Abstract
We have previously shown clinical activity of a mammalian target of rapamycin (mTOR) complex 1 inhibitor in Waldenstrom macroglobulinemia (WM). However, 50% of patients did not respond to therapy. We therefore examined mechanisms of activation of the phosphoinositide 3-kinase (PI3K)/Akt/mTOR in WM, and mechanisms of overcoming resistance to therapy. We first demonstrated that primary WM cells show constitutive activation of the PI3K/Akt pathway, supported by decreased expression of phosphate and tensin homolog tumor suppressor gene (PTEN) at the gene and protein levels, together with constitutive activation of Akt and mTOR. We illustrated that dual targeting of the PI3K/mTOR pathway by the novel inhibitor NVP-BEZ235 showed higher cytotoxicity on WM cells compared with inhibition of the PI3K or mTOR pathways alone. In addition, NVP-BEZ235 inhibited both rictor and raptor, thus abrogating the rictor-induced Akt phosphorylation. NVP-BEZ235 also induced significant cytotoxicity in WM cells in a caspase-dependent and -independent manner, through targeting the Forkhead box transcription factors. In addition, NVP-BEZ235 targeted WM cells in the context of bone marrow microenvironment, leading to significant inhibition of migration, adhesion in vitro, and homing in vivo. These studies therefore show that dual targeting of the PI3K/mTOR pathway is a better modality of targeted therapy for tumors that harbor activation of the PI3K/mTOR signaling cascade, such as WM.
Introduction
Tumorigenesis results from synergistic interactions of a complex of signal transduction processes, including multiple oncoproteins and tumor suppressors such as Ras, Myc, phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR), Her-2/Neu, p53, and phosphate and tensin homolog tumor suppressor gene (PTEN).1-7 The PI3K pathway plays a pivotal role in the initiation and progression of malignancies, enhancing cell survival by stimulating cell proliferation and inhibiting apoptosis.2,3,8,9 Signaling begins with the activation of receptor tyrosine kinases. Upon activation by a ligand, receptor tyrosine kinases engage and activate PI3K, which in turn converts membrane-bound phosphatidylinositol (4,5)-bisphosphonate to phosphatidylinositol (3,4,5)-triphosphonate. Phosphatidylinositol (3,4,5)-triphosphonate then activates Akt by phosphorylation.10 Akt acts to promote cell proliferation and survival, and regulates multiple signaling pathways that maintain cell cycle, proliferation, and resistance to apoptosis, such as Bcl-2 associated agonist of death, caspases, Inhibitor of Kappa light chain polypeptide gene enhancer B cells Kinase, Glycogen Synthase Kinase 3 (GSK3), Forkhead-related transcription factor 1, endothelial Nitric Oxide Synthase, and mTOR.8,10 The mTOR kinase leads to cell growth and proliferation.11 mTOR exists in 2 distinct functional complexes, mTORC1 and mTORC2. mTORC1 (rapamycin sensitive) consists of mTOR and raptor, and its activation results in phosphorylation of p70S6 and 4E-BP1. mTORC2 consists of mTOR and the rapamycin-insensitive companion of mTOR (rictor), and it results in Akt phosphorylation.12-14 PTEN acts as a crucial negative regulator of PI3K/Akt and mTOR pathways.15,16 Therefore, it is critical to examine therapeutic agents that explicitly target this pathway, specifically in tumors that harbor activation of the PI3K/Akt pathway.
Waldenstrom macroglobulinemia (WM) is a rare low-grade immunoglobulin M (IgM)–secreting lymphoplasmacytic lymphoma, characterized by the presence of lymphoplasmacytic cells in the bone marrow (BM) and IgM secretion in the peripheral blood. We have previously shown that Akt is constitutively activated in this disease.3 We have also shown that targeting mTOR leads to significant clinical activity in these patients with up to 45% partial remission when treated with a TORC1 inhibitor (RAD001; Novartis).17 However, patients did not have a complete remission, which indicates a mechanism of resistance to TORC1 exists in WM. We therefore sought to examine the activity of the PI3K/Akt/mTOR pathway in WM and whether dual targeting of the PI3K and mTOR pathways will show higher cytotoxic activity in WM cells compared with PI3K or mTOR inhibitors alone.
In this study, we first demonstrated that WM cells show constitutive activation of the PI3K/Akt pathway, as shown by decreased expression of PTEN at the gene and protein levels, together with constitutive activation of Akt and mTOR, PI3K downstream signaling cascades. We then showed that dual targeting of the PI3K and mTOR pathways by the novel inhibitor NVP-BEZ235 exhibited toxicity on WM cells by directly targeting the tumor clone, and indirectly through an indirect effect on the BM milieu in vitro and in vivo. These studies therefore show that dual targeting of the PI3K and mTOR pathways is a better modality of targeted therapy for tumors that harbor activation of the PI3K/mTOR pathway, such as in WM.
Methods
Cells
The WM cell lines (BCWM.1) and IgM-secreting low-grade lymphoma cell lines (MEC-1; RL) were used in this study.3 MEC-1 was a gift from Dr Neil Kay (Mayo Clinic, Rochester MN). RL was purchased from the ATCC. Primary WM cells were obtained from BM samples from previously treated WM patients using CD19+ microbead selection (Miltenyi Biotec) with more than 90% purity, as confirmed by flow cytometric analysis with monoclonal antibody reactive to human CD20-phycoerythrin (BD Biosciences). Peripheral blood mononuclear cells (PBMCs) were obtained from healthy subjects by Ficoll-Hipaque density sedimentation, and subsequently, CD19+ selection was performed. Cells were cultured at 37°C in RPMI 1640 containing 10% fetal bovine serum (FBS; Sigma-Aldrich), 2mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (GIBCO). Approval for these studies was obtained from the Dana-Farber Cancer Institute Institutional Review Board. Informed consent was obtained from all patients and healthy volunteers in accordance with the Declaration of Helsinki protocol.
Reagents
NVP-BEZ235 was provided by Novartis, diluted in dimethylsulfoxide, and stored at 4°C until use; it was then diluted in culture medium immediately before use. The maximum final concentration of dimethylsulfoxide (< 0.1%) did not affect cell proliferation and did not induce cytotoxicity on the cell lines and primary cells tested (data not shown). Mitogen-activated protein kinase kinase (MEK) 1/2 inhibitor U0126, LY-294002, and rapamycin were purchased from Calbiochem.
Growth inhibition assay
The inhibitory effect of BEZ-235 on WM cells, IgM-secreting cell lines, and primary cell growth was assessed by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Chemicon International) dye absorbance, as previously described.3
DNA synthesis
DNA synthesis was measured by [3H]-thymidine (PerkinElmer) uptake, as previously described.3
Flow cytometric analysis
Cell-cycle analysis was profiled by flow cytometry using propidium iodide (PI) staining (5 μg/mL; Sigma-Aldrich) after 24-hour culture with BEZ-235, rapamycin alone, or in combination with LY-294002. Apoptosis was quantitated using annexin/PI staining and flow cytometric analysis (Beckman Coulter), as described.3
Immunoblotting
WM and IgM-secreting cell lines were harvested and lysed using lysis buffer (Cell Signaling Technology) reconstituted with 5mM NaF, 2mM Na3VO4, 1mM phenylmethylsulfonyl fluoride, 5 μg/mL leupeptine, and 5 μg/mL aprotinin. Whole-cell lysates (50 μg/lane) were subjected to sodium dodecyl sulfate–polyacrylamide gel eletrophoresis and transferred to polyvinyldene fluoride membrane (Bio-Rad). The antibodies used for immunoblotting included the following: anti-phospho (p)–Akt (Ser473), anti-Akt, anti–p-GSK3α/β (Ser21/9), p-S6 ribosomal, anti-PTEN, p-mTOR, anti-mTOR, p-p70S6, anti–p-4EBP1, anti-rictor, anti-raptor, anti–p-extracellular signal-regulated kinase (ERK)1/2 (Thr202/Tyr204), anti-ERK1/2, p-cRAF, Rheb, anti–caspase-3, anti–caspase-8, anti–caspase-9, anti–poly(adenosine 5′-diphosphate-ribose) polymerase (PARP), anti-mcl1, anti–second mitochondria-derived activator of caspases (Smac), anti–cellular (c) inhibitors of apoptosis proteins 1 (IAP1), anti–p-foxO1/O4, anti–p-foxO3, anti-p27kip1, anti-p21Cip1, anti-BIM (Bcl-2 interacting mediator of cell death), p53, anti-PUMA (p53 upregulated modulator of apoptosis), and anti-Bax (Cell Signaling Technology); and anti–α-tubulin antibodies (Santa Cruz Biotechnology).
