Abstract
Reticulocytes release small membrane vesicles termed exosomes during their maturation into erythrocytes. Exosomes are intraluminal vesicles of multivesicular endosomes released into the extracellular medium by fusion of these endosomal compartments with the plasma membrane. This secretion pathway contributes to reticulocyte plasma membrane remodeling by eliminating certain membrane glycoproteins. We show in this study that galectin-5, although mainly cytosolic, is also present on the cell surface of rat reticulocytes and erythrocytes. In addition, in reticulocytes, it resides in the endosomal compartment. We document galectin-5 translocation from the cytosol into the endosome lumen, leading to its secretion in association with exosomes. Galectin-5 bound onto the vesicle surface may function in sorting galactose-bearing glycoconjugates. Fittingly, we found that Lamp2, a major cellular glycoprotein presenting galectin-reactive poly-N-acetylactosamine chains, is lost during reticulocyte maturation. It is associated with released exosomes, suggestive of binding to galectin-5. Finally, we reveal that the uptake of rat reticulocyte exosomes by macrophages is dependent on temperature and the mechanoenzyme dynamin and that exosome uptake is decreased by adding galectin-5. These data imply galectin-5 functionality in the exosomal sorting pathway during rat reticulocyte maturation.
Introduction
Exosomes are membrane vesicles secreted by various cells and represent intraluminal vesicles of multivesicular endosomes (MVEs) released into the extracellular medium upon fusion of MVE with the plasma membrane.1 Exosomal release during reticulocyte maturation is part of a cellular program resulting in remodeling of the plasma membrane by removal of a distinct set of (glyco)proteins.2 We have previously shown that exosomal segregation of internalized membrane (glyco)proteins can occur by different routes, such as a natural enrichment in membrane domains (eg, lipid rafts),3 antibody- or lectin-induced clustering on the cell surface,4 or via the endosomal sorting complex required for transport (ie, ESCRT) cytosolic machinery.5
During an earlier stage of mammalian erythroid differentiation, ie, when the erythroblast expels its nucleus, some components of the erythroblast plasma membrane are already specifically sorted to either the nascent reticulocyte or the expelled nucleus. For example, it has been shown by the use of mouse erythroblasts that proteins such as erythroblast-macrophage protein and β1-integrin partitioned predominantly to the plasma membrane surrounding the expelled nucleus, whereas glycophorin A localized to the plasma membrane of the nascent reticulocyte.6 Both erythroblast-macrophage protein and β1-integrin have been identified as adhesion molecules involved in erythroblast retention by central macrophages within erythroblastic islands.7-9 Thus, it has been suggested that their segregation to expelled nuclei favors their engulfment by macrophages while enabling reticulocytes to detach from erythroblastic islands and enter the circulation. Interestingly, we have found that the α4β1 integrin is specifically sorted into reticulocyte exosomes during their final maturation into erythrocytes, suggesting that exosome secretion completes integrin clearance from the cell surface started during nuclear expulsion.10 In line with this observation, glycophorin A is not sorted into exosomes during reticulocyte maturation.11
In light of emerging data demonstrating the versatility of glycans as sorting signals,12,13 findings of earlier studies in which the authors used electron microscopic visualization of rat and rabbit erythroid cells are intriguing. They revealed that glycoproteins could be differently enriched in the plasma membrane surrounding either the expelled nucleus or the nascent reticulocyte, as a function of their sialylation state.14,15 The presence of terminal sialic acid is able to play a decisive role in switching reactivity to certain endogenous lectins on and off, for example, α2,6-sialylation for adhesion/growth-regulatory galectins.16,17 Fittingly, a lectin originally termed erythroid developmental agglutinin (EDA) could be purified from rabbit bone marrow erythroblasts, supposedly having a role in intererythroblast recognition and adhesion in vivo.18 Of particular interest, during the phenotypic transition leading to nuclear extrusion, EDA surrounds the expelled nucleus, whereas residual lectin in the reticulocyte remains intracellular.19
