our findings indicate that PS protects the BBB integrity via Tyro3 and S1P1, suggesting potentially novel treatments for neurovascular dysfunction resulting from hypoxic/ischemic BBB damage.
Introduction
Protein S (PS) is a vitamin K–dependent anticoagulant plasma glycoprotein.1 PS exerts activated protein C–dependent2 and –independent3,4 anticoagulant activities. The physiologic importance of PS is best demonstrated by the life-threatening purpura fulminans with disseminated intravascular coagulation, a condition seen in the newborns that are homozygous for PS mutations.5 Persons with PS heterozygous mutations or polymorphisms have an increased risk for deep venous thrombosis and other thrombotic events.6,7
Independent of its anticoagulant action, PS exerts direct cellular activities. It binds to negatively charged surfaces on the plasma membranes of apoptotic cells, promoting their phagocytosis by macrophages.8-10 PS has a potent mitogenic activity11-13 and protects neurons from ischemic/hypoxic injury.14 The growth arrest specific gene-6, a structural analog of PS,1 activates the Tyro-3, Axl, and Mer (TAM) receptor tyrosine kinase Tyro3 in neurons15 and Axl in oligodendroglia16 and vascular cells,1 resulting in cytoprotection. Earlier studies have suggested that PS is a Tyro3 ligand.17 Recent studies show that PS interacts with the TAM receptor Mer in the retina18 and macrophages10 and is a biologically relevant ligand for both Mer and Tyro3 in the retinal epithelium.19 The TAM receptors Tyro3, Axl, and Mer are expressed in mammalian reproductive, immune, vascular, and nervous systems.20-24 Mice with triple mutations in Tyro3, Axl, and Mer display apoptosis of the blood vessels and nervous tissue.21
In addition to its synthesis by hepatocytes,1 PS is highly expressed in vascular endothelial cells,25,26 which make substantial contribution to circulating PS.4 The exact role of PS in vascular endothelial cells, however, is unknown. Recent studies demonstrated that lack of PS in mice causes embryonically lethal coagulopathy, ischemic/thrombotic injuries, and vascular dysgenesis with the blood-brain barrier (BBB) disruption, intracerebral hemorrhages, and brain damage.3,4 The BBB breakdown and vascular defects in mice lacking PS could be attributed to thrombotic/ischemic injuries,3,4 but may also reflect loss of PS-mediated vasculoprotection. We hypothesized that PS can protect the BBB integrity through the TAM receptor(s) in brain endothelium, which in turn may control neurovascular dysfunction in stroke and other brain disorders associated with hypoxic/ischemic BBB breakdown.27
Methods
For details, see the supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Reagents
Full-length properly γ-carboxylated mouse recombinant PS was prepared and characterized by the laboratory of Dr J. H. Griffin (The Scripps Research Institute), as we described.13 Human plasma–derived PS was purchased from Enzyme Research Laboratories (see supplemental Methods).
Primary human BECs and astrocytes
Brain endothelial cells (BECs) and astrocytes were isolated from small fragments of cerebral cortex obtained from surgical resections of adults with seizure disorder (supplemental Methods). Early passage 2 to 3 cells were used in all experiments.
In vitro model of the BBB
The BBB in vitro model was constructed in inserts with collagen-coated polycarbonate Transwell membrane filters with 0.4-μm pores (Corning Life Science), as we described.28 Astrocyte-conditioned medium from primary cultured human astrocytes was added to the lower chamber and was supplemented with 550nM hydrocortisone (Sigma-Aldrich). Cells were cultured for 5 to 7 days until an in vitro BBB monolayer was established, as we reported.28 To confirm the BBB formation, we analyzed monolayers by confocal microscopy for a characteristic cobblestone pattern of the tight junction zona occludens 1 (ZO-1) protein and a cortical distribution of the cytoskeletal F-actin filaments, as reported.28-31 The transmonolayer electrical resistance (TER) and monolayer permeability to 40 kDa dextran were measured as reported.28-31
OGD injury
Analysis of the endothelial barrier by confocal microscopy
Details are reported in supplemental Methods.
TER
Permeability of the endothelial barrier
We used fluorescein isothiocyanate (FITC)–labeled dextran (40 kDa; Invitrogen) as described.34 The BBB permeability to dextran was expressed as a permeability coefficient in cm · s−1 (supplemental Methods). The volume cleared (ΔVc) of each time point was calculated using equation 1: ΔVc = Clower × Vlower/Cupper, where Cupper and Clower are FITC-labeled dextran concentrations in upper and lower chambers, respectively, and Vlower is the volume in lower chamber.
The volume cleared (ΔVc) was plotted against time, and the permeability surface area (PS) product was obtained from the slope by linear regression. The permeability coefficient (P) was then calculated by equation 2: P = PS/s, where s is the surface area of the filter (1.12 cm2).
Finally, the permeability coefficient of cells (Pcell) was obtained by correcting the overall permeability coefficient (Pcell+filter) for that of the cell-free filter (Pfilter) using equation 3: 1/Pcell = 1/Pcell+filter − 1/Pfilter.
Pfilter was determined on a separate series of experiments using the cell-free filter inserts only. Human PS was added to the lower chamber at different concentrations from 5 to 100nM. For nonspecific leakage, see the supplemental Methods.
Silencing through RNA interference
Details are reported in the supplemental Methods.
Western blotting for Tyro3, Axl, and Mer
Details are reported in the supplemental Methods.
Antibody blockade of TAM receptors
The antibodies used to block the TAM receptors were raised against the extracellular N-terminus domains of the respective TAM receptor family members (R&D Systems). See the supplemental Methods.
Tyro3 tyrosine phosphorylation
Human BBB monolayers were lysed with radioimmunoprecipitation assay buffer and incubated with a mouse monoclonal antiphosphotyrosine antibody (Millipore Corp). The samples were then immunoprecipitated using a protein G immunoprecipitation kit (Roche) followed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis separation and transfer onto nitrocellulose membranes (Millipore Corp) and incubation with a goat anti–human Tyro3 antibody (R&D Systems). After incubation with a horseradish peroxidase–conjugated donkey anti–goat secondary antibody (Santa Cruz Biotechnology), the immunoreactivity was detected using the SuperSignal West Pico chemiluminescent substrate (Thermo Scientific). Cells were treated with human PS for 15 minutes.
Rac1 activation assay
Rac1 activation was determined using the Rac1 activation assay kit (Cytoskeleton; see the supplemental Methods).
Sphingosine 1-phosphate receptor threonine phosphorylation
Details are reported in supplemental Methods.