Immunoprecipitation
mTOR complexes were immunoprecipitated with mTOR antibody and analyzed by Western blot, as previously described.14
In vitro Akt and mTOR kinase assays
In vitro Akt kinase assay (Cell Signaling Technology) was performed, as previously described.3 In vitro mTOR activity has been evaluated using the Kinase-linked immunosorbent assay (K-LISA) mTOR activity kit (Calbiochem), following the manufacturer's procedure.
Immunofluorescence
The effect of BEZ-235 on Akt and mTOR phosphorylation has been evaluated in WM cells. Briefly, BCWM.1 cells were cultured in presence or absence of NVP-BEZ235 (20nM) for 6 hours. Immunocytochemical analysis was performed using an epifluorescence microscope (Nikon Eclipse E800) and a Photometrics Coolsnap CF color camera (Nikon), as previously described.18
Effect of NVP-BEZ235 on paracrine WM cell growth in the BM
To evaluate growth stimulation and signaling in WM cells adherent to BM stromal cells (BMSCs), 3 × 104 BCWM.1 cells were cultured in BMSC-coated 96-well plates for 48 hours in the presence or absence of NVP-BEZ235. DNA synthesis was measured, as described.3
Transwell migration assay
We performed transwell migration assay (Costar; Corning) using BCWM.1 cells in the presence or absence of 30nM stromal derived factor-1 (SDF-1).18 In brief, cells were suspended in 1% fetal calf serum media and were placed (2 × 105 cells) in the upper chambers of the transwell plates, with serial concentrations of SDF-1 in the lower chambers in 1 mL of 1% fetal calf serum media. After 4 hours at 37°C, cells that migrated to the lower chambers were counted. Triplicates of each concentration were performed, and the means and standard deviations were calculated.
Adhesion assay
BCWM.1 cells were pretreated with NVP-BEZ235 for 4 hours, before an in vitro adhesion assay to fibronectin (FN), and primary BMSCs, following the manufacturer's recommendations (EMD Biosciences). Calcein Acetoxymethylester (AM) was used to measure adherent cells, and the degree of fluorescence was measured using a spectrophotometer. (485-520 nm) Bovine serum albumin–coated wells served as a negative control.
Lentivirus short hairpin RNA vector construction and Akt gene transduction
To further determine the role of NPI-0052 in the regulation of the Akt pathway, we established an Akt knockout BCWM.1 cell line using a lentivirus transfection system, as previously described.3,19-21 The sense and antisense oligonucleotide sequence for construction of Akt short hairpin RNA were as follows: clone 10162, target sequence GGACAAGGACGGGCACATTAA; 10163, target sequence CGAGTTTGAGTACCTGAAGCT.
mTOR small interfering RNA
BCWM.1 and other IgM-secreting low-grade lymphoma cell lines (RL; MEC.1) were used and transfected with either scramble probe or mTOR small interfering RNA (siRNA), according to manufacturer's procedure (Cell Signaling Technology).
Gene expression profiling
Total RNA has been isolated from primary CD19+ cells isolated from BM of patients with WM and PBMCs of healthy donors, using RNeasy kit (QIAGEN), as described by the manufacturer. Purified cRNA (15 μg) was hybridized to HG-U133Plus2.0 GeneChip (Affimetrix). RNA integrity was verified with the Agilent 2100 Bioanalyzer (Agilent).22
In vivo flow cytometry
The effect of NVP-BEZ325 on homing in vivo has been tested using BALB/c mice with in vivo flow cytometry, as previously described.21,23,24 Briefly, BCWM.1 cells were treated with 20nM NVP-BEZ235, or control phosphate-buffered saline, for 4 hours, and then injected into the mice. Treated cells and untreated cells were fluorescently labeled by incubation with calcein red/orange (Invitrogen) and calcein green/AM (Molecular Probes), 5μM, respectively, for 30 minutes. Each mouse received both calcein red/orange– and calcein green/AM–labeled cells. Fluorescence signal was detected on an appropriate artery in the ear and digitized for analysis with Matlab software developed in house, as described.21,23,24
Statistical analysis
Statistical significance of differences in drug-treated versus control cultures was determined using Student t test. The minimal level of significance was P less than .05. Gene expression profiling-supervised clustering analysis has been performed using the dChip software (www.dchip.org; P < .05). Drug synergism was analyzed by isobologram analysis using the CalcuSyn software program (Biosoft), as described.24
Results
WM is characterized by reduced expression of PTEN and constitutive activation of the PI3K/Akt/mTOR pathway
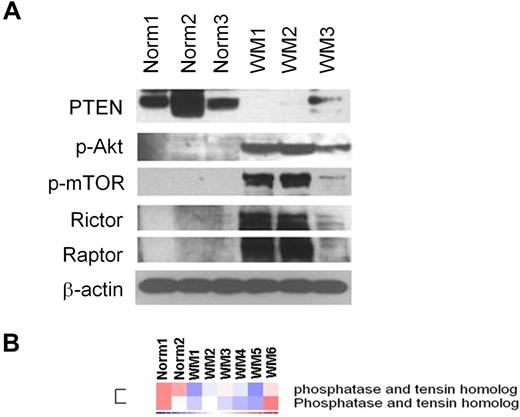
We first examined whether the PI3K/Akt/mTOR pathway is activated in WM primary tumor cells. As shown in Figure 1A, the negative regulator of the PI3K pathway PTEN was significantly reduced in CD19+ WM cells compared with normal CD19+ control cells. It is known that PTEN acts also as a negative regulator of Akt and mTOR;16 we therefore sought to investigate the expression level of p-Akt and p-mTOR in primary WM tumor cells. Consequently, we demonstrated a higher p-Akt and downstream p-mTOR protein levels compared with their normal cellular counterpart together with a higher expression of rictor and raptor, 2 different components of the protein kinase mTOR (Figure 1A). We further confirmed that PTEN expression was decreased at the gene level in another 6 WM samples compared with 2 normal control CD19+ cells (Figure 1B).
Primary WM cells are characterized by low expression of PTEN and high expression of p-Akt and p-mTOR, compared with their normal cellular counterpart. (A) Immunoblotting for PTEN, p-Akt, p-mTOR, rictor, raptor, and β-actin expression in BM-derived CD19+ WM primary cells (WM), compared with normal primary CD19+ cells (Norm). (B) Purified cRNA (15 μg) isolated from primary BM-derived CD19+ WM primary cells (WM) and normal primary CD19+ cells (Norm) was hybridized to HG-U133Plus2.0 GeneChip (Affimetrix). Fold change is shown by the intensity of induction (red) or suppression (blue).
Primary WM cells are characterized by low expression of PTEN and high expression of p-Akt and p-mTOR, compared with their normal cellular counterpart. (A) Immunoblotting for PTEN, p-Akt, p-mTOR, rictor, raptor, and β-actin expression in BM-derived CD19+ WM primary cells (WM), compared with normal primary CD19+ cells (Norm). (B) Purified cRNA (15 μg) isolated from primary BM-derived CD19+ WM primary cells (WM) and normal primary CD19+ cells (Norm) was hybridized to HG-U133Plus2.0 GeneChip (Affimetrix). Fold change is shown by the intensity of induction (red) or suppression (blue).
