Abstract
EndoS from Streptococcus pyogenes is an immunomodulating enzyme that specifically hydrolyzes glycans from human immunoglobulin G and thereby affects antibody effector functions. Autoimmune hemolytic anemia is caused by antibody-mediated red blood cell (RBC) destruction and often resists treatment with corticosteroids that also cause frequent adverse effects. We show here that anti-RhD (anti-D) and rabbit anti–human-RBC antibodies (anti-RBC) mediated destruction of RBC, ie, phagocytosis, complement activation, and hemolysis in vitro and in vivo was inhibited by EndoS. Phagocytosis by monocytes in vitro was inhibited by pretreatment of anti-D with EndoS before sensitization of RBCs and abrogated by direct addition of EndoS to blood containing sensitized RBCs. The toxic effects of monocytes stimulated with anti-D–sensitized RBCs, as measured by interleukin-8 secretion and oxygen metabolite production, was restrained by EndoS. Agglutination of RBCs and complement-mediated hemolysis in vitro in whole human blood caused by rabbit anti-RBCs was inhibited by EndoS. Development of anemia in mice caused by a murine anti-RBC immunoglobulin G2a monoclonal autoantibody and complement activation and erythrophagocytosis by Kupffer cells in the liver were reduced by EndoS. Our data indicate that EndoS is a potential therapeutic agent that might be evaluated as an alternative to current treatment regimens against antibody-mediated destruction of RBCs.
Introduction
The presence and pathogenicity of autoantibodies directed against red blood cells (RBCs) have been extensively investigated and implicated in a number of autoimmune diseases, such as autoimmune hemolytic anemia (AIHA)1 and systemic lupus erythematosus.2 The RBC surface contains many epitopes recognizable by alloantibodies and autoantibodies, including the more than 300 blood group antigens located on glycoproteins and glycolipids but also nonpolymorphic/nonblood group protein structures.3 The most common targets of pathogenic RBC autoantibodies are epitopes on the Rh protein, encoded by 2 homologous genes on chromosome 1, RHD and RHCE.4 More than half of all autoantibody specificities in patients with AIHA of the common so-called warm type include immunoglobulin G (IgG) antibodies against these Rh epitopes, which are consequently often used to model AIHA experimentally.
Human antibodies against RhD (anti-D) or rabbit antibodies against human RBC (anti-RBC) can result in accelerated phagocytosis and removal of RBC from the circulation by monocytes/macrophages in the spleen and liver.5,6 Fcγ receptor (FcγR)–mediated erythrophagocytosis, in addition to other IgG effector functions, such as activated complement, oxidative burst, and cytokine production by FcγR expressing effectors cells, play an essential role in shortened RBC survival.7-11 The ability of normal human monocytes to bind and phagocyte IgG-sensitized RBCs via FcγR and mediate immune RBC destruction is highly related to the glycosylation status of the Fc domain on the anti-RBC antibodies.12-17
Endoglycosidase S (EndoS) is secreted by Streptococcus pyogenes and has a specific endoglycosidase activity on native IgG by hydrolyzing the conserved asparagine-linked glycans on the heavy chains of IgG.18,19 EndoS is the first known bacterial enzyme with a unique specificity for native IgG. EndoS affects the functionality of opsonizing IgG by decreased binding to FcγRs on a monocyte-like cell line and impaired classic complement activation in vitro.19 EndoS has recently been shown to modulate human IgG/FcγR interactions by influencing the binding/dissociation of IgG to soluble and cell-bound FcγR.20 Furthermore, the pretreatment of pathogenic antibodies by EndoS has a protective function in a mouse model of collagen-induced arthritis, and EndoS cures mice from lethal IgG-driven immune thrombocytopenic purpura (ITP).21,22
The present study was undertaken to establish the effects of EndoS on antibody-mediated destruction of RBCs with a future therapeutic application in mind. Here we show, using monocytes from blood and the THP-1 monocytic cell line, that pretreatment of anti-RBC with EndoS abrogates erythrophagocytosis and subsequent activation of monocytes. The complement-dependent hemolytic effects of anti-RBC added to whole human blood were greatly decreased by pretreatment of this antibody with EndoS. Finally and most importantly, EndoS showed inhibitory effects on a model of AIHA in mice. These results suggest that anti-RBC antibodies can be rendered less pathogenic by treatment with EndoS, which may be regarded as a possible future inhibitor of antibody-mediated destruction of RBCs and AIHA.
Methods
Human blood donors and preparation of plasma, red cells, and monocytes
For RBC sensitization, blood was drawn from healthy Rh-positive persons and collected in heparin-containing tubes. After centrifugation at 1100g for 10 minutes, plasma and buffy coat aspiration, RBCs were washed 5 times with 10 volumes of phosphate-buffered saline (PBS): 10mM phosphate buffer, pH 7.4, 120mM NaCl, 3mM KCl. RBCs, 1 mL of 5% suspension, were incubated with 100 μL commercial human anti-D, 0.1 mg/mL in PBS for 1 hour at 37°C after washes in PBS. For sensitization of RBCs with rabbit IgG, 300 μL of 5% RBC suspension was incubated with an equal volume of rabbit IgG against human RBC (anti-RBC), diluted to 0.1 mg/mL. This IgG fraction antibody was purified from antiserum by a multistep process, including delipidation, salt fractionation, and ion exchange chromatography followed by dialysis against 0.02M potassium phosphate and 0.15M sodium chloride, pH 7.2. Peripheral blood monocytes were separated from heparinized human whole blood from healthy volunteers using Ficoll-Paque Plus (GE Healthcare) according to instructions provided by the manufacturer. After isolation, the monocytes were washed 3 times with PBS and finally resuspended in RPMI 1640 medium containing 10% fetal calf serum to the concentration 4 to 6 × 106 cells/mL. The human monocytic cell line THP-1 (ATCC) was cultured in RPMI 1640 medium supplemented with 10% fetal calf serum and 10 μg/mL gentamycin at 37°C in an atmosphere containing 5% CO2 and 95% humidity. Nunclon flasks (Nunc) for cell culture were used.
RBC labeling with FITC
In some cases, fluorescein isothiocyanate (FITC)–labeled RBCs were used for sensitization with anti-D (Rhesonativ, 0.1 mg/mL) and subsequent phagocytosis experiments. FITC labeling of RBCs was performed according to the earlier description.23 Briefly, RBCs were resuspended to 1 × 108 cells/mL in fresh 0.5M sodium bicarbonate, pH 9.0, and labeled with 200 μg/mL FITC (Pierce Chemical) for 30 minutes at room temperature with constant agitation followed by 5 washes in cold PBS. The degree of ingested FITC-labeled RBCs by monocytes was determined by measuring the fluorescence (excitation 485 nm, emission 535 nm) of washed monocytes using a Wallac 1420 Multilabel Counter (PerkinElmer Life and Analytical Sciences).