Tyro3 interaction with sphingosine 1-phosphate receptor
Human BBB monolayers were pretreated for 1 hour with an N-terminus–specific Tyro3 blocking antibody (10 μg/mL; R&D Systems). This antibody recognizes the amino acid residues 41 to 420 in the N-terminus extracellular domain of Tyro3 (the ligand-binding domain for PS and Gas6 is around amino acid 210)23 and as we show blocks PS binding to Tyro3 and Tyro3 activation (Figure 2D). The cells were then subjected to normoxia or OGD for 10 minutes with or without human PS (100nM). The cells were lysed with radioimmunoprecipitation assay lysis buffer and incubated with either control nonimmune immunoglobulin G (IgG) or a C-terminus Tyro3 antibody (Santa Cruz Biotechnology), which we show does not block PS binding to and activation of Tyro3 (Figure 2D) as expected, because this antibody recognizes the C-terminus amino acids 840 to 890 outside the PS/Gas6 ligand-binding domain. This C-terminus Tyro3 antibody was used in coimmunoprecipitation study. The Tyro3 immunocomplexes with the Tyro3 bound proteins were spun down with protein G sepharose beads at 12 000g for 20 seconds. The pellets were eluted with lithium dodecyl sulfate (LDS) sample buffer (Invitrogen) and samples electrophoresed on bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane/tris(hydroxymethyl)aminomethane 4% to 20% gradient gels (Invitrogen) to separate proteins that interacted with Tyro3 and were bound to Tyro3. The separated proteins were transferred on nitrocellulose membrane and then immunoblotted using an anti–sphingosine 1-phosphate receptor (S1P1) antibody (Abcam) to detect whether S1P1 had been bound to Tyro3, and with anti-Tyro3 (C-terminus specific) antibody to ensure equal loading of samples.
The same samples as described in the previous paragraph were subjected to reverse immunoprecipitation to confirm the presence of Tyro3 physical association with S1P1. For these studies, the cell lysates were incubated with an anti-S1P1 antibody (Abcam) to immunoprecipitate S1P1 and any proteins bound to S1P1. The S1P1-bound immunocomplexes were centrifuged with protein G sepharose beads as in the previous paragraph, eluted with LDS sample buffer (Invitrogen), and electrophoresed on a 4% to 12% bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane/tris(hydroxymethyl)aminomethane gradient gel to separate any proteins that were bound to S1P1. The separated proteins were transferred on nitrocellulose membrane and immunoblotted using an anti-Tyro3 (C-terminus specific) antibody to detect whether Tyro3 had been bound to S1P1, or with anti-S1P1 antibody to ensure equal loading of samples.
Akt serine phosphorylation
Details are reported in the supplemental Methods.
Isolation of mouse brain endothelial cells
BECs from wild-type mice and Tyro3, Axl, and Mer mutants20,21 were isolated and characterized as we described35 ; see the supplemental Methods for details. OGD-induced injury was induced as we reported previously32 and described in “OGD injury.” Cell survival was determined by WST-8 assay (Dojindo Molecular Technologies).
Transient MCAO
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Rochester using National Institutes of Health guidelines. The transient middle cerebral artery occlusion (MCAO) was performed as we reported.32,36 Male C57BL6 mice, Tyro3−/−, Axl−/−, and Mer−/− mutants20,21 at 2 to 3 months of age weighing 26 to 30 g were used throughout the study. Mice were anesthetized with ketamine (100 mg/kg, intraperitoneally) and xylazine (10 mg/kg, intraperitoneally). Physiologic parameters and blood gasses were measured. Cerebral blood flow was monitored by laser Doppler flowmetry (Transonic Systems). The rectal temperature was maintained at 37°C using a thermal feedback heating pad (Harvard Apparatus). Briefly, an 8-0 silicon-coated nylon monofilament was advanced into the internal carotid artery to occlude the MCA for 1 hour. This was followed by 8-hour reperfusion before 2-photon in vivo imaging of the BBB disruption. After imaging, mice were additionally reperfused for 15 hours and killed at 24 hours after MCAO for neurologic and neuropathologic evaluations. Mouse PS (0.2 mg/kg) or vehicle was administered via the femoral vein 10 minutes after the MCAO. W146 was given intraperitoneally 10 minutes before the MCAO. Unfixed 1-mm coronal brain slices were incubated in 2% tripenylterazolium chloride in phosphate buffer (pH 7.4) to determine volume of injury, as described.32,36 All studies were performed in a blinded fashion (supplemental Methods).
Two-photon in vivo imaging of the BBB leakage
Mice were reanesthetized with intraperitoneal ketamine (100 mg/kg) and xylazine (10 mg/kg) for 8 hours after 1-hour MCAO for 2-photon in vivo imaging to determine leakage of fluorescein-conjugated F-dextran (molecular weight [MW] = 70 kDa) from ischemic vessels. In the present MCAO model, the most pronounced BBB leakage of F-dextran was observed in nontreated mice within 8 hours of reperfusion, as indicated by our pilot study. Therefore, all BBB measurements were performed 8 hours after the MCAO. The BBB permeability measurements were performed on the ischemic side 4 mm from the frontal pole, 3 mm from the midline, and 80 μm in depth from the cortical surface. F-dextran in 0.1 mL of phosphate-buffered saline was administered via the femoral vein. In vivo images were acquired in the cortical layer II (80 μm below the cortical surface) using a custom-built Zeiss 5MP multiphoton microscope coupled to a 900-nm mode locked Ti:sapphire laser (Mai Tai; Spectra Physics). Fluorescein emission was collected using a 500- to 550-nm bandpass filter. At the 8-hour time point after the MCAO, the maximum signal intensity reflecting F-dextran leakage from postischemic blood vessels was obtained in each mouse within 30 minutes of F-dextran administration. Thus, all measurements were made within the linear portion of F-dextran leakage curves within 30 minutes of F-dextran administration. Quantification was performed by a blinded investigator by measuring the fluorescent signal intensity in 20 randomly selected 20 × 20-μm extravascular areas in brain parenchyma per mouse in the region of interest in the ischemic border. The integrated signal intensity was obtained as the sum of the values of the pixels in 20 randomly selected 20 × 20-μm areas in the region of interest using the National Institutes of Health ImageJ software integrated density function. A one-way analysis of variance with a Tukey posthoc test was performed using GraphPad Prism software to determine statistical significance between groups and graphs were plotted. Plotted values are mean plus or minus SEM (n = 6 mice per group).
Protein S levels in the cerebrospinal fluid
Details are reported in the supplemental Methods.
Tyro3 localization (abluminal vs luminal) in brain endothelium in situ
Details are reported in the supplemental Methods.
Tyro3, Axl, and Mer in mouse brain microvessels
Details are reported in the supplemental Methods.