Dual targeting of the PI3K and mTOR pathways in WM cells and other IgM-secreting low-grade lymphoma cells
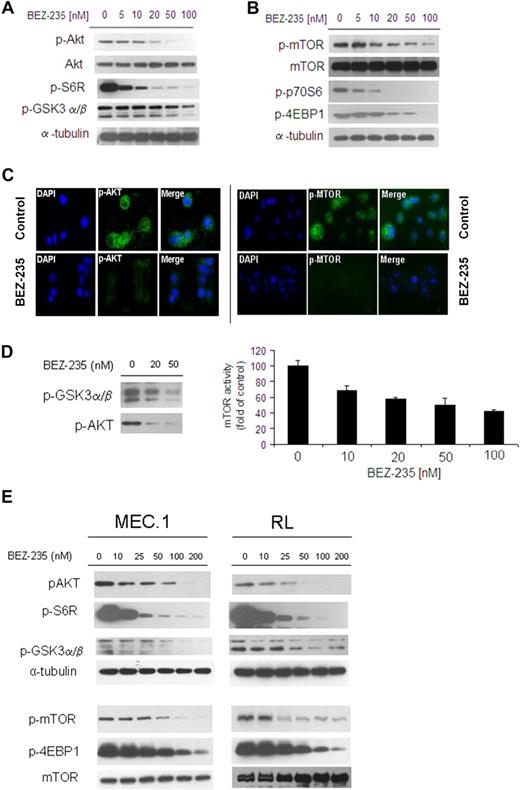
We first investigated the efficacy of the dual NVP-BEZ235 in targeting Akt and mTOR pathways in WM cells as well as in other IgM-secreting low-grade lymphoma cell lines. BCWM.1 were treated with increasing doses of NVP-BEZ235 (5-100nM) for 6 hours: NVP-BEZ235 inhibited phosphorylation of Akt (ser473) and downstream GSK3α/β and ribosomal protein S6 in a dose-dependent manner in WM cells (Figure 2A). In parallel, NVP-BEZ235 also inhibited phosphorylation of mTOR as well as of the downstream targets p70S6 and 4EBP1, in a dose-dependent manner in WM cells (Figure 2B). We further confirmed inhibition of p-Akt and p-mTOR upon NVP-BEZ235 treatment, as shown by immunofluorescence in WM cells (Figure 2C). We then confirmed the effect of NVP-BEZ235 on Akt and mTOR activities using an in vitro Akt kinase assay and a K-LISA mTOR activity kit in a dose-dependent manner (Figure 2D).
NVP-BEZ235 targets Akt and mTOR, and inhibits Akt and mTOR activity in WM cells and other low-grade lymphoma IgM-secreting cell lines. (A-B) BCWM.1 cells were cultured with NVP-BEZ235 (5-100nM) for 6 hours. Whole-cell lysates were subjected to Western blotting using anti–p-Akt, anti-Akt, anti–p-S6R, anti–p-GSK3α/β, anti–p-mTOR, anti-mTOR, p-p70S6, anti–p-4EBP1, and anti–α-tubulin antibodies. (C) BCWM.1 cells were cultured with NVP-BEZ235 (20nM) or control medium for 6 hours. Immunocytochemical analysis was assessed using anti–p-Akt and p-mTOR antibodies. The 4′,6-diamidino-2-phenylindole was used to stain nuclei. (D) In vitro Akt and in vitro mTOR kinase assays. BCWM.1 cells were cultured with control media or NVP-BEZ235 (20-50nM) for 6 hours. Akt kinase assay: whole-cell lysates were immunoprecipitated with anti-Akt antibody. Then the immunoprecipitate was washed and subjected to in vitro kinase assay, according to the manufacturer's protocol. Western blotting used anti–p-GSK3α/β and anti-Akt antibodies. mTOR kinase assay: whole-cell lysates were immunoprecipitated with anti-mTOR antibody. Then the immunoprecipitate was washed and subjected to in vitro kinase assay, according to the manufacturer's protocol. Enzyme-linked immunosorbent assay–based assay has been performed (anti-p70S6K was added to each well), and absorbance read at 450nM with a reference wavelength set at 540nM. (E) IgM-secreting cell lines (MEC.1; RL) were cultured with NVP-BEZ235 (10-200nM) for 6 hours. Whole-cell lysates were subjected to Western blotting using anti–p-Akt, anti-Akt, anti–p-S6R, anti–p-GSK3α/β, anti–p-mTOR, anti–p-4EBP1, anti-mTOR, and anti–α-tubulin antibodies.
NVP-BEZ235 targets Akt and mTOR, and inhibits Akt and mTOR activity in WM cells and other low-grade lymphoma IgM-secreting cell lines. (A-B) BCWM.1 cells were cultured with NVP-BEZ235 (5-100nM) for 6 hours. Whole-cell lysates were subjected to Western blotting using anti–p-Akt, anti-Akt, anti–p-S6R, anti–p-GSK3α/β, anti–p-mTOR, anti-mTOR, p-p70S6, anti–p-4EBP1, and anti–α-tubulin antibodies. (C) BCWM.1 cells were cultured with NVP-BEZ235 (20nM) or control medium for 6 hours. Immunocytochemical analysis was assessed using anti–p-Akt and p-mTOR antibodies. The 4′,6-diamidino-2-phenylindole was used to stain nuclei. (D) In vitro Akt and in vitro mTOR kinase assays. BCWM.1 cells were cultured with control media or NVP-BEZ235 (20-50nM) for 6 hours. Akt kinase assay: whole-cell lysates were immunoprecipitated with anti-Akt antibody. Then the immunoprecipitate was washed and subjected to in vitro kinase assay, according to the manufacturer's protocol. Western blotting used anti–p-GSK3α/β and anti-Akt antibodies. mTOR kinase assay: whole-cell lysates were immunoprecipitated with anti-mTOR antibody. Then the immunoprecipitate was washed and subjected to in vitro kinase assay, according to the manufacturer's protocol. Enzyme-linked immunosorbent assay–based assay has been performed (anti-p70S6K was added to each well), and absorbance read at 450nM with a reference wavelength set at 540nM. (E) IgM-secreting cell lines (MEC.1; RL) were cultured with NVP-BEZ235 (10-200nM) for 6 hours. Whole-cell lysates were subjected to Western blotting using anti–p-Akt, anti-Akt, anti–p-S6R, anti–p-GSK3α/β, anti–p-mTOR, anti–p-4EBP1, anti-mTOR, and anti–α-tubulin antibodies.
Next, we further validated the role of NVP-BEZ235 in targeting Akt and mTOR pathways in other IgM-secreting low-grade lymphoma cells: NVP-BEZ235 inhibited both Akt and mTOR as well as the related downstream-targeted proteins in MEC.1 and RL cell lines (Figure 2E).
Dual targeting of PI3K and mTOR by NVP-BEZ235 differs in activity compared with single PI3K or mTOR inhibitors
mTOR is a large protein kinase that exists as 2 different entities within cells: one that contains mTOR and raptor, and another containing mTOR and rictor. The raptor-mTOR complex is sensitive to the mTOR inhibitor rapamycin, whereas the rictor-containing complex is rapamycin insensitive.12-14 Moreover, it has been previously shown that rictor-mTOR complex represents a positive feedback loop responsible for Akt phosphorylation.14 We therefore investigated the effect of NVP-BEZ235 in targeting both the raptor- and rictor-mTOR complexes. WM cells were treated for 6 hours with either NVP-BEZ235, the PI3K inhibitor LY294002, the mTOR inhibitor rapamycin, or the combination of LY294002 and rapamycin. We first showed a 50% inhibition/inhibitory concentration (IC50) for LY294002 and rapamycin of 10μM and 5nM, respectively (Figure 3A). As shown in Figure 3B, NVP-BEZ235 targeted both rictor and raptor in the context of mTORC1 and mTORC2 complexes, indicating that this may result in down-regulating the rictor-positive feedback loop on Akt activation. Indeed, whereas rapamycin inhibited raptor and did not target rictor, leading to p-Akt up-regulation, NVP-BEZ235 induced significant p-Akt inhibition, resulting from the dual targeting of both rictor and raptor. In addition, NVP-BEZ235–induced WM cells p-Akt down-regulation appeared to be more significant than the effect of LY294002. Interestingly, NVP-BEZ235 was equally or more effective in down-regulating the mTOR downstream-targeted proteins p-p70S6 and p-4EBP1 compared with either LY294002, which inhibited only p-p70S6, or rapamycin when used alone or in combination with LY294002 (Figure 3B). In addition, we found that NVP-BEZ235 up-regulated ERK phosphorylation, which appeared to be less significant compared with rapamycin- or LY294002-induced p-ERK up-regulation, either used as single drug or in combination (Figure 3B).
NVP-BEZ235 targets both rictor and raptor. (A) Thymidine uptake assay. BCWM.1 cells were cultured with rapamycin and LY294002 for 48 hours. (B-C) BCWM.1 cells were cultured with NVP-BEZ235 (20nM), rapamycin (5nM), LY294002 (10μM), or the combination of rapamycin and LY294002 for 6 hours. (B) Whole-cell lysates were subjected to immunoprecipitation (IP) using a mTOR antibody and Western blotting using anti-mTOR, anti-rictor, and anti-raptor antibodies. (C) Whole-cell lysates were subjected to Western blot using anti–p-Akt, anti-Akt, anti–p-p70S6, anti–p-4EBP1, anti–p-ERK, anti-ERK, and anti–α-tubulin antibodies.