Antibodies
Commercial anti-D was obtained either from DiaMed GmbH or Rhesonativ (Octapharma). Rabbit IgG against human RBCs was from Rockland. Human polyclonal anti-D of titers 256 and 512 were obtained as ethylenediaminetetraacetic acid-anticoagulated plasmas from the collection of in-house control reagents made available as a kind gift from the University Hospital Blood Center in Lund, Sweden. As a negative control, ethylenediaminetetraacetic acid-anticoagulated whole blood was drawn after informed consent from apparently healthy volunteer donors with negative blood group antibody screen results. Horseradish peroxidase (HRP)–conjugated anti–rabbit IgG and HRP-anti–human IgG were obtained from Bio-Rad. FITC-conjugated anti–human C1q was from Cappel MP Biomedicals and goat anti–mouse C3 from Cappel Laboratories. Goat anti–mouse C1q was a kind gift from Dr Franz Petry (Johannes Gutenberg University, Mainz, Germany).
Reagents
Full-length EndoS with glutathione-S-transferase as a fusion was recombinantly expressed and purified from Escherichia coli harboring the plasmid pGEXndoS.24 RPMI 1640 medium and Hanks balanced salt solution (HBSS) were from Invitrogen. All other reagents were purchased from Sigma-Aldrich Sweden AB, unless indicated otherwise.
EndoS treatment of antibodies, plasma, and IgG-sensitized RBCs
Recombinant glutathione-S-transferase-EndoS in PBS, enzyme/antibody molar ratio 1:50 was added to anti-D or anti-RBC, and samples were incubated for 2 hours at 37°C. All antibody and plasma samples were passed over a glutathione-Sepharose column (GE Healthcare) according to the description from the manufacturer. Details about EndoS hydrolysis of RBCs sensitized with anti-D are described in “Chemiluminescence test.”
SDS-PAGE and Western blot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting were performed as described previously.20 After blotting, membranes were blocked in PBS supplemented with 0.05% vol/vol Tween 20 (PBST) and 5% wt/vol skim milk (Difco) for 20 minutes at room temperature. For detection of IgG, the blots were subsequently washed in PBST and then incubated with HRP-conjugated anti–human IgG or HRP anti–rabbit IgG (diluted 1:2000) for one hour at 37°C. The membranes were developed using SuperSignal West Pico (Pierce Chemical) according to the manufacturer's instructions before analyzing by the Chemidoc XRS imaging system and Quantity One image analysis software (Bio-Rad).
ELISA for glycan detection.
Microtiter plates (Nunc) were coated with 100 μL of 5% RBCs (solubilized in 20mM Tris-HCl pH 8.0, 0.150 NaCl, 1% Triton, 0.25% NP40), diluted 10 times in PBS, and kept at 4°C overnight. The plates were washed 3 times with lectin buffer containing 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.5, 0.15M NaCl, 0.01mM MnCl2, 0.1mM CaCl2, and 0.1% vol/vol Tween 20 and blocked in the same buffer for 1 hour at room temperature. After 3 washes with lectin buffer, 1 μg/mL of biotinylated Lens culinaris agglutinin-lectin (Vector Laboratories) was added and incubation continued for 1 hour at 37°C. After 3 more washes, 0.1 μg/mL HRP-labeled streptavidin (Vector Laboratories) was added, and the plate was incubated for 1 hour at 37°C followed by washing with lectin buffer. The color reaction was developed using tetramethylbenzidine/hydrogen peroxide containing substrate solution (R&D Systems Europe). After addition of 50 μL stop solution (1M H2SO4), the absorbance was analyzed using a Wallac 1420 Multilabel Counter at 450 nm.
ELISA detection of RBC-bound IgG.
Microtiter plates were coated with 100 μL of 5% solubilized RBCs diluted 10 times in PBS for 20 hours at 4°C. The next day, the plates were blocked with PBST and 2% wt/vol bovine serum albumin for 2 hours at room temperature. After this step, an HRP-anti–human IgG or HRP-anti–rabbit IgG (dilution 1:1000) was used for detection. The plates were washed 3 times with PBST after the coating step and between each of the after incubation steps. The color reaction was performed as described in “ELISA for glycan detection.”
Quantification of hemoglobin.
Hemoglobin quantification was performed spectrophotometrically at 541 nm or using the chromogen 2,7-diaminofluorene at 600 nm. Briefly, a working solution was prepared by adding 1 volume of 1% wt/vol 2,7-diaminofluorene in 90% glacial acetic acid to 10 volumes of 0.2M Tris-HCl, pH 7.2, and 0.02 volume of 30% hydrogen peroxide. A total of 100 μL of working solution was added to 10 to 100 μL of RBC supernatants. After 20 minutes of incubation at room temperature, the optical density at 600 nm was analyzed in a Wallac 1420 Multilabel Counter.
Phagocytosis assay.
First, 100 to 500 μL of a 5% solution of RBCs, sensitized with anti-D (Octapharma, 0.1 mg/mL), was added to 2 to 10 × 106 blood monocytes or THP-1 cells in PBS and incubated 60 minutes at 37°C in suspension with gentle agitation. Subsequently, cells were washed 5 times with PBS and centrifuging at 400g, and noningested RBCs were lysed with 0.5 mL distilled water for 20 seconds followed by 2 washes in PBS. This treatment has been described to entirely lyse adherent RBCs without influencing the integrity of monocytes.25 Finally, the cells were dissolved in 500 μL 0.1M NaOH containing 0.05% (vol/vol) Triton X-100, and the concentration of ingested hemoglobin (RBCs) was measured using 2,7-diaminofluorene.
Monocyte monolayer assay.
The monocyte monolayer assay was performed 5 times according to a previous description.26 Monocyte monolayers were prepared by incubating 0.2 mL of monocytes purified from blood of 5 healthy donors in Lab-Tek Chamber Slides (Nunc) at 37°C for 1 hour. Nonadherent cells and RPMI 1640 were removed by aspiration with a pipette. Subsequently, 0.2 mL of a 5% RBC suspension was presensitized with anti-D immunoglobulin (DiaMed GmbH; 0.1 mg/mL), or control RBCs resuspended in RPMI 1640 containing 10% fetal calf serum were added to the monolayer and incubated for 1 hour at 37°C. The slides were then rinsed gently with PBS to remove nonadherent RBCs. Dried slides were stained with May-Grünwald-Giemsa (VWR International AB). Monocytes were examined microscopically, and the frequency of cells with adherent or phagocytosed red cells was estimated.
Chemiluminescence test.