Statistical analysis
We used S-plus 7.0 (TIBCO Software) for statistical calculations. Data were presented as mean plus or minus SEM. One-way or 2-way analysis of variance followed by Tukey posthoc test were used to determine statistically significant differences. Nonparametric data (motor neurologic scores) were subjected to Kruskal-Wallis testing. P values less than .05 were considered statistically significant.
Results
Protein S protects the endothelial barrier integrity from OGD in an in vitro model of the human BBB
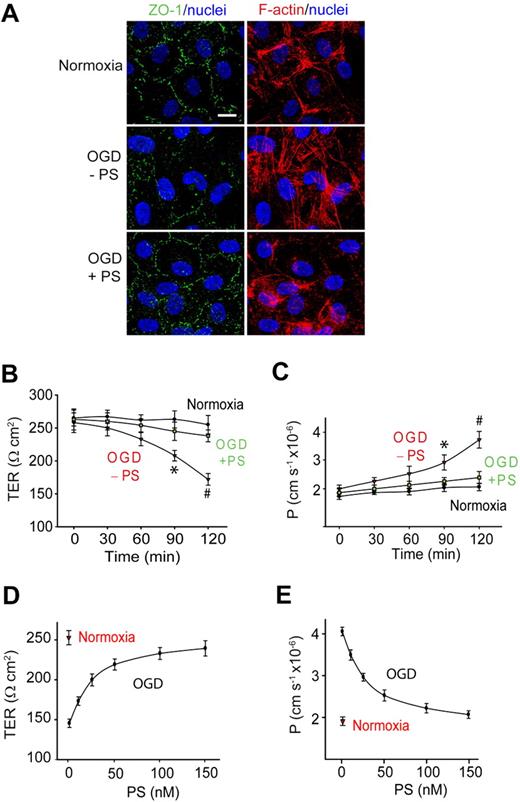
To determine whether PS can protect the BBB from a hypoxic/ischemic insult, we studied primary human brain endothelial cells in an in vitro monolayer model of the human BBB.28 Brain endothelial monolayers were challenged with OGD that has been used frequently as an in vitro model of ischemia in cultured endothelium.30,32 Control cultures expressed a cobblestone pattern of the ZO-1 tight junction protein and a cortical distribution of the cytoskeletal F-actin filaments (Figure 1A top panel), as described.28-31 The TER and the transendothelial permeability constant (P) to a metabolically inert polar molecule dextran (MW = 40 000 Da) were 270 Ω · cm2 and 1.89 cm · s−1 × 10−6 (Figure 1B-C), respectively. There was a progressive drop in TER (Figure 1B) and an increase in dextran permeability (Figure 1C) beginning 1 hour after OGD, indicating BBB disruption. This correlated with substantially diminished ZO-1 staining, disassembly of cortical F-actin, and an increase in stress fibers in the cytoplasm (Figure 1A).
Protein S protects the endothelial barrier integrity from OGD in an in vitro monolayer model of the human BBB. (A) Confocal scanning microscopy analysis of human endothelial monolayers 2 hours after normoxia or OGD with and without PS (100nM). ZO-1: green; F-actin: red; and nuclei; blue. Scale bar represents 10 μm. (B-C) Transmonolayer electrical resistance (TER) (B) and permeability coefficient (P) to FITC-dextran (C) in BBB monolayers during 2 hours of normoxia or OGD with and without PS (100nM). *P < .05, OGD − PS versus normoxia; #P < .01, OGD − PS versus OGD + PS. (D-E) PS dose-dependently enhances TER (D) and permeability to FITC-dextran in BBB monolayers 2 hours after OGD. Mean ± SEM, from 3 to 6 independent cultures.
Protein S protects the endothelial barrier integrity from OGD in an in vitro monolayer model of the human BBB. (A) Confocal scanning microscopy analysis of human endothelial monolayers 2 hours after normoxia or OGD with and without PS (100nM). ZO-1: green; F-actin: red; and nuclei; blue. Scale bar represents 10 μm. (B-C) Transmonolayer electrical resistance (TER) (B) and permeability coefficient (P) to FITC-dextran (C) in BBB monolayers during 2 hours of normoxia or OGD with and without PS (100nM). *P < .05, OGD − PS versus normoxia; #P < .01, OGD − PS versus OGD + PS. (D-E) PS dose-dependently enhances TER (D) and permeability to FITC-dextran in BBB monolayers 2 hours after OGD. Mean ± SEM, from 3 to 6 independent cultures.
Human PS (100nM) abolished OGD-induced BBB breakdown as shown by normalization of TER (Figure 1B) and endothelial permeability to dextran (Figure 1C) and rearrangement of polymerized F-actin toward the cortical cytoskeletal ring (Figure 1A). PS protection of the endothelial barrier integrity was concentration dependent (Figure 1D-E) with the half maximal effective concentration (EC50) value of 27 plus or minus 3nM (Figure 1E). PS (100nM) vasculoprotection persisted throughout the studied 24-hour period of time (supplemental Figure 1A-C).