NVP-BEZ235 targets both rictor and raptor. (A) Thymidine uptake assay. BCWM.1 cells were cultured with rapamycin and LY294002 for 48 hours. (B-C) BCWM.1 cells were cultured with NVP-BEZ235 (20nM), rapamycin (5nM), LY294002 (10μM), or the combination of rapamycin and LY294002 for 6 hours. (B) Whole-cell lysates were subjected to immunoprecipitation (IP) using a mTOR antibody and Western blotting using anti-mTOR, anti-rictor, and anti-raptor antibodies. (C) Whole-cell lysates were subjected to Western blot using anti–p-Akt, anti-Akt, anti–p-p70S6, anti–p-4EBP1, anti–p-ERK, anti-ERK, and anti–α-tubulin antibodies.
NVP-BEZ235 inhibits DNA synthesis and induces cytotoxicity of WM cells and IgM-secreting low-grade lymphoma cells
The PI3K/Akt and mTOR pathways regulate cell growth and proliferation.1-3,25 We therefore sought to investigate the effect of NVP-BEZ235 on WM cells and IgM-secreting low-grade lymphoma cells. WM cells were cultured for 24 to 48 hours and 48 to 72 hours in presence or absence of NVP-BEZ235 (0.1-400nM). As shown in Figure 4A, NVP-BEZ235 inhibited BCWM.1 proliferation, as measured by [3H]-thymidine uptake assay, with an IC50 of 25nM at 48 hours. NVP-BEZ235 demonstrated similar activity on all IgM-secreting cell lines tested, with IC50 between 25 and 75nM at 48 hours (Figure 4B). Next, we studied the cytotoxic effect of NVP-BEZ235 (0.1-400nM) on WM-, IgM-secreting cell lines, and primary CD19+ WM cells by MTT assay. NVP-BEZ235 decreased survival of BCWM.1 cells (IC50, 50nM, at 48 hours; Figure 4C) with similar results on IgM-secreting cell lines (Figure 4D). To further validate the role of mTOR and Akt pathways in NVP-BEZ235–dependent cytotoxicity, we used mTOR and Akt knockdown approaches. We have performed a mTOR siRNA (Cell Signaling Technology) in WM (BCWM.1) and other IgM-secreting low-grade lymphoma cell lines (RL; MEC1): untransfected, scramble probe-, and mTOR siRNA-transfected cells have been studied. MTT assay performed at 48 hours showed that mTOR-siRNA is responsible for approximately 20% of toxicity in the transfected WM cells, which increases in a dose-dependent manner by treating transfected cells with NVP-BEZ235 (Figure 4E). Similar results were obtained in IgM-secreting low-grade lymphoma cell lines (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). This suggests that although mTOR has been silenced, NVP-BEZ235 may still exert its antitumor activity, which results from its targeting on PI3K/Akt signaling pathway. In addition, we used an Akt knockdown BCWM.1 cell line established using lentivirus infection3,19-21 and demonstrated 50% induction of cytotoxicity at baseline level in transfected cells (Figure 4F). NVP-BEZ235 did not induce further cytotoxicity in these cells. This may be due to the absence of Akt, a crucial mediator of PI3K/Akt and mTOR pathways. NVP-BEZ235 induced cytotoxicity in primary CD19+ cells isolated from 3 patients with WM (IC50 27-40nM; Figure 4E). In contrast, NVP-BEZ235 had no cytotoxic effect on PBMC-derived CD19+ cells isolated from 4 healthy volunteers (Figure 4F). These results demonstrate that NVP-BEZ235 triggers significant cytotoxicity in tumor cell lines and patient CD19+ WM cells, without toxicity in normal PBMCs.
NVP-BEZ235 decreases DNA synthesis and triggers cytotoxicity in WM cells and IgM-secreting cell lines, without affecting normal cells. (A) Thymidine uptake assay. BCWM.1 cells were cultured with NVP-BEZ235 (0.1-400nM) for 24 to 72 hours. (B) Thymidine uptake assay. IgM-secreting cell lines (MEC.1; RL) were cultured with NVP-BEZ235 (0.1-400nM) for 48 hours. (C) Cytotoxicity was assessed by MTT assay. BCWM.1 cells were cultured with BEZ235 (0.1-400nM) for 24 to 48 hours and 48 to 72 hours. (D) Cytotoxicity was assessed by MTT assay. IgM-secreting cell lines (MEC.1; RL) were cultured with NVP-BEZ235 (0.1-400nM) for 48 hours. (E) BCWM.1 cells were transfected with either scramble probe or mTOR siRNA. Untransfected BCWM.1 cells were used as control. Cells were treated with NVP-BEZ235 (2.5-100nM) for 48 hours, and cytotoxicity was assessed by MTT assay. Whole-cell lysates were subjected to Western blotting using anti-mTOR and α-tubulin antibodies (inset [E]). (F) BCWM.1 cells were transduced with Akt short hairpin RNA. Mock: control plasmid. BCWM.1-transfected cells or BCWM.1 control cells were treated with NVP-BEZ235 (2.5-100nM) for 48 hours, and cytotoxicity was assessed by MTT. Whole-cell lysates were subjected to Western blotting using anti-Akt and α-tubulin antibodies (inset [F]). (G) Freshly isolated BM CD19+ tumor cells from 4 patients with WM were cultured with NVP-BEZ235 (5-50nM) for 48 hours. Cytotoxicity was assessed by MTT assay. (H) Freshly isolated peripheral blood–derived CD19+ from 4 healthy donors was cultured with NVP-BEZ235 (2.5-1000nM) for 48 hours.
NVP-BEZ235 decreases DNA synthesis and triggers cytotoxicity in WM cells and IgM-secreting cell lines, without affecting normal cells. (A) Thymidine uptake assay. BCWM.1 cells were cultured with NVP-BEZ235 (0.1-400nM) for 24 to 72 hours. (B) Thymidine uptake assay. IgM-secreting cell lines (MEC.1; RL) were cultured with NVP-BEZ235 (0.1-400nM) for 48 hours. (C) Cytotoxicity was assessed by MTT assay. BCWM.1 cells were cultured with BEZ235 (0.1-400nM) for 24 to 48 hours and 48 to 72 hours. (D) Cytotoxicity was assessed by MTT assay. IgM-secreting cell lines (MEC.1; RL) were cultured with NVP-BEZ235 (0.1-400nM) for 48 hours. (E) BCWM.1 cells were transfected with either scramble probe or mTOR siRNA. Untransfected BCWM.1 cells were used as control. Cells were treated with NVP-BEZ235 (2.5-100nM) for 48 hours, and cytotoxicity was assessed by MTT assay. Whole-cell lysates were subjected to Western blotting using anti-mTOR and α-tubulin antibodies (inset [E]). (F) BCWM.1 cells were transduced with Akt short hairpin RNA. Mock: control plasmid. BCWM.1-transfected cells or BCWM.1 control cells were treated with NVP-BEZ235 (2.5-100nM) for 48 hours, and cytotoxicity was assessed by MTT. Whole-cell lysates were subjected to Western blotting using anti-Akt and α-tubulin antibodies (inset [F]). (G) Freshly isolated BM CD19+ tumor cells from 4 patients with WM were cultured with NVP-BEZ235 (5-50nM) for 48 hours. Cytotoxicity was assessed by MTT assay. (H) Freshly isolated peripheral blood–derived CD19+ from 4 healthy donors was cultured with NVP-BEZ235 (2.5-1000nM) for 48 hours.