This method allows differentiation of the clinically significant antibodies from the clinically benign and is used to predict the clinical impact of alloantibodies in transfusion of incompatible blood or severity of hemolytic disease of the newborn.27 Monocytes were washed 4 times in PBS containing 0.2% wt/vol bovine serum albumin and finally resuspended to 2 × 106 cells/mL in HBSS containing 30% RPMI 1640 medium and 3% fetal calf serum (vol/vol). The cells were pipetted into 96-well microplate and incubated for 2 hours at 37°C in 5% CO2. Then, 50 μL of a 5% suspension of RBC in PBS with 0.5% human serum albumin was sensitized with 50 μL plasma from anti-D-positive donors on a 96 V-well plate for 1 hour at 37°C. After repeated washes of plate with PBS, sensitized RBCs were incubated with 50 μL EndoS (20 μg) for 30 minutes to 1 hour at 37°C resuspended in HBSS. EndoS was removed by washing with HBSS. In some cases, plasma was pretreated with EndoS before sensitization of RBCs. Subsequently, 80 μL of a 0.8mM solution of luminol in HBSS and 20 μL of the resuspended RBCs were added to wells containing monocytes. Chemiluminescence responses were monitored for 1 hour with 5-minute intervals using a luminometer Wallac 1420 Multilabel Counter. Monocyte responses to sensitized RBCs were compared with unsensitized control RBCs and expressed as a ratio (opsonic index). Opsonic index was calculated from the maximum point values obtained from the measurements.
IL-8 assay.
Human interleukin-8 (IL-8) was detected using DuoSet ELISA (R&D Systems). Briefly, THP-1 cells or monocytes (0.6 × 106 cells in 1 mL) were incubated with 150 μL of a 0.5% to 1% suspensions of anti-D (Octapharma; 0.1 mg/mL) sensitized RBCs or control RBCs (unsensitized) for 24 hours (THP-1 cells) and 2 hours (blood monocytes) at 37°C on 12-well cell culture plates (Nunc). The supernatants were analyzed for IL-8. The stability of IL-8 was analyzed by incubation of purified recombinant human IL-8 (PeproTech) with EndoS, 5 μg of each protein, for 2 hours at 37°C followed by analysis by SDS-PAGE. The viability of cells after interaction with EndoS was measured by incubation of monocytes, 2.5 × 106 cells/mL in 200 μL with 20 μg EndoS for 2 hours at 37°C followed by analysis using flow cytometry (Epics XL-MCL; Beckman-Coulter). The monocytes were washed once and incubated with 10 μL annexin V binding buffer, 5 μL annexin V, and 2 μL propidium iodide (BD Biosciences PharMingen) in a total volume of 100 μL for 20 minutes at room temperature. The cells were washed and resuspended in 500 μL PBS before analysis by flow cytometry.
C1q uptake studies.
C1q binding to sensitized RBCs was analyzed as described previously28 with small modifications. Briefly, blood was collected in ethylenediaminetetraacetic acid tubes. A total of 300 μL of 5% RBC suspension in isotonic Veronal-buffered saline containing 0.1% gelatin, 0.15mM CaCl2, and 1mM MgCl2 (GVBS2+) were incubated with an equal volume of rabbit anti-RBC (10 μg/mL), with or without pretreatment of antibodies with EndoS, or buffer for 30 minutes at 37°C. After 2 washes in GVBS2+ RBCs, pellets were resuspended in 250 μL human serum and were incubated at 30°C for 15 minutes. After washing of pellets, FITC-conjugated anti–human C1q was added and incubated for 60 minutes at room temperature. After 3 washes of cell pellets, fluorescence intensity was measured using a Wallac 1420 Multilabel Counter.
Experimental AIHA.
Animals studies described herein were approved by the Ethical Committee for Animal Experimentation at the Faculty of Medicine of the University of Geneva in Switzerland. AIHA was induced in 2-month-old BALB/c mice by a single intravenous injection of purified murine 34-3C anti-RBC monoclonal autoantibody as described previously.29 The hybridoma secreting the 34-3C IgG2a anti-RBC monoclonal autoantibody was derived from unmanipulated NZB mice,30 and the generation of IgG1 and IgG2b subclass-switch variants was described previously.29 Twenty-four hours after the injection of anti-RBC monoclonal antibody (mAb), 20 μg of EndoS was intravenously administered. Blood samples were collected by orbital sinus puncture into heparinized microhematocrit tubes, and hematocrit values were directly determined after centrifugation. The deposition of C1q and C3 on RBCs was detected 24 hours after injection of EndoS or PBS in mice pretreated with 34-3C anti-RBC mAb by a flow cytometry assay, using biotinylated goat anti–mouse C1q31 or goat anti–mouse C3 followed by phycoerythrin-conjugated streptavidin, as previously described. The injection of mAb was controlled by assessing the level of antibody opsonization of RBCs using biotinylated rat anti–mouse κ chain mAb.32 Livers, obtained 8 days after injection of mAb, were processed for histologic examination, and the extent of in vivo RBC destruction by Kupffer cell-mediated phagocytosis was determined by iron staining using Perls Prussian blue stain, which is widely used to demonstrate ferric iron in tissues. No counterstaining was performed.
Statistics
Student t tests were performed to determine statistical significance. Error bars in the figures represent the mean plus or minus SE.
Image acquisition
Light microscopy images were visualized with an Optiphot microscope (Nikon Corporation) equipped with a 10× eyepiece, a Plan 4×/0.13 objective lens, and a Plan 20×/0.50 objective lens. Images were captured with a Nikon COOLPIX 4500 camera (Nikon Corporation) and were processed using Adobe Photoshop software (Adobe Systems).
Results
Treatment of anti-D with EndoS does not affect the binding of anti-D to RBCs
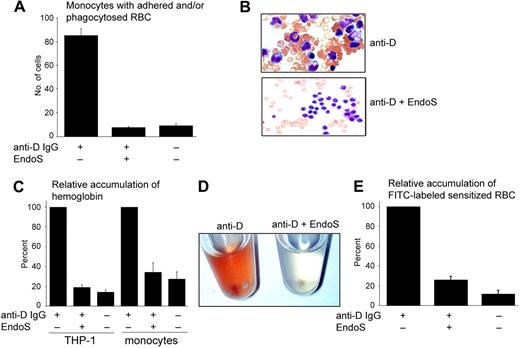
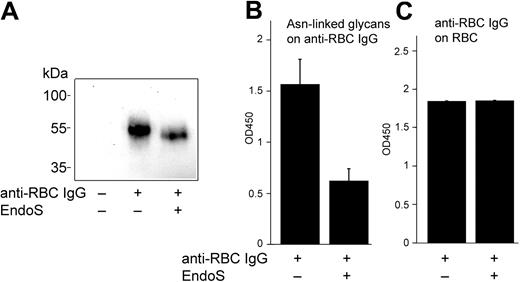
We have recently reported that EndoS inhibits binding of IgG to soluble and monocyte-bound FcγR by hydrolyzing the N-linked glycan on the heavy chain of IgG.20 Therefore, we aimed to evaluate further the effect(s) of EndoS on IgG/FcγR interactions using antibody-mediated lysis of RBCs, including phagocytosis via FcγR and direct hemolysis, as an experimental model. For this purpose, RBCs were incubated with anti-D, with or without preincubation of antibodies with EndoS. Cell-bound IgG was determined by Western blot and enzyme-linked immunosorbent assay (ELISA) subsequent to solubilization of sensitized RBCs and IgG purification. EndoS hydrolysis of the N-glycan on anti-D bound to RBCs was confirmed by Western blot showing a characteristic reduction in the size of IgG heavy chain and decreased reactivity of EndoS-treated anti-D with Lens culinaris agglutinin lectin using ELISA (Figure 1A-B). Furthermore, the results showed that the treatment of anti-D with EndoS did not affect the antigen recognition by this antibody and the amount of RBC-bound IgG was not influenced by EndoS treatment (Figure 1A,C).