Protein S protects the endothelial barrier integrity in OGD-treated human BBB monolayers via Tyro3
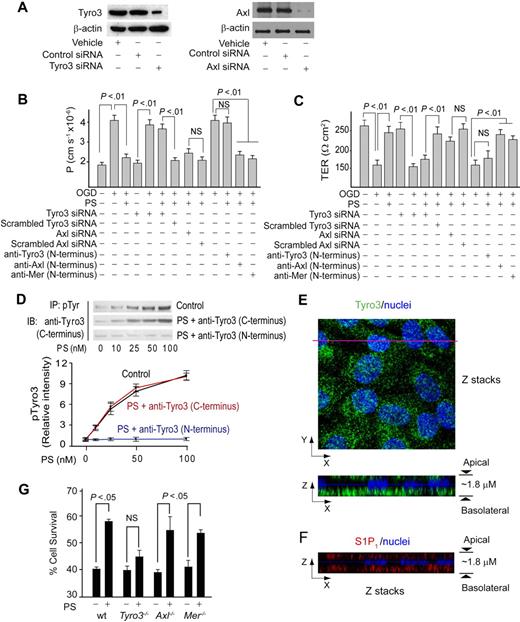
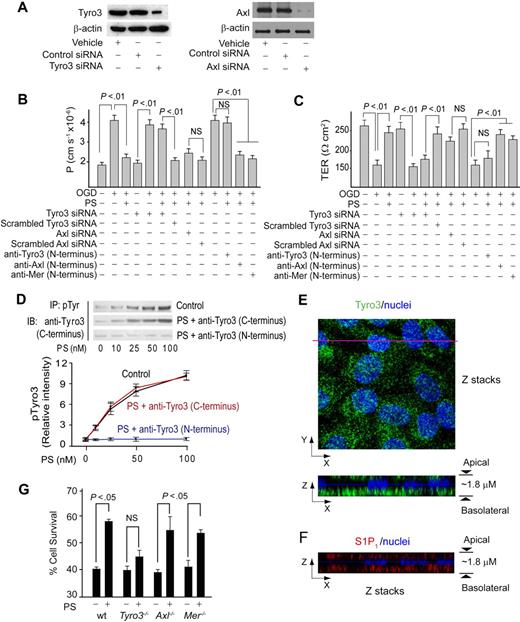
The immunoblot analysis confirmed expression of Tyro3 and Axl in human brain endothelial cells (Figure 2A), and low expression of Mer (not shown). Transfection of endothelium with Tyro3-specific and Axl-specific siRNAs inhibited Tyro3 and Axl expression by approximately 80% and 90%, respectively (Figure 2A). Tyro3 siRNA transfection alone did not alter permeability or TER of BEC monolayers (Figure 2B-C). After OGD, PS (100nM) failed to protect barrier integrity in BBB monolayers with inhibited Tyro3 expression, but not in those with decreased Axl expression (Figure 2B-C), suggesting a requirement for Tyro3. To confirm this finding, cultures were pretreated with Tyro3-, Axl-, and Mer-specific N-terminus–blocking antibodies raised against the amino acid sequences of the TAM receptors containing the ligand-binding domain for PS and Gas6.23 Blockade of PS binding to Tyro3, but not to Axl or Mer, resulted in loss of barrier protection by PS (100nM) as determined by endothelial permeability to dextran (Figure 2B) and TER (Figure 2C). Human PS dose-dependently increased Tyro3 phosphorylation in human brain endothelium, with the EC50 value of approximately 25nM (Figure 2D). PS activation of Tyro3 was abolished by the Tyro3 N-terminus–blocking antibody raised against the Tyro3 domain containing the ligand-binding site for PS and Gas 6,23 but not with a C-terminus Tyro3-specific antibody that recognizes Tyro3 epitopes outside the ligand-binding domain (Figure 2D). Normoxia or OGD did not affect Tyro3 phosphorylation (supplemental Figure 2A). Confocal scanning microscopy showed that Tyro3 is predominantly localized at the basolateral side of a BBB monolayer (Figure 2E). In all in vitro experiments, PS was added to the medium to the lower chamber at the basolateral side.
Protein S protects the endothelial barrier integrity in OGD-treated human BBB monolayers and mouse brain endothelial cells via Tyro3. (A) Tyro3 and Axl immunoblotting in human endothelial monolayers transfected with Tyro3 or Axl siRNAs. (B-C) Permeability (P) to FITC-dextran (B) and transmonolayer electrical resistance (TER; C) in human BBB monolayers transfected with Tyro3 or Axl siRNAs or incubated with Tyro3, Axl, and Mer N-terminus–specific blocking antibodies 2 hours after OGD with and without human PS (100nM). (D) Human PS dose-dependently phosphorylates Tyro3 in human BBB monolayers 15 minutes after OGD. IP indicates immunoprecipitation of phosphotyrosine proteins; IB, immunoblotting of Tyro3. Cells were treated with PS and anti-Tyro3 (C-terminus) or anti-Tyro3 (N-terminus) antibody. P < .05 versus vehicle. (E) Confocal microscopy analysis of Tyro3 distribution in a human BBB monolayer. Top (x-y axis): an image of compressed Z-stacks. Tyro3, green; nuclei, blue. Bottom (x-z axis): Z-stacks image of Tyro3 (green) at the apical and basolateral side. (F) Confocal microscopy analysis of S1P1 distribution in a human BBB monolayer. Z-stacks image (x-z axis) of S1P1 (red) at the apical and basolateral side. (G) Survival of subconfluent mouse brain endothelial cells derived from Tyro3, Axl, and Mer mutants and littermate controls 24 hours after OGD with and without mouse PS (50nM). wt indicates wild-type mice. Mean ± SEM, from 3-6 independent cultures.
Protein S protects the endothelial barrier integrity in OGD-treated human BBB monolayers and mouse brain endothelial cells via Tyro3. (A) Tyro3 and Axl immunoblotting in human endothelial monolayers transfected with Tyro3 or Axl siRNAs. (B-C) Permeability (P) to FITC-dextran (B) and transmonolayer electrical resistance (TER; C) in human BBB monolayers transfected with Tyro3 or Axl siRNAs or incubated with Tyro3, Axl, and Mer N-terminus–specific blocking antibodies 2 hours after OGD with and without human PS (100nM). (D) Human PS dose-dependently phosphorylates Tyro3 in human BBB monolayers 15 minutes after OGD. IP indicates immunoprecipitation of phosphotyrosine proteins; IB, immunoblotting of Tyro3. Cells were treated with PS and anti-Tyro3 (C-terminus) or anti-Tyro3 (N-terminus) antibody. P < .05 versus vehicle. (E) Confocal microscopy analysis of Tyro3 distribution in a human BBB monolayer. Top (x-y axis): an image of compressed Z-stacks. Tyro3, green; nuclei, blue. Bottom (x-z axis): Z-stacks image of Tyro3 (green) at the apical and basolateral side. (F) Confocal microscopy analysis of S1P1 distribution in a human BBB monolayer. Z-stacks image (x-z axis) of S1P1 (red) at the apical and basolateral side. (G) Survival of subconfluent mouse brain endothelial cells derived from Tyro3, Axl, and Mer mutants and littermate controls 24 hours after OGD with and without mouse PS (50nM). wt indicates wild-type mice. Mean ± SEM, from 3-6 independent cultures.
We next studied cultured mouse BECs from Tyro3−/−, Axl−/−, and Mer−/− mice. The objective of this study was to determine whether in a model system using mouse BECs, mouse Tyro3, and mouse PS, PS interacts with Tyro3, as shown for human PS and human BECs. In these studies, we determined cell survival of subconfluent BEC cultures to OGD, using our published method.32 Consistent with species-specific differences between human and mouse PS, we used full-length properly γ-carboxylated mouse recombinant PS, as we reported.13 Our pilot study with murine PS showed maximal protection of OGD-treated subconfluent cultures at 100nM (not shown), similar as for human PS and human BECs. A submaximal concentration of murine PS was used to increase sensitivity of the protection assay in case that multiple, including Tyro3-independent mechanisms, are present in mouse BECs. Murine PS (50nM) protected cultured mouse BECs from wild-type, Axl−/−, and Mer−/− mice at a comparable level (Figure 2G), indicating these receptors are not necessary for PS protection. However, PS failed to achieve a significant protection of BECs derived from Tyro3−/− mice (Figure 2G), indicating that Tyro3 is required for protection of mouse BECs.