A lectin also has been identified in rat red blood cells and its cDNA cloned. This rat lectin is designated galectin-5 because it has significant sequence similarity with members of the galectin family.20 Galectin-5 binding to glycans is modulated by sialylation and N-glycan core substitutions; it has high affinity for polyvalent glycans with unmasked N-acetylactosamine and for terminal histo-blood group AB extensions (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).21-24 Concerning routing, it has been proposed that galectin secretion occurs via the exosomal pathway, bypassing the endoplasmic reticulum–Golgi secretion pathway.25 Of relevance, galectin-3 was identified as an exosomal protein by proteomic analysis of exosomes secreted by dendritic cells,26 and galectin-9, whose C-terminal domain shares extensive identity to galectin-5, was found to be associated with exosomes released by carcinoma cells.27 Once secreted, galectins then play important roles in diverse physiologic processes and, notably, are involved in immunomodulation.12,16
Galectin-5 stands out from the group of prototype galectins because of its exceptionally low propensity for dimer formation in the absence of multivalent ligands and its strict cell-type–specific expression pattern.28,29 Furthermore, the galectin-5 gene has exclusively been found in rat, likely resulting from duplication of an ancestral gene encoding for the C-terminal domain of galectin-9.28,30 Considering these features, it was of interest to investigate the functional relevance of this rat-specific galectin expression, which will then prompt respective analysis in other mammals. We show in this study that galectin-5, although mainly cytosolic, is also present on the cell surface of reticulocytes and mature red cells. Moreover, we demonstrate that galectin-5 is secreted in association with rat reticulocyte exosomes. We present evidence of the translocation of galectin-5 from the cytosol into the endosomal compartment and its further secretion partly bound onto the exosomal membrane. Parallel to these observations, we found that the highly glycosylated endo-lysosomal protein Lamp2, which contains potential galectin-binding glycan motifs,31 is lost during reticulocyte maturation through the exosomal pathway. This finding suggests that galectin-5, as a β-galactoside–binding lectin, could be involved in sorting distinct glycoproteins into exosomes. Moreover, our data, obtained from our use of PKH67 fluorescently labeled exosomes, reveal that the vesicles are efficiently internalized by macrophages through a dynamin-dependent pathway and that this event is modulated by the presence of galectin-5 on the exosome surface.
Methods
Reagents
The lipophilic fluorochrome PKH67, tetramethylrhodamine isothiocyanate (TRITC)–dextran, Percoll, cytochalasin B, Dynasore, thiodigalactoside, protein A-sepharose, and glutathione–agarose were purchased from Sigma-Aldrich. SYTO 16 green was from Invitrogen. Conjugation of lactose to Sepharose 4B beads was performed after activation with divinyl sulfone.32
Cells
Reticulocyte production in Sprague-Dawley white rats was induced by phenylhydrazine, as previously described.33 All rat protocols were reviewed and approved by the Animal Care and Use Committee of Université Montpellier. For control experiments, erythrocytes were obtained from the blood of untreated rats. Erythroblasts were enriched from bone marrow of anemic rats by the use of discontinuous Percoll density gradient34 and were immediately used for immunofluorescence microscopy. Lymphocytes were isolated from rat blood by the use of Lymphoprep (Axis-Shield) according to the manufacturer's instructions. Peritoneal macrophages were obtained by flushing peritoneal cavities of rats with 30 mL of RPMI 1640. The lavage was centrifuged at 300g for 5 minutes, red cells were lysed by the use of ammonium chloride, and peritoneal macrophages were plated in 6-well tissue-culture plates (Becton Dickinson) in 2 mL of complete medium (RPMI 1640; 10% fetal calf serum [FCS], 2mM glutamine, 100 UI/mL penicillin, and 20 μg/mL streptomycin) per well. After 2 hours of incubation at 37°C with 5% CO2 to allow adherence, wells were washed twice to remove nonadherent cells and overlaid with complete medium. The J774 macrophage cell line was grown in 75-cm2 plastic flasks in complete medium.