Treatment of anti-D with EndoS does not affect the binding of anti-D to RBCs. (A) Western blot analysis of anti-D on RBCs using peroxidase-conjugated anti–human IgG. (B) Glycan hydrolysis of anti-D on RBCs determined by lectin ELISA (optical density [OD] = 450 nm). (C) Quantification of RBC-bound anti-D by ELISA (OD = 450 nm) using antiserum against human IgG.
Treatment of anti-D with EndoS does not affect the binding of anti-D to RBCs. (A) Western blot analysis of anti-D on RBCs using peroxidase-conjugated anti–human IgG. (B) Glycan hydrolysis of anti-D on RBCs determined by lectin ELISA (optical density [OD] = 450 nm). (C) Quantification of RBC-bound anti-D by ELISA (OD = 450 nm) using antiserum against human IgG.
EndoS inhibits phagocytosis and adherence of sensitized RBCs by monocytes
The effect(s) of EndoS on phagocytosis of anti-D–sensitized RBCs by monocytes was analyzed using monocyte monolayer assay. Monolayers of monocytes were cultured on slides, and RBCs presensitized with anti-D were added to the monolayers. The degree of phagocytosis was determined using light microscopy and expressed as the number of monocytes with adherent/phagocytosed RBCs by analyzing 100 monocytes. As expected, the phagocytosis of anti-D–sensitized RBCs was significantly greater than nonsensitized RBCs and was efficiently eliminated by pretreatment of anti-D with EndoS (P < .001; Figure 2A). The number of “rosettes” (3 or more RBCs attached to each monocyte) was greater for anti-D–sensitized RBCs than for RBCs sensitized with anti-D pretreated with EndoS (Figure 2B). To establish the extent of the intracellular ingestion of sensitized RBCs, with and without pretreatment of anti-D with EndoS, RBCs were also incubated with THP-1 cells or blood monocytes in suspension. Extracellularly bound RBCs were lysed with distilled water (a method that does not influence the intracellular environment of monocytes) and the amount of ingested RBCs was determined by measuring the concentration of internalized hemoglobin using 2,7-diaminofluorene, a reagent that is specifically oxidized by hemoglobin. This experiment showed a substantial increase in accumulation of intracellular hemoglobin in both THP-1 cells and monocytes, explained by a phagocytosis of sensitized RBCs, contrary to the low phagocytosis of nonsensitized RBCs (in both cell types, P < .001) and RBCs sensitized with EndoS-pretreated anti-D (P < .001 and P < .002, respectively; Figure 2C). Accumulation of hemoglobin in monocytes could also be visualized macroscopically with red-colored resuspended monocytes after incubation with sensitized RBCs, compared with colorless monocytes when EndoS-pretreated anti-D was used for sensitization of RBCs (Figure 2D). To further confirm this observation, RBCs were labeled with FITC and then sensitized with anti-D with and without pretreatment of antibodies with EndoS. FITC-labeled RBCs were subsequently incubated with monocytes. After washing the cells with PBS and removal of uningested RBCs by hypotonic buffer, the degree of ingested RBCs was determined by measuring the fluorescence of cells. This experiment revealed a significantly lower degree of phagocytosis of nonsensitized RBCs (P < .018) and RBCs sensitized with EndoS-pretreated anti-D (P < .002) compared with RBCs sensitized with intact anti-D (Figure 2E). This clearly shows that the treatment of anti-D with EndoS before sensitization of RBCs abrogates the phagocytosis-enhancing activity of anti-D and decreases the accumulation of intracellular hemoglobin in monocytes.
RBCs sensitized with anti-D IgG pretreated with EndoS are not attached to, or phagocytosed by, monocytes. (A) The degree of adherence/phagocytosis of RBCs sensitized with anti-D determined by monocyte monolayer assay and expressed as a number of monocytes with one or more adherent/phagocytosed RBCs of 100 monocytes detected. (B) The appearance of “rosettes” showed using Grünwald-Giemsa staining of monocyte monolayers incubated with RBCs sensitized with anti-D (top panel) and anti-D pretreated with EndoS (bottom panel; original magnification ×60). (C) Phagocytosis of sensitized RBCs by THP-1 cells and blood monocytes with or without treatment of anti-D with EndoS. The results are presented as the relative amount of internalized hemoglobin in cells detected by reactivity with 2,7-diaminofluorene. (D) Resuspended monocytes after incubation with RBCs sensitized with anti-D followed by the removal of noningested RBCs. (Left) RBCs sensitized with anti-D. (Right) Anti-D pretreated with EndoS. (E). Purified blood monocytes were incubated with FITC-labeled RBCs sensitized with anti-D and the degree of ingested RBCs determined by measuring the fluorescence.
RBCs sensitized with anti-D IgG pretreated with EndoS are not attached to, or phagocytosed by, monocytes. (A) The degree of adherence/phagocytosis of RBCs sensitized with anti-D determined by monocyte monolayer assay and expressed as a number of monocytes with one or more adherent/phagocytosed RBCs of 100 monocytes detected. (B) The appearance of “rosettes” showed using Grünwald-Giemsa staining of monocyte monolayers incubated with RBCs sensitized with anti-D (top panel) and anti-D pretreated with EndoS (bottom panel; original magnification ×60). (C) Phagocytosis of sensitized RBCs by THP-1 cells and blood monocytes with or without treatment of anti-D with EndoS. The results are presented as the relative amount of internalized hemoglobin in cells detected by reactivity with 2,7-diaminofluorene. (D) Resuspended monocytes after incubation with RBCs sensitized with anti-D followed by the removal of noningested RBCs. (Left) RBCs sensitized with anti-D. (Right) Anti-D pretreated with EndoS. (E). Purified blood monocytes were incubated with FITC-labeled RBCs sensitized with anti-D and the degree of ingested RBCs determined by measuring the fluorescence.