Protein S mediates Rac1-dependent barrier protection and activates S1P1 in OGD-treated human BBB monolayers
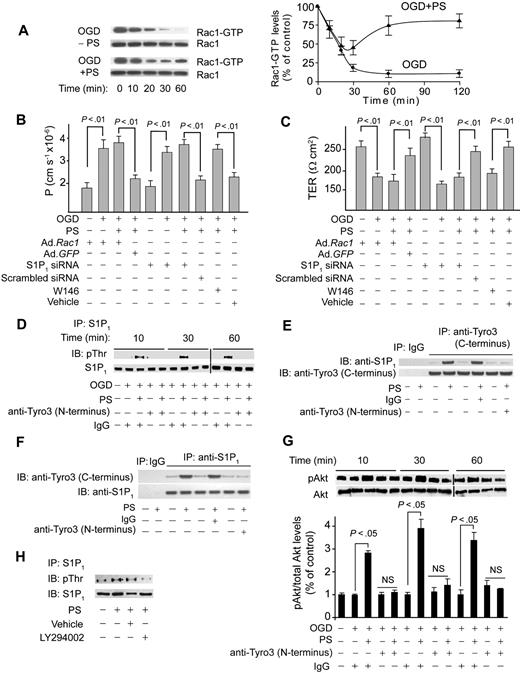
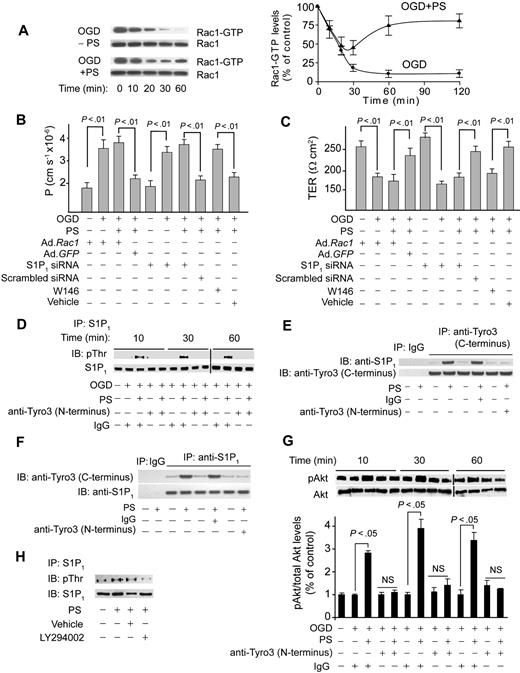
Because Rac1 GTPase regulates cortically distributed actin polymerization and endothelial permeability,29,30,33 we studied Rac1 activation in cultured endothelium challenged by OGD in the presence and absence of PS. OGD decreased activated Rac1 (Figure 3A), as reported in endothelia subjected to hypoxia31 and edematogenic agents.33 PS reduced the OGD-induced drop in activated Rac1 over the studied 2 hours (Figure 3A). In monolayers transduced with a dominant negative Rac1 adenovirus, PS failed to improve barrier permeability to dextran (Figure 3B) and TER (Figure 3C) compared with control Ad.GFP. Transduction of BECs with Rac1 did not change endothelial permeability or TER (Figure 3B-C).
Protein S mediates Rac1-dependent barrier protection and activates S1P1 after Tyro3 ligation in OGD-treated human BBB monolayers. (A) Time course of Rac1 activation (Rac1-GTP) in human BBB monolayers in vitro after OGD (0-60 minutes) with and without PS (100nM). Graph: relative abundance of Rac1-GTP signal intensity normalized by Rac1 (0-120 minutes). P < .01, OGD + PS versus OGD − PS. (B-C) Permeability to FITC-dextran (B) and TER (C) in BBB monolayers 2 hours after OGD and treatment with PS (100nM) after transduction with Ad.Rac1 and Ad.GFP, transfection with S1P1 siRNA and control siRNA, and incubation with S1P1-specific antagonist W146 (50nM). (D) Immunoprecipitation (IP) of S1P1 with anti-S1P1 antibody followed by immunoblotting (IB) with antiphosphothreonine antibody (pThr) in BBB monolayers 10 to 60 minutes after OGD and treatment with PS with and without anti-Tyro3 (N-terminus) antibody or nonimmune IgG. (E) IP of S1P1 with anti-Tyro3 (C-terminus) and control nonimmune IgG in BBB monolayers 10 minutes after OGD and treatment with PS (100nM) with and without control antibody or anti-Tyro3 (N-terminus) ligand-binding domain–blocking antibody. (F) IP of Tyro3 with anti-S1P1 antibody and control nonimmune IgG in BBB monolayers 10 minutes after OGD and PS (100nM) treatment with and without control antibody or anti-Tyro3 (N-terminus) blocking antibody. (G) Akt phosphorylation (pAkt, Ser473) in BBB monolayers 10 to 60 minutes after OGD with or without PS and with and without Tyro3 (N-terminus) blocking antibody. Graph: relative abundance of pAkt signal intensity normalized by total Akt. (H) IP of S1P1 with anti-S1P1 antibody followed by IB with antiphosphothreonine or anti-S1P1 antibodies in BBB monolayers pretreated for 1 hour with vehicle or LY294002 (10μM) followed by OGD and PS treatment for 10 minutes. In all studies, human PS was used at 100nM. Mean ± SEM, from 3 to 6 independent cultures.
Protein S mediates Rac1-dependent barrier protection and activates S1P1 after Tyro3 ligation in OGD-treated human BBB monolayers. (A) Time course of Rac1 activation (Rac1-GTP) in human BBB monolayers in vitro after OGD (0-60 minutes) with and without PS (100nM). Graph: relative abundance of Rac1-GTP signal intensity normalized by Rac1 (0-120 minutes). P < .01, OGD + PS versus OGD − PS. (B-C) Permeability to FITC-dextran (B) and TER (C) in BBB monolayers 2 hours after OGD and treatment with PS (100nM) after transduction with Ad.Rac1 and Ad.GFP, transfection with S1P1 siRNA and control siRNA, and incubation with S1P1-specific antagonist W146 (50nM). (D) Immunoprecipitation (IP) of S1P1 with anti-S1P1 antibody followed by immunoblotting (IB) with antiphosphothreonine antibody (pThr) in BBB monolayers 10 to 60 minutes after OGD and treatment with PS with and without anti-Tyro3 (N-terminus) antibody or nonimmune IgG. (E) IP of S1P1 with anti-Tyro3 (C-terminus) and control nonimmune IgG in BBB monolayers 10 minutes after OGD and treatment with PS (100nM) with and without control antibody or anti-Tyro3 (N-terminus) ligand-binding domain–blocking antibody. (F) IP of Tyro3 with anti-S1P1 antibody and control nonimmune IgG in BBB monolayers 10 minutes after OGD and PS (100nM) treatment with and without control antibody or anti-Tyro3 (N-terminus) blocking antibody. (G) Akt phosphorylation (pAkt, Ser473) in BBB monolayers 10 to 60 minutes after OGD with or without PS and with and without Tyro3 (N-terminus) blocking antibody. Graph: relative abundance of pAkt signal intensity normalized by total Akt. (H) IP of S1P1 with anti-S1P1 antibody followed by IB with antiphosphothreonine or anti-S1P1 antibodies in BBB monolayers pretreated for 1 hour with vehicle or LY294002 (10μM) followed by OGD and PS treatment for 10 minutes. In all studies, human PS was used at 100nM. Mean ± SEM, from 3 to 6 independent cultures.