Antibodies
Mouse monoclonal (H68.4) raised against the cytoplasmic tail of the human transferrin receptor (TfR) was from Zymed Laboratories Inc. Rabbit polyclonal anti-Lamp2 was from Invitrogen. Mouse monoclonal anti–flotillin-1 antibody (clone 18) was from BD Pharmingen. Mouse anti–rat CD47 was from Serotec Limited. Rabbit anti–mouse TCTP antibody was kindly provided by Drs A. Telerman and R. Amson (UMR 8113, ENS Cachan).35 Peroxidase-conjugated donkey anti–mouse immunoglobulin G (IgG) was obtained from Jackson ImmunoResearch Laboratories. Peroxidase-conjugated swine anti–rabbit IgGs were from Dako. Alexa Fluor 488– conjugated goat anti–mouse IgG and donkey anti–rabbit IgG and Alexa Fluor 594–conjugated donkey anti–rabbit IgG were from Molecular Probes. Anti–galectin-2, -4, and -5 antibodies were raised against recombinant proteins and controlled for specificity and absence of cross-reactivity by enzyme-linked immunosorbent assay and Western blotting.28,36 To generate polyclonal antiserum, a rabbit was immunized with galectin-5 purified from rat erythrocytes as described in the next section. Anti–galectin-5 antibody was purified from the serum by affinity chromatography by the use of recombinant galectin-5, tagged with glutathione-S-transferase (GST–Gal-5) immobilized on glutathione-Sepharose beads (GE Healthcare).
Purification of red cell galectin-5 and production of recombinant GST–Gal-5
Galectin-5 was purified from rat red cells by the use of lactose-Sepharose as previously described20 and dialyzed against PBS containing 4mM β-mercaptoethanol and 2mM ethylenediaminetetraacetic acid (MEPBS). Erythroblasts from rat bone marrow were lysed with TRIzol, the pellet was washed in cold ethanol (80%), and total RNA content was recovered. Reverse-transcription polymerase chain reaction that uses the M-MulV Reverse Transcriptase (Amersham) and oligo dT as primers was performed to finally prepare the cDNA. The quality of the obtained cDNA was evaluated by polymerase chain reaction by the use of primers for amplification of actin.
GST–Gal-5 was produced from cloning of the Gal-5 mRNA open reading frame downstream of the GST gene with the use of Gateway Cloning Technology. This construct was then inserted in a pDEST-15 eubacterial expression vector (Invitrogen), and the fused recombinant protein was extracted and purified by the use of glutathione-agarose beads according to the manufacturer's protocol.
Exosome preparation
After removing the buffy coat, red blood cells from anemic rats were washed with Ringer buffer and fractionated on a discontinuous Percoll gradient (d: 1.100; 1.105; 1.110; 1.123 g/mL, 10 minutes at 15 000g). Young reticulocytes were collected from the upper band (F1), washed twice with Ringer buffer, and cultured for the indicated periods of time at 37°C in RPMI 1640 supplemented with 5mM glutamine, 5mM adenosine, 10mM inosine, 3% exosome-free FCS obtained after overnight ultracentrifugation (100 000g), 50 U/mL penicillin, and 50 μg/mL streptomycin. In the indicated experiments, in vitro maturation was carried out in the presence of pharmacologic agents. Exosomes were isolated by differential centrifugation as described previously.33 In some experiments, exosomes (100 μg of protein) were treated with 0.5M sodium carbonate (pH 11) as previously described.37
Western blot analysis
Samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) by the use of 8% and 15% polyacrylamide gels and analyzed by Western blot as previously described.3
Red cell subcellular fractionation
Endocytic vesicles and cytosol were prepared from reticulocytes as previously described.29 Reticulocyte and erythrocyte plasma membranes (ghosts) were prepared according to Steck and Kant38 with blood from anemic or healthy rats, respectively. Low-density Triton-insoluble fractions were isolated from total cells or ghosts prepared from immature or mature red cells as previously described.3 For Western blot analysis, fractions were precipitated with trichloracetic acid (TCA) before SDS-PAGE.
Sucrose gradient analysis
Reticulocyte endosomes were layered on top of a linear39 or step40 sucrose gradient. Exosomes were layered on top of a linear sucrose gradient (0.5-2.5M sucrose) in a Beckman SW41 tube. Gradients were centrifuged at equilibrium for 16 hours at 200 000g after which 700-μL fractions were collected from the top of the tube. In each case (ie, endosome or exosome isolation), proteins in collected fractions were precipitated by TCA, and samples were analyzed by SDS-PAGE and Western blot.