EndoS inhibits oxygen metabolite production by monocytes induced by sensitized RBCs
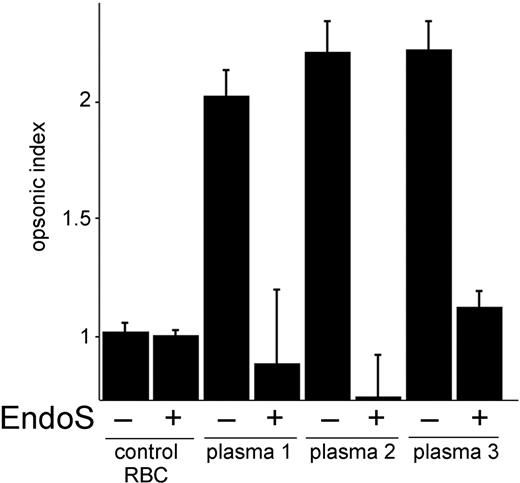
In an attempt to further elucidate the effects of EndoS on monocyte-driven cell cytotoxicity to sensitized RBCs, we measured the release of oxygen metabolites from monocytes using a chemiluminescence test. RBCs were opsonized with immune plasma with high titers of anti-D from 3 persons. Monocyte responses to sensitized RBCs were compared with their response to unsensitized cells and expressed as a ratio (opsonic index). RBCs incubated with immune plasma gave positive results in chemiluminescence test with opsonic index 2.5. In contrast, monocyte responses to RBCs incubated with immune plasma pretreated with EndoS were significantly decreased (plasma 1, P < .07; plasma 2, P < .02; plasma 3, P < .015) and in some cases even below the control plasma (Figure 3). These results suggest that treatment of immune plasma with EndoS abrogates in vitro metabolic oxidative responses by monocytes.
EndoS inhibits metabolic oxygen responses induced by RBCs sensitized with anti-D immune plasma. Metabolic oxygen responses from monocytes were analyzed by measuring the chemiluminescence after addition of RBC-sensitized anti-D-positive plasma (with or without treatment with EndoS) and luminol, at 5-minute intervals for 1 hour. y-axis represents opsonic index, ie, the ratio of monocyte responses to sensitized RBCs versus unsensitized RBCs. control RBC indicates RBCs sensitized with nonimmune plasma.
EndoS inhibits metabolic oxygen responses induced by RBCs sensitized with anti-D immune plasma. Metabolic oxygen responses from monocytes were analyzed by measuring the chemiluminescence after addition of RBC-sensitized anti-D-positive plasma (with or without treatment with EndoS) and luminol, at 5-minute intervals for 1 hour. y-axis represents opsonic index, ie, the ratio of monocyte responses to sensitized RBCs versus unsensitized RBCs. control RBC indicates RBCs sensitized with nonimmune plasma.
EndoS inhibits IL-8 secretion from monocytes induced by sensitized RBCs
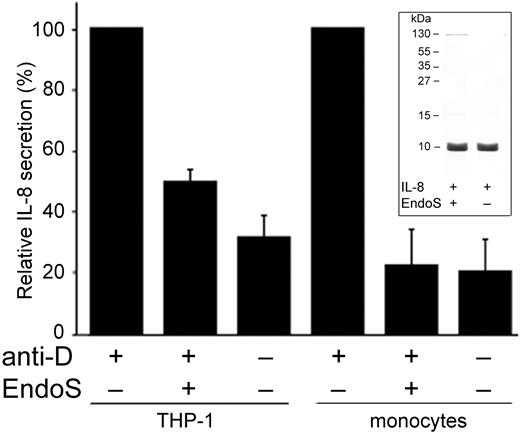
It has been reported that IL-8, besides being produced by monocytes in response to lipopolysaccharide, tumor necrosis factor-α, or IL-1, is implicated as an indicator of RBC incompatibility and mediator of the pathologic events in hemolysis in, for example, hemolytic transfusion reactions.33-35 Therefore, we found it relevant to establish the role of EndoS regarding the in vitro response of monocytes exposed to sensitized RBCs by measuring the release of IL-8. Thus, THP-1 cells and monocytes were incubated with anti-D–sensitized RBCs, and the secretion of IL-8 was measured using ELISA. As expected, a significantly elevated IL-8 concentration was observed in both THP-1 cells and peripheral blood monocytes. Noticeably, RBCs sensitized with anti-D pretreated with EndoS showed significantly decreased secretion of IL-8 in THP-1 cells (P < .001) and blood monocytes (P < .003; Figure 4), which was comparable with the secretion of IL-8 by THP-1 cells and monocytes because of stimulation with nonsensitized RBCs (P < .001 and P < .002, respectively). Having future experiments involving not only pretreatment of antibody but also a direct addition of EndoS to blood in mind, we found it important to exclude any direct effects of EndoS on IL-8. EndoS was therefore incubated with purified IL-8 and analyzed by SDS-PAGE. No changes in the IL-8 band intensity were visible, confirming that there was no direct activity of EndoS on IL-8 (Figure 4 inset). Furthermore, to investigate whether the results described here were independent of any cytotoxic effect of EndoS, a viability test was performed using annexin V and propidium iodide staining, detecting apoptotic and necrotic cells, respectively. For EndoS-treated monocytes, 0.68% of cells were stained for annexin V and 1.14% for propidium iodide, and these results were comparable with results obtained for untreated monocytes, 0.82% and 1.25% of cells, respectively. These results demonstrate that EndoS does not have any direct cytotoxic effects on monocytes.
EndoS inhibits secretion of IL-8 by monocytes induced by RBCs sensitized with anti-D. Relative quantification of IL-8 secretion in supernatants from THP-1 cells or blood monocytes incubated with RBCs sensitized with anti-D for 3 hours, with or without pretreatment of anti-D with EndoS. (Inset) IL-8 incubated with EndoS followed by separation on SDS-PAGE and staining with Coomassie Blue.
EndoS inhibits secretion of IL-8 by monocytes induced by RBCs sensitized with anti-D. Relative quantification of IL-8 secretion in supernatants from THP-1 cells or blood monocytes incubated with RBCs sensitized with anti-D for 3 hours, with or without pretreatment of anti-D with EndoS. (Inset) IL-8 incubated with EndoS followed by separation on SDS-PAGE and staining with Coomassie Blue.