NVP-BEZ235 induces apoptosis in WM cells and IgM-secreting low-grade lymphoma cells
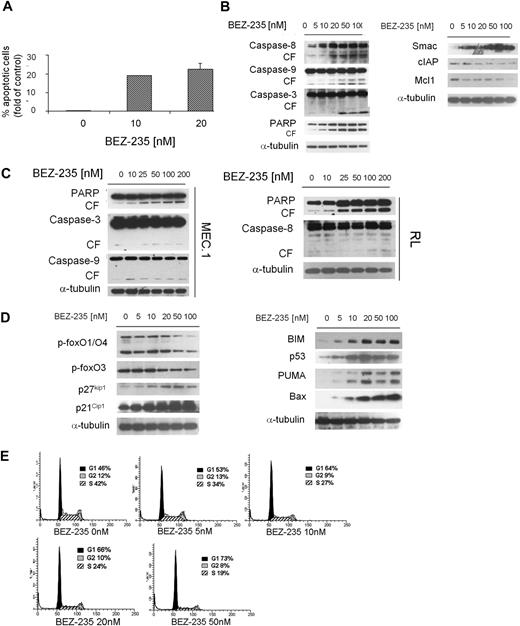
We next examined the functional effect of NVP-BEZ235 on apoptosis in WM cells. We demonstrated that NVP-BEZ235 induced apoptosis, evidenced by annexin/PI staining and flow cytometry analysis. The percentage of apoptotic BCWM.1 cells increased from 0.18% (untreated) to 19% and 24.7% after 48 hours of treatment with 10nM and 20nM NVP-BEZ235, respectively (Figure 5A). Similar data were obtained with other IgM-secreting cell lines (data not shown). We next defined mechanisms whereby NVP-BEZ235 induces apoptosis in WM, and demonstrated that NVP-BEZ235 induced both intrinsic and extrinsic apoptotic pathways with caspase-9, caspase-8, caspase-3, and PARP cleavage in a dose-dependent manner. Moreover, NVP-BEZ235 induced down-modulation of the antiapoptotic protein Mcl-1, with an increased release of the Smac/DIABLO from the mitochondria to the cytosol (Figure 5B). It has been reported that Smac/direct IAP binding protein (DIABLO) can abrogate the protective effects of IAPs, such as c-IAP.26 We therefore treated BCWM.1 cells with NVP-BEZ235 (5-100nM) for 16 hours and demonstrated that NVP-BEZ235 down-regulated the expression of c-IAP1 (Figure 5B). The viability of BCWM.1 cells assessed by MTT was not affected by NVP-BEZ235 treatment at 12 hours (data not shown). To further confirm the apoptotic role of NVP-BEZ235 in other IgM-secreting low-grade lymphoma cell lines, MEC.1 and RL cell lines were evaluated: NVP-BEZ235 induced caspase-3, caspase-9, and PARP in MEC.1 cells together with induction of caspase-8 and PARP cleavage in RL cells (Figure 5C).
NVP-BEZ235 induces apoptosis and cell-cycle arrest in WM cells. (A) BCWM.1 cells were cultured with NVP-BEZ235 (10-20nM) for 48 hours, and apoptosis was performed using annexin/PI staining and flow cytometric analysis. (B) BCWM.1 cells were cultured with NVP-BEZ235 (5-100nM) for 16 hours. Whole-cell lysates were subjected to Western blotting using anti–caspase-8, anti–caspase-9, anti–caspase-3, anti-PARP, anti-Smac/DIABLO, anti–c-IAP1, anti-Mcl1, and anti–α-tubulin antibodies. (C) IgM-secreting cell lines (MEC.1; RL) were cultured with NVP-BEZ235 (5-100nM) for 16 hours. Whole-cell lysates were subjected to Western blotting using anti–caspase-8, anti–caspase-9, anti–caspase-3, anti-PARP, and anti–α-tubulin antibodies. (D) BCWM.1 cells were cultured with NVP-BEZ235 (5-100nM) for 16 hours. Whole-cell lysates were subjected to Western blotting using anti–p-foxO1/O4, anti–p-foxO3, anti-p27kip1, anti-p21Cip1, anti-BIM, anti-p53, anti-PUMA, anti-Bax, and anti–α-tubulin antibodies. (E) BCWM.1 were cultured with NVP-BEZ235 (0-50nM) for 24 hours, and cell-cycle analysis was performed by PI staining.
NVP-BEZ235 induces apoptosis and cell-cycle arrest in WM cells. (A) BCWM.1 cells were cultured with NVP-BEZ235 (10-20nM) for 48 hours, and apoptosis was performed using annexin/PI staining and flow cytometric analysis. (B) BCWM.1 cells were cultured with NVP-BEZ235 (5-100nM) for 16 hours. Whole-cell lysates were subjected to Western blotting using anti–caspase-8, anti–caspase-9, anti–caspase-3, anti-PARP, anti-Smac/DIABLO, anti–c-IAP1, anti-Mcl1, and anti–α-tubulin antibodies. (C) IgM-secreting cell lines (MEC.1; RL) were cultured with NVP-BEZ235 (5-100nM) for 16 hours. Whole-cell lysates were subjected to Western blotting using anti–caspase-8, anti–caspase-9, anti–caspase-3, anti-PARP, and anti–α-tubulin antibodies. (D) BCWM.1 cells were cultured with NVP-BEZ235 (5-100nM) for 16 hours. Whole-cell lysates were subjected to Western blotting using anti–p-foxO1/O4, anti–p-foxO3, anti-p27kip1, anti-p21Cip1, anti-BIM, anti-p53, anti-PUMA, anti-Bax, and anti–α-tubulin antibodies. (E) BCWM.1 were cultured with NVP-BEZ235 (0-50nM) for 24 hours, and cell-cycle analysis was performed by PI staining.
NVP-BEZ235 targets Forkhead box transcription factors and leads to cell-cycle arrest in WM cells
Forkhead box transcription factors (FoxOs) represent downstream effectors of the PI3K/Akt pathway. Phosphorylation of Akt leads to nuclear export and cytoplasm retention of phosphorylated FoxOs, with consequent inhibition of their transcriptional activity.27,28 We therefore sought to investigate the effect of NVP-BEZ235 on FoxOs in WM cells and found that Akt-dependent p-FoxO1/O4/O3 expression was inhibited by NVP-BEZ235 in a dose-dependent manner (Figure 5C). It has been reported that FoxO members act as tumor suppressors by promoting cell-cycle arrest and apoptosis.28-30 Specifically, FoxO3 opposes cell-cycle progression, and we found that NVP-BEZ123 up-regulated cell-cycle inhibitors p27kip1 and p21waf1 together with up-regulation of proapoptotic factors PUMA, Bax, and BIM (Figure 5D). Based on the observed up-regulation of cyclin-dependent kinase inhibitors p27Kip1 and p21Cip1 upon NVP-BEZ235 treatment, we next evaluated the effect of the drug in modulating cell-cycle profiling in WM cells, and demonstrated G1 arrest and reduction of S phase in the NVP-BEZ235–treated cells compared with control (Figure 5E).
NVP-BEZ235 up-regulates the MEK/ERK pathway through activation of Rheb-Raf pathway
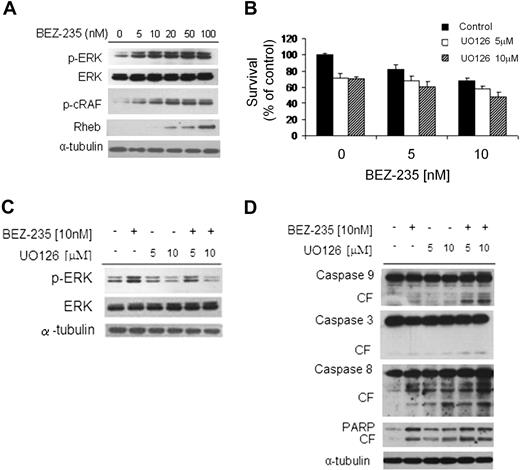
We next sought to examine the effect of NVP-BEZ235 on mitogen-activated protein kinase (MAPK) pathway using immunoblotting analysis, and found that NVP-BEZ235 induced ERK1/2 phosphorylation in a dose-dependent fashion (Figure 6A). It has been previously reported that Akt inhibition activates the MEK/ERK pathway through upstream activation of protein kinase C and c-Raf in WM cells.1 We confirmed these findings using NVP-BEZ235 in WM cells, as demonstrated by up-regulation of p-cRAF in a dose-dependent manner. We next examined whether NVP-BEZ235 could target Rheb, a Raf-MEK-MAPK pathway activator; we found an increased Rheb phosphorylation in NVP-BEZ235-WM–treated cells (Figure 6A), indicating that NVP-BEZ235–induced ERK phosphorylation could result from the activation of the Rheb-Raf pathway.31
NVP-BEZ235 up-regulates the MEK/ERK pathway, and ERK inihibition increases NVP-BEZ235–induced cytotoxicity. (A) BCWM.1 cells were cultured with NVP-BEZ235 (5-100nM) for 6 hours. Whole-cell lysates were subjected to Western blotting using anti–p-ERK, anti-ERK1/2, anti–p-c-Raf, anti-Rheb, and α-tubulin antibodies. (B) BCWM.1 cells were cultured for 48 hours with NVP-BEZ235 (5nM, 10nM) in the absence or presence of MEK1/2 inhibitor U0126 (5μM, 10μM). Cytotoxicity was assessed by the MTT assay. Data represent mean (± SD) of triplicate experiments. (C) BCWM.1 cells were cultured with NVP-BEZ235 (5nM, 10nM) in the absence or presence of MEK1/2 inhibitor U0126 (5μM, 10μM) for 6 hours. Whole-cell lysates were subjected to Western blotting using anti–p-ERK, anti-ERK1/2, and α-tubulin antibodies. (D) BCWM.1 cells were cultured with NVP-BEZ235 (5nM, 10nM) in the absence or presence of MEK1/2 inhibitor U0126 (5μM, 10μM) for 16 hours. Whole-cell lysates were subjected to Western blotting using anti–caspase-9, anti–caspase-3, anti–caspase-8, anti-PARP, and anti–α-tubulin antibodies.