Because sphingosine 1-phosphate receptor 1 (S1P1), the endothelial barrier–enhancing receptor for sphingosine 1-phosphate (S1P), mediates endothelial barrier protection by activating Rac1-dependent cytoskeletal rearrangement,30,33 we next studied whether S1P1 is involved in PS-mediated vasculoprotection. S1P1 enhances endothelial barriers when stimulated by its biologic ligand S1P29 or after transactivation after ligation of endothelial protein C receptor33 or growth factor receptors.37 Thus, we studied whether brain endothelial barrier protection by PS-mediated activation of Tyro3 involves crosstalk between Tyro3 and S1P1. Immunoprecipitation of S1P1 from endothelial monolayers after OGD challenge, with and without PS (100nM) and/or blockade of the Tyro3 N-terminus domain followed by antiphosphothreonine immunoblotting, provided evidence for PS-mediated Tyro3-dependent Thr phosphorylation of S1P1 (Figure 3D). In a similar experiment, we did not detect any PS-mediated increase in Ser phosphorylation of S1P1 (not shown). These results are consistent with Thr236 phosphorylation and S1P1 activation in response to S1P.29 Immunoprecipitation by Tyro3 followed by immunoblotting for S1P1 of cell lysates derived from BECs subjected to OGD and treated by PS (100nM) showed coimmunoprecipitation of S1P1, suggesting that PS promoted a physical interaction between Tyro3 and S1P1 (Figure 3E). Furthermore, the reverse S1P1 immunoprecipitation followed by immunoblotting for Tyro3 of the same cell lysates used in Tyro3 immunoprecipitation showed coimmunoprecipitation of Tyro3, confirming that PS promoted the physical interaction between Tyro3 and S1P1 (Figure 3F). Normoxia or OGD did not affect S1P1 phosphorylation (Figure 3D lanes 1-2) and/or S1P1 interaction with Tyro3 (supplemental Figure 2B). As for Tyro3, S1P1 expression was abundant at the basolateral side (Figure 2F).
The phosphoinositide-3 kinase inhibitor LY294002 inhibited S1P1 phosphorylation, suggesting involvement of the PS-Tyro3-Akt pathway (Figure 3H). As expected, Akt was rapidly activated by PS, which was inhibited by Tyro3 antibody (Figure 3G). pAkt remained increased with PS at 2 hours (not shown) comparable with the levels determined at 1 hour (Figure 3G). S1P1-specific siRNA inhibited by more than 90% S1P1 expression (supplemental Figure 2C), resulting in losses of PS-mediated protection of the endothelial barrier integrity (Figure 3B-C) and its blocking effect on OGD-induced time-dependent reductions in activated Rac1 (not shown). S1P1 siRNA transfection did not alter permeability or TER of BEC monolayers (Figure 3B-C). W146, a S1P1-specific antagonist,38 blocked PS-mediated normalization of barrier permeability to dextran (Figure 3B) and TER (Figure 3C).
Protein S prevents postischemic BBB leakage via Tyro3 and S1P1
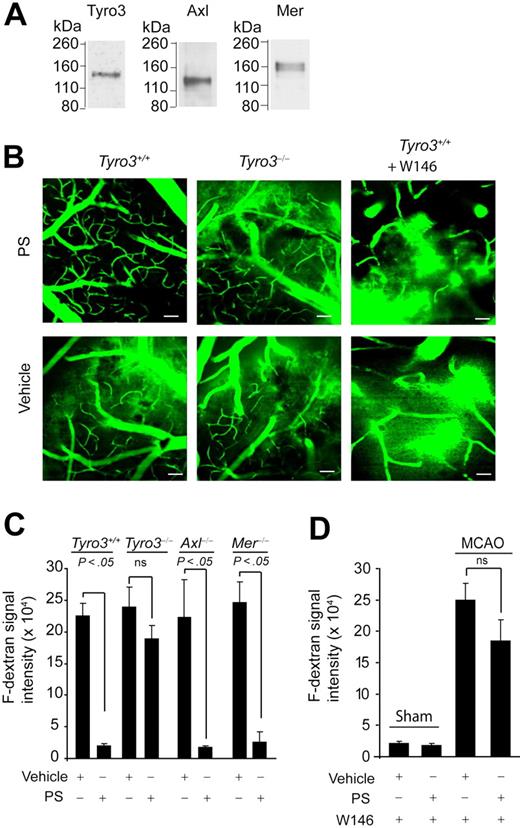
Next, we studied the effects of mouse recombinant PS on ischemic BBB disruption in a transient middle cerebral artery occlusion (MCAO) model36 in control mice and Tyro3−/−, Axl−/−, and Mer−/− mice. The wild-type Tyro3+/+ mice and Tyro3−/−, Axl−/−, and Mer−/− mice treated with PS had no significant differences in blood gasses (ie, PaO2 and PaCO2, pH, hematocrit, or mean arterial blood pressure before) during and 1 hour after the MCAO compared with their respective vehicle-treated controls (supplemental Table 1). Wild-type mice expressed all 3 TAM receptors in cerebral microvessels as demonstrated by immunoblotting (Figure 4A). BBB disruption after ischemia was assessed by in vivo 2-photon imaging of the leakage of F-dextran from the cortical microvessels. As explained in “Transient MCAO,” the leakage of F-dextran from blood vessels in wild-type Tyro3+/+ mice subjected to 1-hour MCAO was most pronounced at 8 hours of reperfusion (Figure 4B). Quantification analysis of F-dextran signal intensity outside the blood vessels indicated approximately 100-fold increase in F-dextran leakage across the BBB in the periphery of the infraction in the cortical layer II in vehicle-treated Tyro3+/+ mice subjected to 1-hour MCAO and 8-hour reperfusion (Figure 4C) compared with an intact BBB and no leakage in sham-operated Tyro3+/+ mice treated with vehicle (supplemental Figure 3A; Figure 4D).