Fluorescence-activated cell-sorting analysis of exosomes and red cells, fluorescence microscopy of red cells
Exosome-coated latex beads were prepared by incubating exosomes with 4-μm diameter aldehyde/sulfate latex beads (Interfacial Dynamics) as previously described.3 Exosome-coated beads or cells were incubated for 45 minutes with the indicated antibodies followed by incubation with Alexa 488–conjugated anti–rabbit IgG antibody and analyzed by flow cytometry on a FACSCanto cytometer (BD Biosciences). After acquisition, all data analyses were performed with FlowJo software 7.2 (TreeStar). For fluorescence microscopy, red cells were washed in PBG buffer (phosphate-buffered saline [PBS], 0.1% bovine serum albumin [BSA], 10mM glucose) and fixed in 0.5% acrolein (4% packed cell volume, 5 minutes at room temperature [RT]). After washing 3 times with PBS, cells were allowed to adhere to polylysin-coated coverslips for 30 minutes at RT and stained by incubation with purified anti–galectin-5 (30 minutes, RT) and Alexa 488 anti–rabbit antibody (30 minutes, RT) diluted in 1% PBS–FCS. Cells mounted in Mowiol were visually examined by the use of a Leica DMRA2 microscope with suitable excitation and emission filters.
Uptake of PKH67-labeled exosomes by macrophages
For fluorescence microscopy, J774 macrophages were grown on glass coverslips until 80% confluence. After incubation with PKH67-labeled exosomes and TRITC-dextran, cells were fixed in paraformaldehyde 4% for 15 minutes at 37°C. After washing in PBS, cells were mounted in Mowiol and monitored under a Zeiss Axioimager microscope equipped with an apotome and suitable excitation and emission filters.
Rat reticulocyte exosomes were labeled with PKH67 by mixing vesicles (500 μg of protein) with the fluorescent probe (4 μL) for 30 minutes at RT. Fluorescent exosomes were then isolated on Percoll solution in sucrose (d: 1.08 g/mL; 33 000g for 30 minutes in a Beckman TLA110 rotor), washed once in PBS, and adjusted to 1 mg prot/mL in PBS. Various amounts of PKH67-labeled exosomes or fluorescent beads were incubated with macrophages (24-well plate) for the indicated periods of time at 37°C. Thereafter, the cells were washed once with PBS, detached by the use of trypsin, pelleted, and resuspended in PBS for flow cytometric analysis with a FACSCanto flow cytometer (BD Biosciences) with a 488-nm argon laser and standard bandpass filters for FITC (530/30 nm). When indicated, macrophages were treated with cytochalasin B (5 μg/mL), dynasore (80 μg/mL), or with the equivalent volume of dimethyl sulfoxide for 1 hour at 37°C in serum-free RPMI during incubation together with PKH67-labeled exosomes.
Results
Galectin-5 is present on the surface of rat red cells
To delineate a role of galectin-5 during reticulocyte exosome secretion, we first produced an antibody against the lectin purified from rat red blood cells. For this, the protein was isolated from red cell lysate by affinity chromatography on lactose–Sepharose 4B. A protein, eluted from the column by the use of 150mM lactose, had a molecular weight of approximately 17 kDa, corresponding to the expected molecular weight of galectin-5 (supplemental Figure 2A). To verify its lectin nature, this protein was probed for its hemagglutination activity. It was shown to agglutinate erythrocytes from rat, mouse, and human but with different levels of reactivity, with rat erythrocytes being most reactive. Hemagglutination of all erythrocytes species was inhibited by lactose or thiodigalactoside, thus confirming lectin activity of the purified protein (not shown). Moreover, it was immunoreactive to galectin-5–specific antibodies; sequence deviations among galectins enabled us to avoid intrafamily cross-reactivity (supplemental Figure 2B). The generated antiserum also recognized the purified protein from rat erythrocyte lysate and detected a band at 17 kDa in rat erythrocytes by Western blot. We also produced a recombinant GST fusion protein that agglutinated rat erythrocytes (not shown), which similarly was detected by Western blot by use of the anti–galectin-5 serum produced (supplemental Figure 2D). Galectin-5 partitioning in rat erythrocytes was assessed by Western blot analysis of mature red cell cytosol versus ghosts; membrane-associated galectin-5 represented less than 1% of the total cellular galectin pool (supplemental Figure 2E).
