Abstract
Among risk factors for developing thromboembolism (VTE) in children with acute lymphoblastic leukemia were Escherichia coli asparaginase, concomitant steroid use, presence of central venous lines, and thrombophilic abnormalities. Developing a predictive model for determining children at increased risk would be beneficial in targeting interventional studies to high-risk groups (HRGs). Predictive variables were incorporated into a risk assessment model, which was evaluated in 456 children and then validated in 339 patients. VTE risk by score was no greater than 2.5 for low-risk group (LRG) and greater than 2.5 for HRG. VTE rates at 3.5 months (validation cohorts) were 2.5% in LRG and 64.7% in HRG. In multivariate analysis adjusted for age, duration of asparaginase administration, enoxaparin prophylaxis, and T-immunophenotype, the HRG was significantly associated with VTE compared with the LRG (hazard/95% confidence interval [CI], 8.22/1.85-36.53). Model specificity was 96.2% and sensitivity was 63.2%. As secondary objective we investigated the use of enoxaparin for VTE prophylaxis in the HRG. HRG patients without enoxaparin prophylaxis showed a significantly reduced thrombosis-free survival compared with children on low-molecular-weight heparin (LMWH). On the basis of the high specificity, the model may identify children with leukemia at risk of VTE. LMWH may help prevent VTE in the HRG; this warrants assessment in larger cooperative clinical trials.
Introduction
Children with acute lymphoblastic leukemia (ALL) are at increased risk of venous thromboembolism (VTE); however, not all children experience VTE. The prevalence of symptomatic VTE depends on the treatment protocols and range from 0% to 36%.1-3 A recent meta-analysis of prospective studies in children with ALL identified 4 potential risk factors for VTE in this population2 : (1) treatment with Escherichia coli asparaginase (ASP), (2) concomitant use of steroids, (3) presence of central venous lines (CVLs),4,5 and (4) thrombophilic genetic abnormalities. In this meta-analysis the overall risk of symptomatic thrombosis was 5.2%, with the highest risk reported in children receiving E coli ASP concomitant with prednisone (PDN).2 Developing a predictive model to determine children at increased risk would be of benefit in targeting interventional studies to only high-risk groups (HRGs).
The aim of the present international multicenter study was (1) to develop and to validate a simple model for predicting ALL chemotherapy–associated VTE with the use of baseline clinical and laboratory variables and (2) to evaluate, on an explorative basis, the increasing off-label use of enoxaparin for VTE prophylaxis in children with ALL.
Methods
Patients
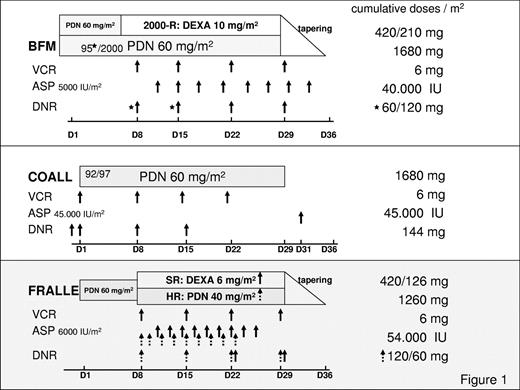
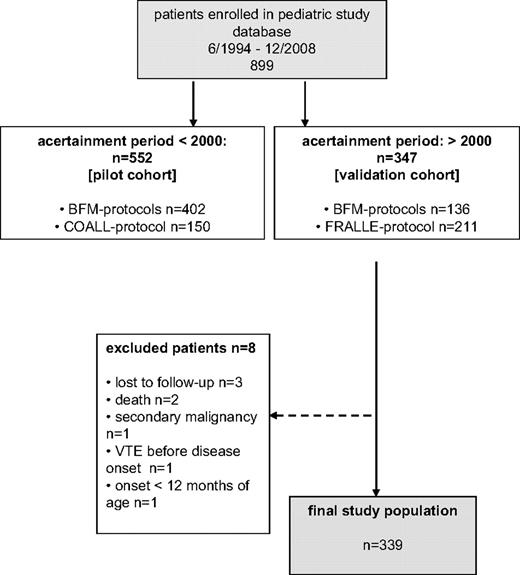
With parental consent, 899 children older than 12 months who had acute onset of ALL treated according to the Berlin-Frankfurt-Münster (BFM) 90/95/2000, the Cooperative Acute Lymphoblastic Leukemia (COALL) 92/97, and the French Acute Lymphoblastic Leukemia (FRALLE) 2000 induction protocols between 1994 and December 2008 were included in this multicenter analysis.6-9 All children with ALL enrolled in the different treatment protocols received the same E coli ASP, originally distributed by Kyowa, Hakko, Kyogo, Japan. Relevant thrombosis-prone drugs, days of administration, single and cumulative doses stratified according to the ALL induction protocols are depicted in Figure 1.6-9 Depending on the clinical practice at the participating centers, polychemotherapy was administered by central venous lines, that is, Broviac, or port catheters implanted within the first weeks of therapy. Subjects lost to follow-up, without parental consent, without complete remission of the disease and children with concomitant chronic diseases, hepatic failure, or severe septicemia were excluded from the analysis (n = 96).