NVP-BEZ235 up-regulates the MEK/ERK pathway, and ERK inihibition increases NVP-BEZ235–induced cytotoxicity. (A) BCWM.1 cells were cultured with NVP-BEZ235 (5-100nM) for 6 hours. Whole-cell lysates were subjected to Western blotting using anti–p-ERK, anti-ERK1/2, anti–p-c-Raf, anti-Rheb, and α-tubulin antibodies. (B) BCWM.1 cells were cultured for 48 hours with NVP-BEZ235 (5nM, 10nM) in the absence or presence of MEK1/2 inhibitor U0126 (5μM, 10μM). Cytotoxicity was assessed by the MTT assay. Data represent mean (± SD) of triplicate experiments. (C) BCWM.1 cells were cultured with NVP-BEZ235 (5nM, 10nM) in the absence or presence of MEK1/2 inhibitor U0126 (5μM, 10μM) for 6 hours. Whole-cell lysates were subjected to Western blotting using anti–p-ERK, anti-ERK1/2, and α-tubulin antibodies. (D) BCWM.1 cells were cultured with NVP-BEZ235 (5nM, 10nM) in the absence or presence of MEK1/2 inhibitor U0126 (5μM, 10μM) for 16 hours. Whole-cell lysates were subjected to Western blotting using anti–caspase-9, anti–caspase-3, anti–caspase-8, anti-PARP, and anti–α-tubulin antibodies.
We then examined whether inhibiting MEK/ERK signaling would enhance NVP-BEZ235–induced cytotoxicity in WM cells. As shown in Figure 6B, cytotoxicity triggered by NVP-BEZ235 (5-10nM) was enhanced in the presence of MEK1/2 inhibitor U0126 (5μM and 10μM). NVP-BEZ235 (5nM) induced cytotoxicity in 17.5% of the cells, which was increased to 31.4% and 38.8% in the presence of 5μM U0126 (combination index [CI]: 0.497) and 10μM U0126 (CI: 0.376), indicating synergism. Similarly, synergism was observed using 10nM NVP-BEZ235 in presence of 5μM and 10μM U126, where we found CIs of 0.231 and 0.190, respectively. The molecular mechanisms whereby NVP-BEZ235–induced cytotoxicity was enhanced by U0126 were next investigated. BCWM.1 cells were treated for 6 hours with either 10nM NVP-BEZ235 or 5μM and 10μM U0126, and the combination. Up-regulation of ERK phosphorylation triggered by NVP-BEZ235 was inhibited by U0126 (Figure 6C), whereas cleavage of caspase-9, -3, -8, and PARP was up-regulated by the combination (Figure 6D).
NVP-BEZ235 targets WM cells even in the context of BM microenvironment in vitro and in vivo
It has been widely demonstrated that BM microenvironment confers growth and induces drug resistance in malignant cells.3,31-33 We therefore sought to evaluate the antitumor activity of NVP-BEZ235 against WM cells in the context of the BM milieu. BCWM.1 cells were cultured with NVP-BEZ235 (5-100nM) in the presence or absence of BMSCs for 48 hours. Using [3H]-thymidine uptake assay, adherence of BCWM.1 cells to BMSCs triggered an increase of 52% in proliferation, which was inhibited by NVP-BEZ235 in a dose-dependent manner (Figure 7A). We next evaluated the efficacy of NVP-BEZ235 in targeting Akt and mTOR in WM cells in the context of BM microenvironment. Adherence of BCWM.1 cells to BMSCs induced phosphorylation of Akt and mTOR; conversely, NVP-BEZ235 abrogated BMSC adhesion-induced phosphorylation of Akt and mTOR in WM cells, indicating that NVP-BEZ235 exerts its antitumor activity even when WM cells were in contact with BM milieu. Similarly, NVP-BEZ235 targeted rictor and raptor in WM cells, even when cultured in presence of BMSCs, which induced up-regulation of raptor, but not rictor (Figure 7B). Previous studies have demonstrated that the PI3K/Akt and mTOR pathways regulate migration and adhesion in B cells.3 We therefore sought to investigate the effect of NVP-BEZ235 on adhesion and migration of WM cells.
Coculture with BMSCs does not protect against NVP-BEZ235–induced WM cell cytotoxicity. (A) BCWM.1 cells were cultured with NVP-BEZ235 (6.25-100nM) for 48 hours in the presence or absence of BMSCs. Cell proliferation was assessed using the [3H]-thymidine uptake assay. (B) BCWM.1 cells were cultured with either NVP-BEZ235 (5-100nM) alone or in presence of BMSCs for 6 hours. Whole-cell lysates were subjected to Western blotting using anti–p-Akt, anti-Akt, p-mTOR, anti-raptor, anti-rictor, and anti–α-tubulin antibodies. (C) Adhesion assay with BCWM.1 in the presence or absence of NVP-BEZ235 (5-50nM). BCWM.1 demonstrated increased adhesion in FN-coated wells compared with control. NVP-BEZ235 inhibited adhesion in FN-coated wells in a dose-dependent manner (P < .05). All data represent mean (± SD) of triplicate experiments. Adhesion of BCWM.1 cell to primary BMSCs. BCWM.1 demonstrated increased adhesion in BMSC-coated wells compared with control. NVP-BEZ235 inhibited adhesion to BMSCs in a dose-dependent manner (P < .02). All data represent mean (± SD) of triplicate experiments. (D) Transwell migration assay. SDF-1 (30nM) was placed in the lower chambers, and migration was determined after 3 hours. Transwell migration assay showing inhibition of migration of BCWM.1 in the presence of NVP-BEZ235 (5-50nM). SDF-1 (30nM) was placed in the lower chambers and induced migration compared with control with no SDF-1. NVP-BEZ235 inhibited SDF-1–induced migration in WM cells (P < .05). (E) BCWM.1 cells were cultured with NVP-BEZ235 (6.25-100nM) for 4 hours. Whole-cell lysates were subjected to Western blotting using anti–p-focal adhesion kinase, anti–p-paxillin, anti–p-cofilin, and anti–α-tubulin antibodies. (F) In vivo flow cytometry. Calcein red/orange–labeled cells treated with 20nM NVP-BEZ235 and calcein green/AM–labeled, untreated cells were injected in the tail vein of 2 BALB/c mice. Cells were counted every 5 minutes for 60 minutes. Fluorescence signal was detected on an appropriate artery in the ear and digitized for analysis with Matlab software (*P < .05).