EndoS inhibits hemolysis of sensitized RBCs and prevents binding of C1q to IgG-sensitized RBCs
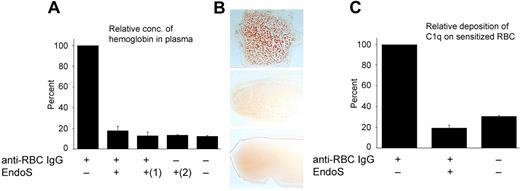
Human anti-D antibodies of IgG isotype do not generally activate the complement pathway, and RBCs coated with these antibodies are preferentially cleared by mononuclear phagocytic cells via FcγRs. Therefore, in an attempt to study the antihemolytic effects of EndoS by analyzing complement activation of sensitized RBCs, a polyclonal rabbit anti–human RBC was used. EndoS hydrolysis of IgG glycans was confirmed by Western blot and lectin ELISA (Figure 5A-B), and IgG on sensitized RBCs was detected by ELISA (Figure 5A,C). The rabbit anti–human RBC with and without treatment with EndoS was added to whole blood; and after pelleting of the blood cells, the supernatants were analyzed for hemoglobin using 2,7-diaminofluorene. This revealed an expected increase in hemoglobin concentration using sensitized RBCs. In contrast, only negligible 2,7-diaminofluorene activity was seen using EndoS-treated anti-RBC, indicating inhibition of complement-mediated lysis of RBCs (P < .001; Figure 6A). We next aimed to elucidate whether a simultaneous addition of EndoS and antibody to blood, without the previous treatment of antibody with EndoS, could have a similar antihemolytic effect. Indeed, it was clearly shown that pretreatment of antibody by EndoS is not a prerequisite for inhibition of hemolysis and that a direct addition of EndoS to blood was sufficient to exclude the hemolytic effect of anti-RBC (P < .001; Figure 6A). EndoS by itself did not have any effect on hemolysis (P < .001; Figure 6A). Furthermore, addition of increasing concentrations of anti-RBC IgG to whole blood caused a strong agglutination reaction as expected. Remarkably, addition of EndoS-treated antibody abrogated this effect (Figure 6B). In conclusion, these data indicate that EndoS possesses antihemolytic activities as demonstrated by pretreatment of anti-RBC with enzyme or by a direct addition of EndoS to anti-RBC-containing blood.
Hydrolysis of anti–human RBC-IgG by EndoS. (A) Rabbit antiserum against human RBCs was treated with EndoS or PBS and used for sensitization of RBCs. RBC-bound IgG was determined by solubilization of sensitized RBCs followed by Western blot using antiserum rabbit IgG. (B) Glycan hydrolysis of RBC-bound IgG determined by lectin ELISA (OD = 450 nm). (C) RBC-bound antibody was quantified by ELISA (OD = 450 nm) using antiserum against rabbit IgG.
Hydrolysis of anti–human RBC-IgG by EndoS. (A) Rabbit antiserum against human RBCs was treated with EndoS or PBS and used for sensitization of RBCs. RBC-bound IgG was determined by solubilization of sensitized RBCs followed by Western blot using antiserum rabbit IgG. (B) Glycan hydrolysis of RBC-bound IgG determined by lectin ELISA (OD = 450 nm). (C) RBC-bound antibody was quantified by ELISA (OD = 450 nm) using antiserum against rabbit IgG.
EndoS inhibits hemolysis and agglutination of sensitized RBCs and prevents binding of C1q to sensitized RBCs. (A) Relative amount of hemoglobin quantified by 2,7-diaminofluorene in plasma after incubation of human blood with antiserum against human RBCs, pretreated with EndoS or not. (1)Direct addition of EndoS to blood containing RBC antiserum. (2)EndoS alone added to blood. NS indicates not significant. (B) Blood smears. (Top panel) A total of 80 μg of anti-RBC IgG added to 700 μL human blood, diluted 35 times in PBS, and incubated 1 hour at 37°C. (Middle panel) Anti-RBC treated with EndoS before addition to blood. (Bottom panel) No addition of antibodies. (C) Relative C1q deposition onto IgG-sensitized RBCs detected using FITC-conjugated anti–human C1q.
EndoS inhibits hemolysis and agglutination of sensitized RBCs and prevents binding of C1q to sensitized RBCs. (A) Relative amount of hemoglobin quantified by 2,7-diaminofluorene in plasma after incubation of human blood with antiserum against human RBCs, pretreated with EndoS or not. (1)Direct addition of EndoS to blood containing RBC antiserum. (2)EndoS alone added to blood. NS indicates not significant. (B) Blood smears. (Top panel) A total of 80 μg of anti-RBC IgG added to 700 μL human blood, diluted 35 times in PBS, and incubated 1 hour at 37°C. (Middle panel) Anti-RBC treated with EndoS before addition to blood. (Bottom panel) No addition of antibodies. (C) Relative C1q deposition onto IgG-sensitized RBCs detected using FITC-conjugated anti–human C1q.
It has been reported that C1q, the recognition subunit of the first component of the classic complement pathway, binds to IgG-sensitized RBCs.28 Because the binding of C1q to IgG is dependent on the glycosylation state of IgG, we decided to explore the effect(s) of EndoS on deposition of C1q onto IgG-sensitized RBCs and with this further elucidate the mechanism for the antihemolytic effects of EndoS. Human RBCs were sensitized with anti-RBC with or without pretreatment of antibody with EndoS. Pellets of sensitized RBCs were then incubated with human serum for 15 minutes at 30°C followed by detection of complement factor C1q uptake using FITC-labeled anti–human C1q. Indeed, there was significantly decreased fluorescence intensity in the samples containing RBCs sensitized with EndoS-treated anti-RBC (P < .001) compared with RBCs treated with native antibody. These results indicated an inability of C1q to bind to these RBCs (Figure 6C), providing further evidence for the complement-inhibitory/antihemolytic properties of EndoS.