Thromboses-prone drugs, administration days, single and cumulative doses according to different ALL-induction protocols are shown. In the BFM-95 protocol (*) DNR was administered twice (days 8 and 15), and in the 2000 study on days 8, 15, 22, and 29. In addition, in the BFM 2000 protocol PDN and DEXA were randomized. In the FRALLE 2000 HRG (dashed arrow), children received PDN instead of DEXA, and ASP was started on day 8 instead of day 10. ALL indicates acute lymphoblastic leukemia; ASP, Escherichia coli asparaginase; D, day; DEXA, dexamethasone; DNR, daunorubicin; HR, high risk; PDN, prednisone; SR, standard risk; and VCR, vincristine.
Thromboses-prone drugs, administration days, single and cumulative doses according to different ALL-induction protocols are shown. In the BFM-95 protocol (*) DNR was administered twice (days 8 and 15), and in the 2000 study on days 8, 15, 22, and 29. In addition, in the BFM 2000 protocol PDN and DEXA were randomized. In the FRALLE 2000 HRG (dashed arrow), children received PDN instead of DEXA, and ASP was started on day 8 instead of day 10. ALL indicates acute lymphoblastic leukemia; ASP, Escherichia coli asparaginase; D, day; DEXA, dexamethasone; DNR, daunorubicin; HR, high risk; PDN, prednisone; SR, standard risk; and VCR, vincristine.
Potential risk factors for VTE in children with ALL were defined according to the meta-analysis by Caruso et al.2 The predictive variables were incorporated in a risk assessment model and applied to consecutively admitted children with ALL (BFM and COALL and FRALLE) as follows: (1) induction before phase with steroids (PDN), treatment with E coli ASP in combination with steroids (PDN or dexamethasone [DEXA]), (2) presence of CVLs, and (3) genetic thrombophilic abnormalities, for example, positive family history for VTE, or identification of a single thrombophilic trait, or carrier status of combined thrombophilic traits. Acquired deficiency states of antithrombin, protein C, or protein S were not included in the model presented here. According to protocol differences during ALL induction therapy (Figure 1) the minimum-maximum scores to be achieved per protocol were as follows (Table 1): BFM (PDN 60 mg/m2 on days 1 to 7, followed by ASP and PDN [90/95 protocol], or followed by ASP and DEXA; 2000 protocol: DEXA arm) 0.5 to 4.0, COALL (no concomitant administration of ASP and steroids) 0 to 3.0, and FRALLE (PDN 60 mg/m2 on days 1 to 7, followed by ASP and DEXA 6 mg/m2 [standard risk] and ASP and PDN 40 mg/m2 [high risk; HR]) 0.5 to 3.5. In addition, risk factors for thrombosis in children with ALL include older age and T immunophenotype.10,11
The score was initially investigated in a database pilot cohort of 456 consecutively enrolled children with ALL treated according to the BFM 92/95 and COALL 92/97 protocols6,7 and then validated in a cohort of 339 newly recruited children with ALL (BFM 2000 and FRALLE 2000).6,8,9 On the basis of the receiver operating characteristic (ROC) report generated in the pilot cohort, the cutoff value with the highest accuracy to discriminate between patients at risk was chosen: The VTE risk by score was low (≤ 2.5) and high (> 2.5). The study flow chart is depicted in Figure 2.