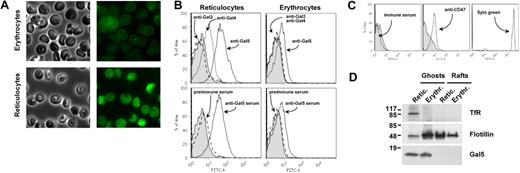
To detect the presence of galectin-5 on the surface of red cells, we carried out immunofluorescence microscopy of nonpermeabilized reticulocytes and erythrocytes. Both mature and immature cells were labeled by the galectin-5–specific antibody (Figure 1A, right), demonstrating the presence of lectin on the extracellular side of the plasma membrane. As a more quantitative approach to determine the amount of galectin-5 present on the cell surface, we analyzed the extent of cell surface labeling of reticulocytes and erythrocytes by flow cytometry. Both sets of cells were positive to anti–galectin-5 but negative for labeling with anti–galectin-2 or -4 antibodies (Figure 1B). Moreover, note that the shift in fluorescence intensity reflecting galectin-5–specific labeling was higher for immature cells compared with that obtained for mature red cells. In contrast, lymphocytes isolated from rat blood were not reactive with galectin-5, as demonstrated by flow cytometry (Figure 1C).
Galectin-5 is present on the surface of rat red cells. (A) Freshly isolated reticulocytes or erythrocytes were adsorbed on glass coverslips and processed for immunofluorescence as described in “Fluorescence-activated cell-sorting analysis of exosomes and red cells, fluorescence microscopy of red cells.” Transmission images of red cells (left) and corresponding fluorescence imaging (right) were recorded on cells by the use of purified rabbit anti–galectin-5 antibody followed by incubation with Alexa 488 anti–rabbit antibody. (B) Young reticulocytes and erythrocytes were analyzed by flow cytometry by the use of antibodies raised against Gal-2 (dashed line), Gal-4 (dotted line), and Gal-5 (solid line), already tested for their specificity (top), or the produced anti–galectin-5 serum (solid line) and the preimmune serum (bottom, dashed line). Tinted patterns indicate cell labeling obtained in the absence of primary antibodies. (C) Lymphocytes isolated from rat blood, as described in “Cells,” were analyzed by flow cytometry for Gal-5 (left, solid line), CD47 (middle, solid line) and Syto 16 green (right, solid line). Tinted patterns indicate cell labeling in the absence of primary antibodies. (D) Ghost and raft extracts isolated from reticulocytes or mature erythrocytes, as described in “Red cell subcellular fractionation,” were processed by SDS-PAGE and analyzed by Western blot for the indicated proteins. The molecular mass (kDa) standards are indicated on the left.
Galectin-5 is present on the surface of rat red cells. (A) Freshly isolated reticulocytes or erythrocytes were adsorbed on glass coverslips and processed for immunofluorescence as described in “Fluorescence-activated cell-sorting analysis of exosomes and red cells, fluorescence microscopy of red cells.” Transmission images of red cells (left) and corresponding fluorescence imaging (right) were recorded on cells by the use of purified rabbit anti–galectin-5 antibody followed by incubation with Alexa 488 anti–rabbit antibody. (B) Young reticulocytes and erythrocytes were analyzed by flow cytometry by the use of antibodies raised against Gal-2 (dashed line), Gal-4 (dotted line), and Gal-5 (solid line), already tested for their specificity (top), or the produced anti–galectin-5 serum (solid line) and the preimmune serum (bottom, dashed line). Tinted patterns indicate cell labeling obtained in the absence of primary antibodies. (C) Lymphocytes isolated from rat blood, as described in “Cells,” were analyzed by flow cytometry for Gal-5 (left, solid line), CD47 (middle, solid line) and Syto 16 green (right, solid line). Tinted patterns indicate cell labeling in the absence of primary antibodies. (D) Ghost and raft extracts isolated from reticulocytes or mature erythrocytes, as described in “Red cell subcellular fractionation,” were processed by SDS-PAGE and analyzed by Western blot for the indicated proteins. The molecular mass (kDa) standards are indicated on the left.