Coculture with BMSCs does not protect against NVP-BEZ235–induced WM cell cytotoxicity. (A) BCWM.1 cells were cultured with NVP-BEZ235 (6.25-100nM) for 48 hours in the presence or absence of BMSCs. Cell proliferation was assessed using the [3H]-thymidine uptake assay. (B) BCWM.1 cells were cultured with either NVP-BEZ235 (5-100nM) alone or in presence of BMSCs for 6 hours. Whole-cell lysates were subjected to Western blotting using anti–p-Akt, anti-Akt, p-mTOR, anti-raptor, anti-rictor, and anti–α-tubulin antibodies. (C) Adhesion assay with BCWM.1 in the presence or absence of NVP-BEZ235 (5-50nM). BCWM.1 demonstrated increased adhesion in FN-coated wells compared with control. NVP-BEZ235 inhibited adhesion in FN-coated wells in a dose-dependent manner (P < .05). All data represent mean (± SD) of triplicate experiments. Adhesion of BCWM.1 cell to primary BMSCs. BCWM.1 demonstrated increased adhesion in BMSC-coated wells compared with control. NVP-BEZ235 inhibited adhesion to BMSCs in a dose-dependent manner (P < .02). All data represent mean (± SD) of triplicate experiments. (D) Transwell migration assay. SDF-1 (30nM) was placed in the lower chambers, and migration was determined after 3 hours. Transwell migration assay showing inhibition of migration of BCWM.1 in the presence of NVP-BEZ235 (5-50nM). SDF-1 (30nM) was placed in the lower chambers and induced migration compared with control with no SDF-1. NVP-BEZ235 inhibited SDF-1–induced migration in WM cells (P < .05). (E) BCWM.1 cells were cultured with NVP-BEZ235 (6.25-100nM) for 4 hours. Whole-cell lysates were subjected to Western blotting using anti–p-focal adhesion kinase, anti–p-paxillin, anti–p-cofilin, and anti–α-tubulin antibodies. (F) In vivo flow cytometry. Calcein red/orange–labeled cells treated with 20nM NVP-BEZ235 and calcein green/AM–labeled, untreated cells were injected in the tail vein of 2 BALB/c mice. Cells were counted every 5 minutes for 60 minutes. Fluorescence signal was detected on an appropriate artery in the ear and digitized for analysis with Matlab software (*P < .05).
We first tested the effect of NVP-BEZ235 on adhesion of BCWM.1 cell line to either FN or BMSCs in vitro. We found that NVP-BEZ235 induced significant inhibition of adhesion to either FN or BMSCs, in a dose-dependent manner (P ≤ .05; Figure 7C). We next demonstrated that 30nM SDF-1, an important regulator of migration in B cells,21 induced migration in BCWM.1 cells. To study the effect of NVP-BEZ235 on the migration of WM cells, BCWM.1 cells were incubated with increasing doses of NVP-BEZ235 (5, 10, 20, 50nM) for 4 hours. These doses and duration of incubation did not induce apoptosis in WM cells as confirmed by MTT assay (data not shown). Cells were then examined for the migration assay, as previously described.1 NVP-BEZ235 significantly inhibited WM cell migration toward SDF-1, in a dose-dependent manner (P ≤ .05; Figure 7D).
We sought to determine the mechanism of NVP-BEZ235–inhibited adhesion and migration in WM cells, and found that NVP-BEZ235 inhibited phosphorylation of focal adhesion kinase, paxillin, and cofilin, important proteins that act as key regulators of adhesion and cell migration (Figure 7E).34
We have previously shown that adhesion of neoplastic cells to the BM microenvironment confers resistance to apoptosis.35 Therefore, we sought to investigate the effect of NVP-BEZ235 on homing of WM cells in vivo. Calcein red/orange–labeled BCWM.1 cells treated with NVP-BEZ235 (20nM, 4 hours), or calcein green/AM–labeled, untreated BCWM.1 control cells were injected in the tail vein of BALB/c mice, followed by in vivo flow cytometry every 5 minutes for 60 minutes after injection. Treatment of BCWM.1 cells with NVP-BEZ235 resulted in a significant inhibition of homing at the different time points after injection (*P < .05), suggesting that the PI3K/Akt and mTOR dual inhibitor was able to inhibit homing of WM cells to the BM in vivo (Figure 7F).
Discussion
It has been shown that up-regulation of PI3K/Akt signaling pathway, resulting from different genetic and epigenetic alterations, plays a pivotal role in the pathogenesis and progression of several human cancers.1-3 Reduced expression of PTEN appears to be one of the different genetic aberrations that leads to PI3K/Akt activation, responsible for fundamental cellular processes linked to tumorigenesis, such as cell-cycle regulation, cell proliferation and survival, cell adhesion and migration, and angiogenesis.4,5,15,16 The role of deregulated PTEN expression in WM has not been evaluated yet. In the present study, we identified that the expression of PTEN at gene level and protein level is lower in WM patients compared with control CD19+ cells. This led to constitutive activation of the PI3K/Akt pathway in WM. The activation of this pathway at a high level in WM cells seems to be unique to this lymphoplasmacytic lymphoma and has not been observed at this level in other plasma cell dyscrasias, such as multiple myeloma. This finding, therefore, highlights the importance of targeting this pathway specifically in WM. The mechanisms of activation of the PI3K/Akt pathway in WM, such as PTEN mutation or epigenetic regulation, are beyond the scope of this study and are currently being examined.
Low PTEN expression in primary WM cells represents an interesting finding that could potentially lead to constitutive activation of p-Akt and p-mTOR in this disease. Array comparative genomic hybridization studies have been recently conducted in primary WM samples and did not show any copy number abnormality in the patient population studied.36 In contrast, by gene expression analysis we showed lower expression of PTEN in WM patients compared with control. To date, there have not been any PTEN-inactivating mutation or altered methylation status investigations. Therefore, other epigenetic changes may be responsible for PTEN silencing. Among those, low PTEN levels could result from the deregulated expression of microRNAs (miRNAs), which are known to act as negative regulators of gene expression. We have previously performed miRNA profiling in WM,31 and found that miRNA-494 and miRNA-542-3p are overexpressed in WM patients compared with the normal cellular counterpart, suggesting a possible role of miRNAs in silencing PTEN gene expression, because PTEN represents a predicted target for both miRNA-494 and miRNA-542-3p.
Recent evidence has shown that in addition to PI3K/Akt, the mTOR signaling pathway is also negatively targeted by the tumor suppressor PTEN.16 The observed down-modulation of PTEN in primary WM samples raised the hypothesis that this could lead to activation of Akt and mTOR cascades: we confirmed higher p-Akt and p-mTOR protein levels in primary WM cells, thus providing the rationale for testing NVP-BEZ235, a dual PI3K and mTOR inhibitor, in this disease as well as in other IgM-secreting low-grade lymphomas. We used clinically achievable concentrations of NVP-BEZ235 to identify the antitumor activity in WM, as a result of its effect on PI3K/Akt and mTOR pathways. The efficacy of NVP-BEZ235 to target other IgM-secreting, low-grade lymphoma cells has been evaluated as well.
We demonstrated that NVP-BEZ235 inhibited proliferation in WM cells as well as in other low-grade lymphoma IgM-secreting cells, as shown by inhibition of PI3K/Akt and mTOR kinase activity of their downstream-targeted proteins. We specifically compared the activity of this agent with single agent activity of the potent PI3K inhibitor LY-294002 and the mTOR inhibitor rapamycin, as well as their combination.
It is known that rapamycin targets mTOR pathway by inhibiting the raptor-containing complex, whereas the rictor component is not rapamycin sensitive. In these studies, we have demonstrated that NVP-BEZ235 was able to inhibit both rictor and raptor, suggesting its ability to silence the rictor-dependent positive feedback loop on Akt activation; in contrast, rictor down-regulation was not achieved in WM cells treated with either rapamycin or LY-294002, or the combination of rapamycin and LY-294002. As a result of NVP-BEZ235–induced inhibition of both rictor and raptor, we demonstrated a more enhanced inhibition of p-Akt compared with LY-294002 and rapamycin, used either as single agent or in a combinatory manner. Moreover, mTOR downstream-targeted proteins, p-p70S6 and p-4EBP1, were more significantly inhibited upon NVP-BEZ235 treatment compared with either rapamycin or LY-294002 used alone or in combination.
NVP-BEZ235 also induced significant cytotoxicity in a caspase-dependent and caspase-independent manner, through targeting the FoxOs. It has been previously described that Akt phosphorylation leads to nuclear export and cytoplasm retention of phosphorylated FoxOs, with consequent inhibition of their transcriptional activity,27,28 thus regulating cell-cycle progression and apoptosis. Indeed, we described an increased apoptotic phenotype in NVP-BEZ235–treated cells, sustained by up-regulation of proapoptotic factors, such as PUMA, Bax, and BIM, which are known to be essential substrates for FoxOs-induced cell death and cell-cycle arrest accompanied by up-regulation of cycle inhibitors p27kip1 and p21waf1.