Study endpoints
Patients were prospectively followed over a 3.5-month period. In both cohorts, pilot and validation, the diagnosis of symptomatic VTE during ALL induction therapy was defined as the primary endpoint of this study. VTE was diagnosed clinically and confirmed by standard imaging methods.5 In the validation cohort (Münster and Nuremberg: BFM), the secondary study objective (explorative basis) was to calculate the probability of thrombosis-free survival (TFS) in children with ALL scored as HR with and without enoxaparin prophylaxis (1 mg/kg of body weight per day) during ALL induction. The former was administered on an individual patient basis administered by the treating physicians in the participating study centers. During induction therapy start of low-molecular-weight heparin (LMWH) prophylaxis in high-risk patients was scheduled before CVL insertion. Stopping rules included platelet count less than 20 × 109/L (20 000/μL; restart in case of platelet transfusion). Duration of LMWH prophylaxis was aimed until the end of induction therapy.
Laboratory analyses
In the German BFM and COALL cohorts, FII G20210A and FV G1691A mutations, antithrombin, protein C, and protein S were investigated centrally in Münster with the use of standard laboratory techniques.12 Plasma-based coagulation assays were measured on an ACL 9000 (Instrumentation Laboratory, Munich).12,13 In the French cohort investigation was limited to the FII G20210A variant, factor V G1691A mutation, and antithrombin. In France antithrombin activity was measured locally in each child during the course of ALL treatment, and frozen plasma samples were sent to a specialized coagulation laboratory only if a deficiency state was suspected. In all patients an inherited type I deficiency (antithrombin, protein C) was diagnosed whenever functional plasma activity and immunologic antigen concentrations of a protein were repeatedly shown below 50% of the normal age-related reference range determined in the central laboratory for each assay and age group (reference ranges are no different from the pediatric literature).14 A type II deficiency (antithrombin, protein C) was diagnosed in patients with repeatedly low functional activity along with normal antigen concentrations. The diagnosis of protein S deficiency was based on reduced free protein S antigen levels combined with decreased or normal total protein S antigen concentrations, respectively. Apart from the classification based on age-dependent normal reference ranges and confirmation of a suspected protein-based prothrombotic defect in a second plasma sample (3-6 months later and during ALL maintenance therapy), the presence of a hemostatic defect in at least one first-degree relative or the identification of a causative gene mutation were considered confirming criteria for its hereditary nature.
Statistics
All statistical analyses were performed with the StatView 5 software package (SAS Institute) and MedCalc (Version 11.1.1; Mariakerke). With use of a rule of thumb for proportional hazards analysis, including approximately 10 outcomes for each independent predictor, we have included only 3 predictors in our model (34 VTE events in the pilot cohort).15 Continuous data are presented as median (minimum-maximum: min-max) values. For categorical data, frequency distributions were compared between groups with the chi-square test or Fisher exact test, as appropriate. The time to symptomatic VTE, calculated as the probability of TFS as a function of time was determined with the method of Kaplan and Meier (univariate analysis). The log-rank test was used to test for differences in TFS between groups. The criterion for statistical significance was set at alpha = 0.05. P values were based on the 2-sided test. To evaluate the contribution to the risk of VTE (primary study objective) multivariate analysis that used Cox proportional hazards modeling, with the primary predictor variable consisting of the type of risk score (≤ 2.5 or > 2.5) was performed. Adjustment variables included age at ALL onset, number of ASP received, enoxaparin prophylaxis (yes vs no), the source of CVL, and T immunophenotype (yes vs no).10 Adjusted risks were expressed as hazard ratios (HRs) together with 95% CIs. The degree of agreement beyond chance between first and second reader (scoring of ALL cohorts by authors; BFM: M.F. and M.L.; COALL: C.B.; FRALLE: B.P.) was measured with the κ statistics. The accuracy of the score to discriminate between patients at VTE risk from children without was evaluated with the ROC curve analysis generated by MedCalc. The true positive rate (sensitivity) was plotted in function of the false positive rate (100-specificity) for different cutoff points. In addition, negative and positive predictive values and numbers needed to treat (NNT) were calculated as previously described.16
Ethics
The present multicenter study was performed in accordance with the ethical standards laid down in a relevant version of the 1964 Declaration of Helsinki and approved by the medical ethics committee at the Westfälische Wilhelms-University, Münster, Germany. Appropriate research ethics approval was obtained at each site to include data of consecutively enrolled patients in France (clinical data made anonymous from ALL study database).