PS prevents postischemic BBB leakage in vivo via Tyro3 and S1P1. (A) Immunoblotting of Tyro3, Axl, and Mer in mouse brain microvessels. (B) Two-photon in vivo imaging of fluorescein-conjugated dextran (F-dextran; MW = 70 000 kDa) leakage from cortical cerebral microvessels (layer II) in Tyro3+/+ wild-type mice (left), Tyro3−/− mice (middle), and Tyro3+/+ mice pretreated with W146 (right) after 1-hour transient middle cerebral artery occlusion (MCAO) and 8-hour reperfusion. Murine PS (0.2 mg/kg) or saline was administered via the femoral vein 10 minutes after the MCAO. W146 was administered intraperitoneally 10 minutes before the MCAO. Bar (left and middle panels) represents 50 μm. Bar (right panels) represents 25 μm. (C-D) Quantification of F-dextran extravascular signal intensity (leakage) in brain parenchyma after 1-hour MCAO and 8-hour reperfusion in Tyro3+/+, Tyro3−/−, Axl−/−, and Mer−/− mice treated with vehicle or PS (C) and Tyro3+/+ mice pretreated with W146 and treated with vehicle or PS (D). Sham-operated controls (mice that were not subjected to stroke) receiving W146 are also shown in panel D. PS, saline, and W146 were administered as in panel B. Mean ± SEM; n = 6 mice per group.
PS prevents postischemic BBB leakage in vivo via Tyro3 and S1P1. (A) Immunoblotting of Tyro3, Axl, and Mer in mouse brain microvessels. (B) Two-photon in vivo imaging of fluorescein-conjugated dextran (F-dextran; MW = 70 000 kDa) leakage from cortical cerebral microvessels (layer II) in Tyro3+/+ wild-type mice (left), Tyro3−/− mice (middle), and Tyro3+/+ mice pretreated with W146 (right) after 1-hour transient middle cerebral artery occlusion (MCAO) and 8-hour reperfusion. Murine PS (0.2 mg/kg) or saline was administered via the femoral vein 10 minutes after the MCAO. W146 was administered intraperitoneally 10 minutes before the MCAO. Bar (left and middle panels) represents 50 μm. Bar (right panels) represents 25 μm. (C-D) Quantification of F-dextran extravascular signal intensity (leakage) in brain parenchyma after 1-hour MCAO and 8-hour reperfusion in Tyro3+/+, Tyro3−/−, Axl−/−, and Mer−/− mice treated with vehicle or PS (C) and Tyro3+/+ mice pretreated with W146 and treated with vehicle or PS (D). Sham-operated controls (mice that were not subjected to stroke) receiving W146 are also shown in panel D. PS, saline, and W146 were administered as in panel B. Mean ± SEM; n = 6 mice per group.
Intravenous administration of mouse PS (0.2 mg/kg) 10 minutes after the transient MCAO abolished dextran leakage after ischemia in control Tyro3+/+ mice, but not in Tyro3−/− mice (Figure 4B-C). Systemic administration of W146, a S1P1-specific antagonist (1 mg/kg intraperitoneally),38 blocked almost completely PS-mediated protection of the BBB integrity (Figure 4B,D), but did not alter BBB permeability in sham-operated Tyro3+/+ controls treated with either vehicle or PS (supplemental Figure 3B-C; Figure 4D). This finding was consistent with a previous report showing that low-dose W146 does not affect basal S1P1 receptor tone and endothelial capillary permeability.38 Vehicle-treated Axl−/− and Mer−/− mice exhibited a similar degree of ischemia-induced dextran leakage as vehicle-treated Tyro3+/+ or Tyro3−/− mice (Figure 4C). PS treatment, however, eliminated the BBB breakdown in both Axl−/− and Mer−/− mice in contrast to Tyro3−/− mice (Figure 4C).
Protein S improves neurologic and neuropathologic outcome after transient ischemia via Tyro3 and S1P1
Mice were next evaluated 24 hours after the MCAO for neurologic and neuropathologic outcome. PS improved motor neurologic score and reduced the infarct size and edema (swelling) in Tyro3+/+, Axl−/−, and Mer−/− mice, but not in Tyro3−/− mice (Figure 5A-C) or Tyro3+/+ mice treated with W146 (Figure 5D-F). PS reductions of the infarction volume and edema in wild-type and Axl−/− and Mer−/− mice correlated well with the respective improvements in the neurologic motor scores. Reductions in the infarct volume in wild-type Tyro3+/+ mice were also seen with PS therapy over longer periods of time, as for example within 7 days of stroke (Y.W. and B.V.Z., unpublished observations, March 20, 2009).
Effects of protein S on neurologic and neuropathologic outcome in Tyro3, Axl, and Mer mutants after transient MCAO. Motor neurologic score, infarct volume, and edema volume (swelling) in Tyro3+/+ and Tyro3−/−, Axl−/−, and Mer−/− mice treated with vehicle or PS and subjected to 1-hour MCAO and 23-hour reperfusion (A-C), and in Tyro3+/+ mice pretreated with W146 and sham-operated or subjected to 1-hour MCAO and 23-hour reperfusion and treated with vehicle or PS (D-F). The studied mice were the same as in Figure 4. Murine PS (0.2 mg/kg) or saline was administered via the femoral vein 10 minutes after the MCAO. (G) PS cerebrospinal fluid levels (nM) in wild-type mice 1 hour after intravenous administration of PS (0.2 mg/kg) or vehicle. Mean ± SEM; n = 6 mice per group. (H) A representative confocal scanning analysis of lectin-positive endothelium (red), Tyro3 immunodetection in the capillary endothelium (green), and aquaporin-4–positive astrocyte foot processes (blue) in brain in situ in a control mouse. Merged: yellow. Bar represents 4 μm. Chart: Tyro3 relative signal intensity (green) plotted over the endothelial-specific lectin signal intensity (red). A indicates abluminal side; L, luminal side.