EndoS inhibits AIHA in vivo
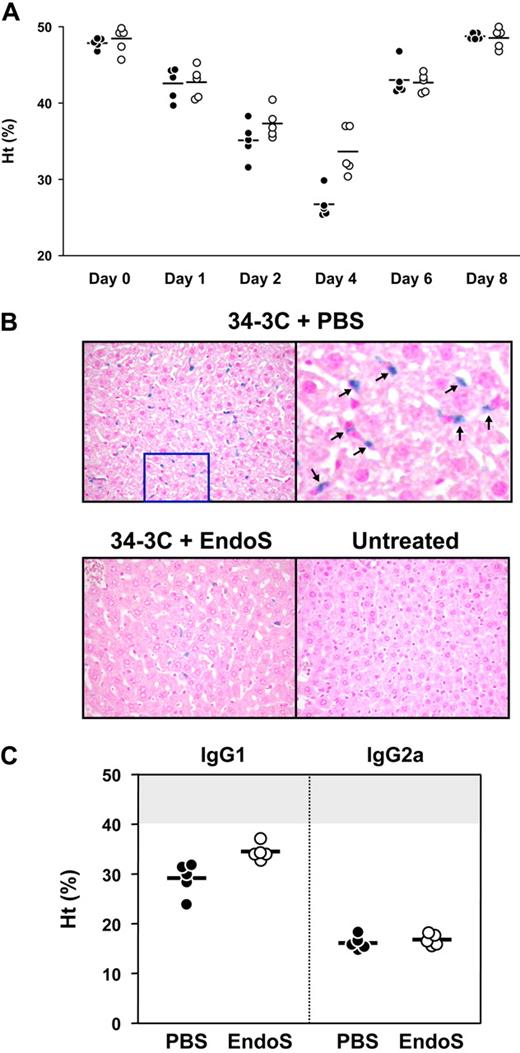
To elucidate the in vivo effects of EndoS in the development of antibody-mediated hemolysis/anemia, BALB/c mice were injected with 34-3C IgG2b anti-RBC mAbs, a model used for studying the development of AIHA resulting from FcγR- and complement receptor-mediated erythrophagocytosis by Kupffer cells in the liver.36 Injection of anti-RBC mAb induced a significant drop in hematocrit values at day 1 (42.7% ± 2.0%) compared with those before the injection (48.1% ± 1.3%, P < .001; Figure 7A). Then, mice were divided into 2 groups with subsequent administration of EndoS to one of the groups. At day 4, hematocrit values in EndoS-treated mice were 33.7% plus or minus 3.1% and significantly higher than hematocrits of the mice in the control group (26.8% ± 2.0%; P < .01; Figure 7A). Furthermore, the deposition of C1q and C3 on RBCs was analyzed by flow cytometry 24 hours after injection of EndoS. This revealed a significant decrease in C1q and C3 deposition on RBCs in mice treated with EndoS compared with PBS-treated mice (mean fluorescence intensity: C1q, 28.4 ± 6.5 vs 42.6 ± 7.8, P < .02; C3, 13.1 ± 4.8 vs 29.6 ± 9.7, P < .01), suggesting a reduced activation of complement. The injection of mAb was controlled by assessing the level of antibody opsonization of RBCs using biotinylated rat anti–mouse κ chain mAb (EndoS-treated mice, 88.0 ± 17.0; PBS-treated mice, 92.6 ± 11.4). To confirm the erythrophagocytosis and/or lysis of RBCs in mice injected with 34-3C IgG2b anti-RBC mAb, histologic analysis of iron deposition in Kupffer cells in the liver was performed. Livers were obtained 8 days after injection of mAb, and the extent of in vivo RBC destruction was visualized by staining of liver sections with Perls iron staining. The results showed that there was a substantial reduction of iron deposits in livers from mice treated with EndoS, compared with mice without treatment of EndoS (Figure 7B). In addition, the effect of EndoS on 34-3C IgG1 and IgG2a class-switch variants was also determined. We found protective effect of EndoS on IgG1-induced anemia but not on IgG2a-induced anemia (Figure 7C). When measuring the extent of C1q and C3 deposits in mice injected with complement-activating 34-3C IgG2a, no significant differences were visible compared with mice without EndoS treatment. Our data indicate that EndoS partially inhibits development of anemia mediated by anti-RBC reactive antibodies in an IgG subclass-dependent manner in mice.
EndoS decreases the development of AIHA in BALB/c mice pretreated with 34-3C anti-RBC mAb. BALB/c mice were intravenously injected with 34-3C anti-RBC mAb; and 24 hours later, 20 μg of EndoS was intravenously injected. (A) Hematocrit values of individual mice treated with EndoS (○) or PBS (●) were measured 1, 2, 4, 6, and 8 days after injection of 200 μg 34-3C IgG2b mAb. Horizontal lines indicate mean values. (B). Representative histologic appearance of iron deposits in Kupffer cells from mice injected with 34-3C IgG2b anti-RBC mAb and then with EndoS. Note substantial reduction of iron deposits in livers from mice treated with EndoS, compared with completely untreated mice (original magnification ×200). (Top right panel) Higher magnification of iron deposits as indicated by arrows. (C) BALB/c mice were intravenously injected with 300 μg of 34-3C IgG1 or 200 μg of 34-3C IgG2a class-switch variant; and 24 hours later, 20 μg of EndoS was intravenously injected. Hematocrit values of individual mice treated with EndoS (○) or PBS (●) were measured 4 days after injection of 34-3C mAb. Horizontal lines indicate mean values. The normal range of hematocrit values (mean ± 3 SD) of 2- to 3-month-old BALB/c mice is represented as a shaded area.
EndoS decreases the development of AIHA in BALB/c mice pretreated with 34-3C anti-RBC mAb. BALB/c mice were intravenously injected with 34-3C anti-RBC mAb; and 24 hours later, 20 μg of EndoS was intravenously injected. (A) Hematocrit values of individual mice treated with EndoS (○) or PBS (●) were measured 1, 2, 4, 6, and 8 days after injection of 200 μg 34-3C IgG2b mAb. Horizontal lines indicate mean values. (B). Representative histologic appearance of iron deposits in Kupffer cells from mice injected with 34-3C IgG2b anti-RBC mAb and then with EndoS. Note substantial reduction of iron deposits in livers from mice treated with EndoS, compared with completely untreated mice (original magnification ×200). (Top right panel) Higher magnification of iron deposits as indicated by arrows. (C) BALB/c mice were intravenously injected with 300 μg of 34-3C IgG1 or 200 μg of 34-3C IgG2a class-switch variant; and 24 hours later, 20 μg of EndoS was intravenously injected. Hematocrit values of individual mice treated with EndoS (○) or PBS (●) were measured 4 days after injection of 34-3C mAb. Horizontal lines indicate mean values. The normal range of hematocrit values (mean ± 3 SD) of 2- to 3-month-old BALB/c mice is represented as a shaded area.
Discussion
In the present study, we reveal a biologic/therapeutic significance of EndoS modulation of human IgG/FcγR. Using Rh antibodies of human origin, we show in vitro that the destructive processes of opsonized RBCs are eliminated or strongly inhibited by action of EndoS, either by pretreatment of RBC antibodies with EndoS or by direct addition of EndoS to blood. The results clearly demonstrate that EndoS modification of IgG glycosylation counteracts FcγR-mediated phagocytosis of anti-D–coated RBCs, inhibits binding of complement factor C1q to rabbit IgG-coated RBCs, and minimizes metabolic oxygen responses and secretion of IL-8 from monocytes. This suggests that EndoS possesses the ability to protect RBCs from lysis, predominantly extravascularly, but also intravascularly.
Different therapies involving immunosuppressing treatments as anti–CD-20 (rituximab), intravenous immunoglobulin (IVIG), glucocorticosteroids, or splenectomy are presently used for treatment of AIHA.37 They are unfortunately not free from undesired side effects. EndoS might therefore be an attractive alternative to be used solely or in combination with already present alternatives.