Results
Patient population
From the 899 children with ALL enrolled in the study database in Germany, 456 of 552 (83.5%) were available for scoring in the pilot cohort and 339 of 347 (97.7%) in the validation cohort. Children with ALL had a median age of 5.9 years (minimum-maximum, 1-18 years), 53% were male. The distribution of risk scores, the corresponding number of VTE events, and the onset of VTE in the pilot and validation cohort are shown in Table 2. The degree of agreement beyond chance between first and second reader measured with the κ statistics was 97.2% (κ, 0.88; 95% CI, 0.83-0.94; z = 31.52; P < .001). In both cohorts the rate of symptomatic VTE during ALL induction therapy was comparable (P = .41) with a significantly higher rate in patients in the high-risk score group (P < .001).
Primary study objective: validation cohort
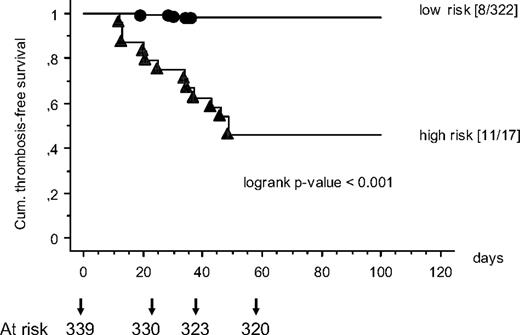
The final study population, that is, the validation cohort, included 339 children with ALL, 136 treated according to the BFM 2000 study protocol and 203 included in the FRALLE 2000 study. The locations of symptomatic VTE in the validation cohort were as follows: cerebral sinovenous thromboses 10 children (52.6%), pulmonary embolisms were diagnosed in 2 patients (10.5%) and central line–associated thrombosis starting at the CVL implantation site were found in 7 (36.8%; superior caval vein, 4 of 7; internal jugular vein, 2 of 7; subclavian vein, 1 of 7). Interestingly in the FRALLE HRG (ASP and PDN 40 mg/m2) no symptomatic thrombosis had occurred. The probability of TFS as a function of time is shown in Figure 3. Children with ALL grouped above the score of 2.5 showed a significantly reduced TFS compared with children with a risk score less than 2.5 (P < .001 log-rank test).
The probability of TSF as a function of time is shown. Children with ALL grouped above the score of 2.5 (▲) showed a significantly reduced thrombosis-free survival (TFS) than children with a risk score less than 2.5 (●).
The probability of TSF as a function of time is shown. Children with ALL grouped above the score of 2.5 (▲) showed a significantly reduced thrombosis-free survival (TFS) than children with a risk score less than 2.5 (●).
Multivariate analyses
Multivariate analysis (Cox-regression) adjusted for age at ALL onset, number of ASP administrations, source of CVLs (Broviac vs Port), concomitant enoxaparin prophylaxis (yes vs no), and the presence of the T immunophenotype are shown in Table 3. Cox regression analysis showed that children with a risk score greater than 2.5 had a significantly increased risk of symptomatic VTE in the validation cohort (hazard ratio, 8.22; 95% CI, 1.85-36.53).
Sensitivity and specificity analyses, negative and positive predictive values
As in the pilot cohort, the ROC report generated showed that the cutoff score of greater than 2.5 had the highest accuracy to discriminate between patients at risk. For this cutoff sensitivity was 63.2% (95% CI, 38.4%-83.7%), and specificity was calculated as 96.2% (95% CI, 93.6%-98.0%). The negative predictive value to rule out an increased risk of symptomatic VTE in children with ALL was 97.7%, and the positive predictive value in this setting was 49.7%,.
Secondary study objective: enoxaparin prophylaxis (explorative basis: both study cohorts)
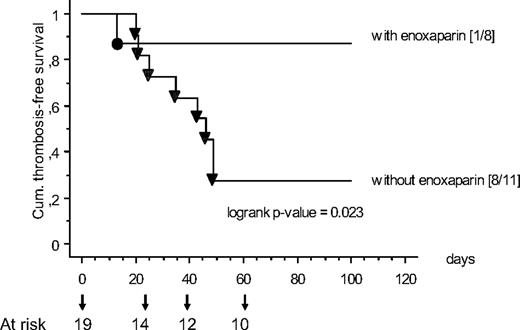
In children treated in Münster and Nuremberg, because of the individual decisions of the treating physicians 8 of 19 children with ALL consecutively scored greater than 2.5 received the first enoxaparin administration for VTE prophylaxis before CVL implantation during induction therapy. In each case LMWH was stopped at the end of ALL induction. In Figure 4 the TFS in children with enoxaparin prophylaxis (HR score only) compared with patients without prophylaxis is shown: Children with ALL without enoxaparin prophylaxis showed a significantly reduced TFS compared with children with prophylactic LMWH (P = .023 log-rank test). No bleeding events were observed. On the basis of this analysis the NNT to prevent one symptomatic VTE in children with a high-risk score was 2 (95% CI, 1-4).