Effects of protein S on neurologic and neuropathologic outcome in Tyro3, Axl, and Mer mutants after transient MCAO. Motor neurologic score, infarct volume, and edema volume (swelling) in Tyro3+/+ and Tyro3−/−, Axl−/−, and Mer−/− mice treated with vehicle or PS and subjected to 1-hour MCAO and 23-hour reperfusion (A-C), and in Tyro3+/+ mice pretreated with W146 and sham-operated or subjected to 1-hour MCAO and 23-hour reperfusion and treated with vehicle or PS (D-F). The studied mice were the same as in Figure 4. Murine PS (0.2 mg/kg) or saline was administered via the femoral vein 10 minutes after the MCAO. (G) PS cerebrospinal fluid levels (nM) in wild-type mice 1 hour after intravenous administration of PS (0.2 mg/kg) or vehicle. Mean ± SEM; n = 6 mice per group. (H) A representative confocal scanning analysis of lectin-positive endothelium (red), Tyro3 immunodetection in the capillary endothelium (green), and aquaporin-4–positive astrocyte foot processes (blue) in brain in situ in a control mouse. Merged: yellow. Bar represents 4 μm. Chart: Tyro3 relative signal intensity (green) plotted over the endothelial-specific lectin signal intensity (red). A indicates abluminal side; L, luminal side.
To determine whether systemically administered PS can cross the BBB, we injected intravenously wild-type mice with PS at the same dose (0.2 mg/kg) that was protective in a stroke model, and determined PS levels in the cerebrospinal fluid (CSF). Compared with vehicle, PS CSF levels were increased by 9-fold (from 4 to 36nM) within 1 hour of systemic PS administration (Figure 5G), indicating PS transport across the BBB. Of note, systemically administered PS achieved the CSF levels corresponding to its concentrations that were protective in a human in vitro model of the BBB (Figure 1). A concentration-dependent transport of PS across the mouse BBB consistent with the presence of a carrier- or receptor-mediated BBB transporter, as reported for several proteins and peptides,27 has been confirmed independently by measuring radiolabeled PS entry into the brain (R.D., B. LaRue, A.S., and B.V.Z., unpublished observations, May 11, 2009).
Discussion
The present study shows that PS protects the BBB from hypoxic/ischemic damage, acting as a Tyro3 ligand both in vitro and in vivo. In cultured brain endothelium, PS activates the barrier-protective S1P1 receptor after ligation of Tyro3, resulting in protection of the BBB integrity through Rac1-dependent cytoskeletal rearrangements.29,31,33 In vivo, S1P1 is also required for PS-mediated cerebral vasculoprotection as shown after transient ischemia. By “sealing” the BBB, PS can protect the brain internal environment from peripheral systemic influences such as those after an acute ischemic stroke or a chronic perfusion stress.
The normal neuronal-vascular relationship is critical for normal brain functioning.27 It has been estimated that nearly every neuron in human brain has its own capillary. The BBB is a highly specialized brain endothelial structure that normally limits the entry of plasma components, red blood cells, and leukocytes into the brain. If they cross the BBB due to an ischemic injury, intracerebral hemorrhage, trauma, neurodegenerative process, inflammation, or vascular disorder, this typically generates neurotoxic products in brain that can compromise synaptic and neuronal functions.27 Moreover, the BBB maintains the chemical composition of the neuronal “milieu,” which is required for proper functioning of neuronal circuits, synaptic transmission, synaptic remodeling, angiogenesis, and neurogenesis in the adult brain. BBB breakdown, due to disruption of the tight junctions, altered expression of proteins that act to transport molecules across the BBB, aberrant angiogenesis, vessel regression, brain hypoperfusion, and inflammatory responses, may initiate and/or contribute to a “vicious circle” of the disease process, resulting in progressive synaptic and neuronal dysfunction and loss as seen in complex neurologic disorders such as Alzheimer disease,27,39-41 amyotrophic lateral sclerosis,42-44 Parkinson disease, or multiple sclerosis.27 These recent findings support developments of new treatments for chronic neurodegenerative disorders directed at the BBB and/or the neurovascular unit. Present data suggest that one of such potential new therapies could be protein S.
PS also has direct neuronal-protective activities.14 The exact contributions of PS-mediated vasculoprotection versus direct neuronal protection in controlling ischemic brain injury in vivo are presently unclear. Our work in progress indicates that Tyro3 is required for PS-mediated protection of cultured mouse cortical neurons against the excitotoxic injury, whereas S1P1 does not participate in PS-mediated direct neuronal protection in vitro (Z.Z. and B.V.Z., unpublished observations, June 30, 2009). Future studies using transgenic mouse models with specific deletions of Tyro3 and S1P1 from brain endothelium and neurons should evaluate the exact roles of Tyro3-S1P1–mediated BBB protection and Tyro3-mediated neuronal protection in the overall beneficial effects of PS therapy on neurologic and neuropathologic outcome in stroke and possibly other neurologic disorders. These transgenic models are currently not available.
Axl is considered the key TAM receptor in vascular cells.1 However, PS does not interact with Axl in endothelium. The ability of PS to control an ischemic BBB leakage in Axl-null mice would argue against the importance of Axl in PS-mediated vasculoprotection. However, one cannot rule out a possibility that PS-mediated vasculoprotection in Axl-null mice is not in part mediated by PS direct protective effects on neurons, given that Tyro3 is the major TAM receptor in neurons,15,24 and is expressed in Axl mutants. Again, the exact roles of Tyro3 in brain endothelium and neuronal cells in protecting the BBB integrity remain to be addressed by future studies in transgenic mice, with specific deletions of Tyro3 from endothelium and neurons that are currently unavailable.
Collectively, our data imply that PS protects the BBB integrity via Tyro3 and S1P1, suggesting potentially novel treatments for neurovascular dysfunction after stroke and in neurologic disorders associated with the central nervous system perfusion stress and chronic ischemic BBB injury.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr G. Lemke for providing mice lacking Tyro3, Axl, and Mer receptors and for his most useful comments. We also thank Dr J. H. Griffin for providing mouse recombinant PS.
This work was supported by National Institutes of Health grant HL81528 (B.V.Z.).
National Institutes of Health
Authorship
Contribution: D.Z. contributed to project planning, and performed human BEC in vitro experiments, BBB functional assays, ex vivo Western blotting, immunostaining studies, and data analysis; Y.W. contributed to project planning, and performed in vivo experiments with the MCAO model, in vivo 2-photon imaging, and data analysis; R.D.B. performed pS1p1 and pAkt analysis and assisted with data analysis; I.S. contributed to project planning and to vitro Tyro3 and Rac1 analysis; R.D. contributed to project planning and analysis of data; Z.Z. performed the mouse BEC survival study and data analysis; A.S. performed Western blot analysis of TAM receptors in brain microvessels and analyzed protein S levels in mouse CSF; E.A.W. participated in immunofluorescent staining and confocal analysis; and B.V.Z. designed the entire study, supervised all parts of the study, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Berislav V. Zlokovic, Center for Neurodegenerative and Vascular Brain Disorders, 601 Elmwood Ave, Box 670, Rochester, NY 14642; e-mail: berislav_zlokovic@urmc.rochester.edu.
References
Author notes
D.Z., Y.W., and I.S. contributed equally to this work.