Bacterial glycosidases have been used previously to modify human molecules for potential medical use. For instance, conversion of the blood-group-specific carbohydrate residues found on group A, B, and AB RBCs to universal blood that types as group O was recently made possible by the discovery of a novel family of exoglycosidases. These are now being evaluated for applications in transfusion medicine.38,39 However, the benefit of EndoS action is the specific activity against IgG, apparently without affecting the RBC surface itself. Other endoglycosidases, such as EndoF1-3 from Elizabethkingia meningoseptica or EndoE from Enterococcus faecalis, have activity on a broad range of glycoproteins.40,41 Thus, EndoS is the only known endoglycosidase that exclusively hydrolyzes the glycans of IgG.22,24 This could be exploited as a novel therapeutic modality for pathologic conditions involving antibodies directed toward RBCs. An additional benefit of EndoS, which makes this enzyme reasonable for the future administration as a therapeutic agent, is that at therapeutic levels in mice no significant IgG response against EndoS could be observed.42
EndoS counteracts the toxic effects of anti-D by decreasing the release of IL-8, a cytokine associated with inflammation.34,43 This is in agreement with the observation that EndoS interferes with proinflammatory responses of IgG antibodies in vivo in a mouse model by modulating the glycosylation of IgG.42
Besides erythrophagocytosis, the B-cell response to opsonized RBCs is another undesired event in AIHA. EndoS hydrolysis of the glycan on human IgG2 results in an enhanced binding affinity toward FcγRIIB as opposed to other IgG subclasses.20 This is particularly interesting because FcγRIIB on B cells is responsible for the down-regulation of B-cell activity when coligated with B-cell receptor by IgG immune complexes.44 It is therefore of major interest and a future aim to investigate the role of EndoS in recognition of autoantibody-sensitized RBCs by B cells and the subsequent T-cell responsiveness.
The amount of EndoS used for in vivo experiment for treatment of BALB/c mice was 20 μg. However, according to Albert et al,42 10 μg of injected EndoS has an inhibitory effect on autoimmune inflammation in mice. Thus, it can be reasonable to suggest that 15 mg EndoS injected into the circulation of humans is enough to completely hydrolyze the whole pool of IgG. This could be an attractive alternative to IVIG therapy of AIHA where routinely 2 g/kg per month is used. Immunogenicity of EndoS could be a potential problem; but despite repeated injections and high antibody titers against EndoS in rabbits, the enzymatic activity is not inhibited.22 Another potential concern when treating with EndoS is the transient lack of many of the effector functions of IgG that could result in increased susceptibility to infection. Patients with agammaglobulinemias frequently have persistent infections with predominantly extracellular bacterial pathogens, such as Helicobacter and Campylobacter.45 In otherwise immunocompetent persons, a transient lack of IgG effector functions probably does not pose a major threat; but in the immunocompromised and for prolonged treatment, replacement therapy with IVIG will most likely be indicated. Such replacement would be facilitated by the short half-life of EndoS in vivo (< 12 hours) that would then not affect the integrity of the administered IVIG.
EndoS has previously been shown to hydrolyze the glycans on all subclasses of murine IgG, but there are subclass differences in the inhibitory effect of ITP provoked by monoclonal IgG antibodies against platelets.22,42 Monoclonal IgG2a antibodies against platelets still induce thrombocytopenia, whereas the thrombocytopenic effect of IgG1 and IgG2b is abrogated. This is also the case for AIHA induced by 34-3C anti-RBC mAb. These data indicate that the activity of IgG1 and IgG2b subclasses in mice is more dependent on the intact sugar moiety on the IgG Fc region than the activity of IgG2a. EndoS hydrolyzes all human IgG subclasses; and in contrast to mice, all hydrolyzed subclasses are affected in their FcR binding in vitro.20 Here we show that EndoS decreases the activation of complement and inhibits binding of IgG/sensitized RBCs to FcγR, thus providing a rationale for the use of EndoS in future therapy of autoantibody-mediated autoimmune diseases. Notably, ITP is often associated with AIHA. EndoS is worth special attention when evaluating therapeutic advantages because it has a dual function, such as an inhibitor of both platelet and RBC elimination.
In conclusion, the results presented here establish that EndoS treatment should be considered as future therapy not only for AIHA but also for other pathologic conditions characterized by an abnormal removal of IgG-opsonized RBCs by macrophages.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms Ulla Johannesson for excellent technical assistance, Dr Franz Petry for providing anti–mouse C1q antibodies, and Dr Jill Storry and Anthony Wilkes for discussions concerning RBC methodology.
This work was supported by the Swedish Research Council (projects 2005-4791 and 2010-57X-20240, M.C.; and project 2008-71X-14251, M.L.O.), Swiss National Foundation for Scientific Research (S.I.), the Foundations of Crafoord (M.C.), Zoégas (M.C.), Bergvall (M.C.), Österlund (M.C.), and Wiberg (M.C.), the Swedish Society for Medicine (M.C.), the Royal Physiografic Society (M.C., M.A.), King Gustaf V's Memorial Fund (M.C.), the Medical Faculty at Lund University (research grants; M.C.), Lund University Hospital (governmental ALF research grants; M.L.O.), and Hansa Medical AB (M.C.).
Authorship
Contribution: M.A. generated and analyzed in vitro experimental data; C.L. performed flow cytometry of monocytes; J.G.B., L.B., and S.I. performed and analyzed the animal studies; M.A., M.C., S.I., and M.L.O. conceived and designed the study; M.L.O. characterized and selected the human plasma samples; M.A. wrote the manuscript; and all authors read, revised, and approved the manuscript.
Conflict-of-interest disclosure: Patents for the in vitro and in vivo use of EndoS have been applied for by Genovis AB and Hansa Medical AB, respectively. M.A. and M.C. are listed as inventors on these pending applications. Hansa Medical AB in part funded this study but had no influence on the design of the study, interpretation of the data, or the final form of the manuscript. M.C. is a part-time scientific consultant for Hansa Medical AB. The remaining authors declare no competing financial interests.
Correspondence: Maria Allhorn, Division of Infection Medicine, Biomedical Center B14, SE-221 84 Lund, Sweden; e-mail: maria.allhorn@med.lu.se.

![Figure 1. Treatment of anti-D with EndoS does not affect the binding of anti-D to RBCs. (A) Western blot analysis of anti-D on RBCs using peroxidase-conjugated anti–human IgG. (B) Glycan hydrolysis of anti-D on RBCs determined by lectin ELISA (optical density [OD] = 450 nm). (C) Quantification of RBC-bound anti-D by ELISA (OD = 450 nm) using antiserum against human IgG.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/24/10.1182_blood-2009-08-239020/4/m_zh89991053770001.jpeg?Expires=1768014116&Signature=Jw2FAFNHKNc19unQ0hIHl9eSay4LrGxzwz1v-J-GyM~CAwYnt2JpHNVT3td~Y10cWYk7~Sy7wl2w7naCRak2iH8blaH4VDdjq8o0Pb0qy88cuBlDmUIJVJo0xf7zGvK~w5V72HwmkJRG1zhW-S9J5S2iEprQexGA5eLJaInBTvXtb529V8k0xOAT-aOJC6xuit-Cef3BErbTgkzRExXk8mKwacZylH65wPYHKPwKKR5y-O~Ew1Ogim3xLSf31Y4cIkAqL3JgNVQQ7tqgpphfVlHseS9wIYT5AuwFXAL1jI-31wwjeZCFIqyApw3V1u1IRKOQPP3hyrFqPkFmsV92wg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)