The TFS in children with enoxaparin prophylaxis compared with patients without prophylaxis is shown. Children with ALL without prophylaxis (▼) showed a significantly reduced TFS than children with prophylactic low-molecular-weight heparin (LMWH; ●).
The TFS in children with enoxaparin prophylaxis compared with patients without prophylaxis is shown. Children with ALL without prophylaxis (▼) showed a significantly reduced TFS than children with prophylactic low-molecular-weight heparin (LMWH; ●).
Discussion
In the present observational cohort study we identified as primary study endpoint a clinical and laboratory risk score that was independently predictive of symptomatic VTE in children treated according to ALL. Apart from leukemia induction therapy, the risk assessment score included also te presence of CVLs and thrombophilic genetic abnormalities.2,17-20 The parameters were shown in a risk assessment model (Table 1) that allows treating physicians to classify VTE risk in children with ALL. In an independent pediatric patient cohort the model was validated. In this validation cohort the rate of symptomatic VTE during the 3.5-month follow-up was 64.7% in children scored in the HRG, and 2.5% in children in the low-risk group. The negative predictive value to rule out an increased risk of symptomatic VTE in children during ALL induction therapy was 97.7%.
The importance of treatment in the pathogenesis of ALL-related VTE is indicated by the observation that VTEs rarely occur at diagnosis and are detected almost exclusively during treatment with 90% of cases found during induction1,2,17 and 10% during consolidation or therapy intensification protocols.2,3
When ASP and corticosteroids are used separately, the risk of thrombosis is low, but when used in combination the risk increased 8- to 10-fold.2,9,17,20 This was not only shown by data from a nonconcurrent controlled study comparing risk of symptomatic thrombosis in German children on the COALL 92/97 protocols (1.5%) and those treated on BFM 90/95 (11%),21-23 but are inline with data from the Dana-Farber Protocol, whereby ASP was administered during consolidation: occurrence of VTE was restricted to the consolidation phase.24 In the present validation cohort, most children with thrombosis treated according to BFM protocols manifested between days 13 and 36, and 3 of them had thrombosis starting on days 43,46, and 49, 3 to 5 days after CVL implantation.
Protocol/treatment differences also have an effect on the VTE risk associated with inherited thrombophilia.13,23 Although the overall prevalence of prothrombotic defects was no different in the populations at risk (BFM vs COALL), patients with symptomatic VTE had a higher association between VTE and thrombophilia in the BFM cohort but not the COALL cohort.23 For the combined population, only the concurrent administration of ASP and PDN in children with a genetic thrombophilic risk was found to increase the risk of a thrombotic event (odds ratio, 34.5; 95% CI, 4.39-271.42).23 As outlined in Figure 1, there was no difference in cumulative doses of vincristine (6 mg), or daunorubicin (DNR; BFM/FRALLE, 60/120 mg) in the BFM-adapted treatment protocols. Interestingly, however, children treated in the COALL protocol received the highest cumulative dose of DNR (144 mg), thereby developing the lowest rate of thrombosis, that is, 2.5%,23 suggesting that there was no association between thrombosis onset and DNR administration. Furthermore, cumulative doses of PDN were identical in BFM and COALL study protocols, whereas in the FRALLE 2000 HRG the cumulative dose of PDN was 25% lower.9 Concomitant with PDN, children treated within the BFM study groups received a 25% lower cumulative dose of ASP than did patients treated according to the FRALLE protocol.6,9 In contrast, in the COALL protocol ASP and PDN were not administered together.7 Thus, the risk assessment model developed by us weighted the induction with PDN 60 mg/m2 followed by the combination of ASP and PDN until day 30 of the induction protocol with 1 point compared with 0 points in the COALL cohort (no concomitant ASP and PDN). The FRALLE induction therapy applied ASP and DEXA 6 mg/m2 (standard risk) and ASP with PDN 40 mg/m2 (HR); 0.5 points were included for this combination in the risk model. Similar to the FRALLE protocol, the DEXA dose of 10 mg/m2 was weighted with 0.5 points in the BFM study.