To further examine mechanisms of resistance to NVP-BEZ235, we examined the effect of this compound on other signaling pathways, specifically looking at the MAPK pathways. Our previous observations have demonstrated that Akt inhibition in WM cells induced activation of ERK1/2 in a dose- and time-dependent manner, which was demonstrated to be due to activation of upstream protein kinase C and c-Raf.3 We confirmed these findings using NVP-BEZ235 in WM cells, as demonstrated by up-regulation of p-cRAF in a dose-dependent manner. In addition, we also found that NVP-BEZ235 induced phosphorylation of Rheb, a Raf-MEK-MAPK pathway activator. Importantly, we found that MEK inhibitor used in combination with NVP-BEZ235 resulted in a synergistic inhibition of WM cell growth. These data elucidate the potential mechanisms responsible for resistance to NVP-BEZ235 treatment, thus providing the rationale for combining this compound with other MEK inhibitors.
In this study, we showed that BMSCs confer growth advantage to WM cells, by up-regulating Akt and mTOR pathways. Importantly, we found that NVP-BEZ235 overcomes the BM milieu-dependent proliferative effect on WM cells and inhibited Akt and mTOR activation in WM cells, even when they were cultured together with BMSCs. In addition, NVP-BEZ235 inhibited in vitro adhesion and migration and delayed in vivo homing to the BM in WM cells, providing the evidence of NVP-BEZ235–inhibited ability of WM cells to home to BM niches.
In summary, the PI3K/Akt/mTOR signaling axis is up-regulated in primary WM tumor cells, suggesting that NVP-BEZ235 represents a valid drug for the treatment of this disease, due to its dual inhibitory effect on PI3K/Akt and mTOR cascades.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Jennifer Stedman for reviewing the manuscript.
This work was supported in part by R21 1R21CA126119-01 and the International Waldenstrom's Macroglobulinemia Foundation. This work was also supported by the Michelle and Steven Kirsch laboratory for Waldenstrom's and the Heje fellowship for Waldenstrom's.
National Institutes of Health
Authorship
Contribution: A.M.R. designed and performed research, analyzed data, and wrote the paper, A.S., E.N.H., C.P., S.V., A.K.A., F.A., M.M., H.T.N., P.Q., P.M., J.R., and M.-C.L. performed research; K.-K.W. and C.L. designed research; and I.M.G. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: I.M.G. was a member of the Speakers Bureau and received honoraria from Celgene, Millennium, and Novartis; she was on the advisory board for Celgene. The remaining authors declare no competing financial interests.
Correspondence: Irene M. Ghobrial, Medical Oncology, Dana-Farber Cancer Institute, 44 Binney St, Mayer 548A, Boston, MA 02115; e-mail: irene_ghobrial@dfci.harvard.edu.


![Figure 4. NVP-BEZ235 decreases DNA synthesis and triggers cytotoxicity in WM cells and IgM-secreting cell lines, without affecting normal cells. (A) Thymidine uptake assay. BCWM.1 cells were cultured with NVP-BEZ235 (0.1-400nM) for 24 to 72 hours. (B) Thymidine uptake assay. IgM-secreting cell lines (MEC.1; RL) were cultured with NVP-BEZ235 (0.1-400nM) for 48 hours. (C) Cytotoxicity was assessed by MTT assay. BCWM.1 cells were cultured with BEZ235 (0.1-400nM) for 24 to 48 hours and 48 to 72 hours. (D) Cytotoxicity was assessed by MTT assay. IgM-secreting cell lines (MEC.1; RL) were cultured with NVP-BEZ235 (0.1-400nM) for 48 hours. (E) BCWM.1 cells were transfected with either scramble probe or mTOR siRNA. Untransfected BCWM.1 cells were used as control. Cells were treated with NVP-BEZ235 (2.5-100nM) for 48 hours, and cytotoxicity was assessed by MTT assay. Whole-cell lysates were subjected to Western blotting using anti-mTOR and α-tubulin antibodies (inset [E]). (F) BCWM.1 cells were transduced with Akt short hairpin RNA. Mock: control plasmid. BCWM.1-transfected cells or BCWM.1 control cells were treated with NVP-BEZ235 (2.5-100nM) for 48 hours, and cytotoxicity was assessed by MTT. Whole-cell lysates were subjected to Western blotting using anti-Akt and α-tubulin antibodies (inset [F]). (G) Freshly isolated BM CD19+ tumor cells from 4 patients with WM were cultured with NVP-BEZ235 (5-50nM) for 48 hours. Cytotoxicity was assessed by MTT assay. (H) Freshly isolated peripheral blood–derived CD19+ from 4 healthy donors was cultured with NVP-BEZ235 (2.5-1000nM) for 48 hours.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/3/10.1182_blood-2009-07-235747/4/m_zh89990947450004.jpeg?Expires=1768249358&Signature=wYGTcKUN4XMQw~9rP4eUF7zhvcFwnW1c-SGpT6SDspHwPaUfwfDgEguJL6b5B3hRVK4P~JLQ2dzkA0V~An6vcRIo1LvsNBuZXQZY1olCsrX~bNVSsAozCECSdiHeIwjyZQroSmMnC3olRBeSj~dLq5qdJvMl6e0l5OW~0Aw35X3knta2GeUSJTPMEnDdSa0~qbcIOIIZoTTOr3kDQfaZ55uPk7IAbsy9g-ollpybegYSG1VreLvDjs9~C7pv~7iAmtY6v9qOHyD-TqPojCCg1wyCZWfIpe-QVnrqKacG9a63jQXmmferaCh1ZycX3kmZh4BsNdfGFCoga3qDDhHF5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Coculture with BMSCs does not protect against NVP-BEZ235–induced WM cell cytotoxicity. (A) BCWM.1 cells were cultured with NVP-BEZ235 (6.25-100nM) for 48 hours in the presence or absence of BMSCs. Cell proliferation was assessed using the [3H]-thymidine uptake assay. (B) BCWM.1 cells were cultured with either NVP-BEZ235 (5-100nM) alone or in presence of BMSCs for 6 hours. Whole-cell lysates were subjected to Western blotting using anti–p-Akt, anti-Akt, p-mTOR, anti-raptor, anti-rictor, and anti–α-tubulin antibodies. (C) Adhesion assay with BCWM.1 in the presence or absence of NVP-BEZ235 (5-50nM). BCWM.1 demonstrated increased adhesion in FN-coated wells compared with control. NVP-BEZ235 inhibited adhesion in FN-coated wells in a dose-dependent manner (P < .05). All data represent mean (± SD) of triplicate experiments. Adhesion of BCWM.1 cell to primary BMSCs. BCWM.1 demonstrated increased adhesion in BMSC-coated wells compared with control. NVP-BEZ235 inhibited adhesion to BMSCs in a dose-dependent manner (P < .02). All data represent mean (± SD) of triplicate experiments. (D) Transwell migration assay. SDF-1 (30nM) was placed in the lower chambers, and migration was determined after 3 hours. Transwell migration assay showing inhibition of migration of BCWM.1 in the presence of NVP-BEZ235 (5-50nM). SDF-1 (30nM) was placed in the lower chambers and induced migration compared with control with no SDF-1. NVP-BEZ235 inhibited SDF-1–induced migration in WM cells (P < .05). (E) BCWM.1 cells were cultured with NVP-BEZ235 (6.25-100nM) for 4 hours. Whole-cell lysates were subjected to Western blotting using anti–p-focal adhesion kinase, anti–p-paxillin, anti–p-cofilin, and anti–α-tubulin antibodies. (F) In vivo flow cytometry. Calcein red/orange–labeled cells treated with 20nM NVP-BEZ235 and calcein green/AM–labeled, untreated cells were injected in the tail vein of 2 BALB/c mice. Cells were counted every 5 minutes for 60 minutes. Fluorescence signal was detected on an appropriate artery in the ear and digitized for analysis with Matlab software (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/3/10.1182_blood-2009-07-235747/4/m_zh89990947450007.jpeg?Expires=1768249358&Signature=TV5rvEICHkGwD5kg07t0v~kKCdiSKJls9F4UD9lwvRPPdV3IZTyvZ00tdksNZeXkaVJDVadC7hfCUWUd3qkrCP6g2bDrdFNIdmtHtGYfTeYmOe-bkBqSGyjhd9raMQYZ~uGDIQNsGcfTKPWDTvi6TgVGa~dXNtU7k~CxU1Vm6mzqnsTwjhaQBDvEXYvysr~J23U0qbDtOfUEHQIUrFdG5Z2vq6y3s9XVgfFZesWbJqq4RIbSJfDPxiN1B1fqp8Dtq5eON34WgI37oLqX9C8gYJgpfyKmeGVBMPl5JhRCwIuKk92NC0--wT8O9ESYPMyGYBgcul4TIl1RidA8fGjL7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