ASP is an important component of induction and intensification therapy. The cytotoxic effect of ASP is mediated by depletion of the essential amino acid asparagine.25 ASP also reduces circulating levels of several hemostatic proteins, including plasminogen, fibrinogen, and antithrombin, by a combination of reduced hepatic production and increased clearance.26-28 Apart from ASP, corticosteroid therapy is an important component of ALL treatment, which is frequently given concurrently with ASP for remission induction. Corticosteroids have a number of inhibitory effects during inflammatory reactions. When comparing the concomitant use of corticosteroids with ASP in ALL treatment, the produced prothrombotic state, for example, the elevation of procoagulant factors and reduction of the fibrinolytic potential, is more pronounced when PDN instead of DEXA is administered, Thus, in the multivariate analysis performed by us, we adjusted for the corticosteroid therapy applied by protocol.29
The secondary study objective was to evaluate the off-label use of LMWH in children with ALL. Children with ALL scores in the HRG without enoxaparin prophylaxis showed a significantly reduced TFS compared with children with prophylactic use of enoxaparin. The NNT to prevent one symptomatic VTE in children with a HR score was 2, which suggests a benefit for enoxaparin prophylaxis in all children with ALL scored as HR. However, administration of enoxaparin in these patients needs to be assessed in carefully designed clinical trials.
These results are supported by an Israeli study in a nonrandomized ALL cohort, in which 41 children received enoxaparin; there were no symptomatic VTE events or bleeding episodes reported.30 Furthermore, in 2008 Meister et al31 published a prospective cohort study of LMWH and antithrombin in comparison to a historical control cohort treated with antithrombin alone for primary prevention in children treated according to BFM protocols. Of all patients in the antithrombin group only 12.7% developed symptomatic VTE compared with 0% in the group of combined administration. Compared with the Israeli cohort and our cohort,30 the investigators did not observe major hemorrhage in the patients investigated.
There are a few limitations of the present study that need to be noted. First, the pilot testing and validation of the model was performed in white BFM and BFM-adapted and COALL cohorts in Europe.6-9 Thus, the risk score presented here for children with ALL and chemotherapy needs to be further validated in nonwhite, non-European, and non–BFM-adapted protocols.11,32,33 Second, the model was developed to predict symptomatic radiographically confirmed but not asymptomatic VTE. A prospective cohort study showed, however, that survivors of childhood ALL with asymptomatic VTE during chemotherapy had postthrombotic syndrome in 50% of cases,34 an estimated prevalence comparable to that in the general pediatric population with symptomatic VTE. Therefore, the model needs to be assessed in relation to asymptomatic VTE in future studies. Finally, as a potential confounder in the study the uncontrolled off-label use of LMWH in children with ALL, which began in the late 1990s, needs to be addressed.30,31 We have, however, recorded LMWH use in our database and thus were able to adjust for enoxaparin administration in the multivariate analysis presented here.
In conclusion, as the primary study objective we have developed and validated a predictive model for determining children at increased risk of symptomatic VTE during ALL chemotherapy. The model presented here is not only useful in predicting the risk of VTE in children undergoing ALL induction chemotherapy, but it also provides evidence for the usefulness of specific thrombophilia testing in a population of children with an identified risk of thrombosis. Therefore, this model should be further validated externally in further pediatric ALL populations treated with different leukemia treatment protocols.10,32,33 On the basis of results of our secondary study objective, the safety and efficacy of prophylactic administration of enoxaparin should be assessed in carefully designed clinical studies in high-risk patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank our colleagues C. Berger (St Etienne, France), P. Schneider (Rouen, France), and G. Leverger (Armand Trousseau, Paris, France) for patient ascertainment. In addition, we thank Kathrin Gordon for help in editing the manuscript and Claudia Lanvers-Kaminsky and Leslie Raffini (University Children's Hospital Philadelphia) for helpful comments.
Authorship
Contribution: Along with the principal study investigators, L.M., G.K., and U.N.-G., who act as the guarantors, all other investigators had full access to the data and took part in the design, execution, and data analysis and in writing the report. S.H. and U.N.-G. were responsible for the statistical calculation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: U. Nowak-Göttl, Pediatric Hematology/Oncology, University Hospital of Münster, Albert-Schweitzer-Str 33, D-48149 Münster, Germany; e-mail: leagottl@uni-muenster.de.
References
Author notes
L.M. and M.L. share first authorship.