Because galectin-5 has been shown to bind the pentasaccharide of ganglioside GM1 by solid-phase assay,29 we next investigated the possibility that galectin-5 was membrane-associated through binding to lipid raft membrane subdomains enriched in cholesterol and glycosphingolipids, especially GM1. Raft domains were isolated from ghosts of reticulocytes or erythrocytes.41 In contrast to the raft marker flotillin-1, we did not detect galectin-5 in raft fractions isolated from ghosts of mature or immature red cells (Figure 1D), suggesting that galectin-5 is not membrane associated through GM1 binding.
Galectin-5 is secreted via the exosomal pathway
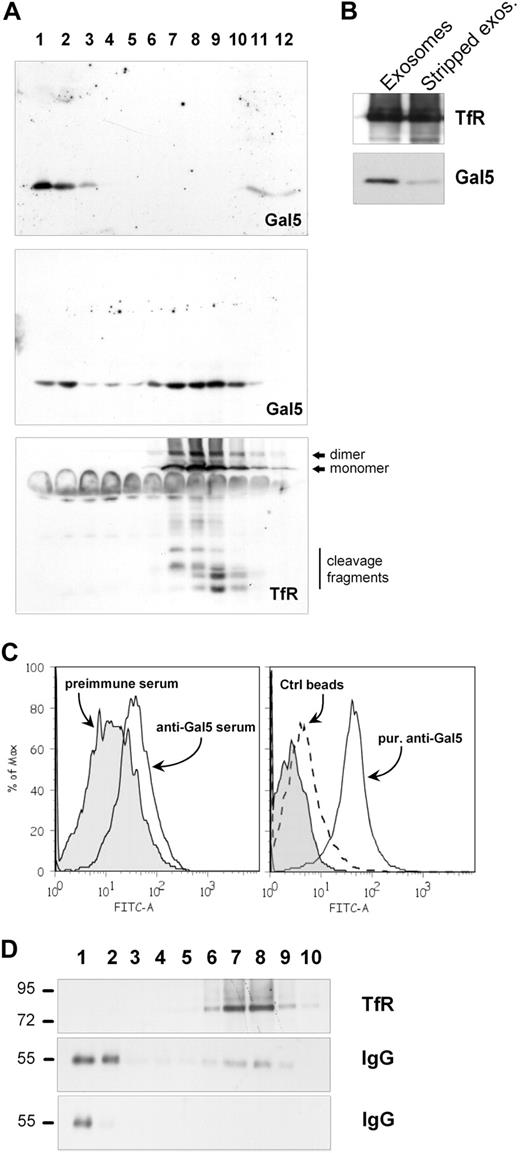
The presence of galectin-5 on the red cell surface indicates that the cytosolic lectin has crossed a cell membrane to reach the extracellular surface, as already described for other galectins in different cell types.25,42 To study this process, we subcultured rat reticulocytes or erythrocytes for 24 hours and screened the conditioned medium for the presence of the lectin by Western blot. No galectin-5 was found in the medium of mature red cells, whereas the lectin was detected in the extracellular medium after reticulocyte subculture. Moreover, when the conditioned medium was ultracentrifuged, galectin-5 was recovered in the pellet, suggesting that the lectin was associated with exosomes secreted during reticulocyte maturation. Circulating reticulocytes, even though anucleated, still have some intracellular compartments, including vesicles involved in the TfR endocytic pathway from which exosomes are generated. To more thoroughly explore the possibility of galectin-5 secretion through the exosomal pathway, we purified endosomal vesicles from rat reticulocytes and analyzed their galectin-5 content by Western blot after sucrose gradient fractionation. As shown in Figure 2A, endosomal vesicles partitioned in the gradient with a peak in fractions 3 to 5, as revealed by the detection of TfR (top). Interestingly, part of the galectin-5 detected in the gradient was found in fractions corresponding to TfR-positive vesicles, although a pool of free galectin-5, likely corresponding to cytosolic galectin-5, was recovered on top of the gradient (bottom). Note that galectin-5 comigrated with a light endosomal fraction, likely corresponding to the MVE compartment. This assumption was confirmed by the use of a previously described method40 facilitating separation of single-walled endocytic vesicles (EVs) from MVE. Enrichment of galectin-5 relative to the TfR content was found in the MVE fraction relative to the EV fraction (Figure 2B), likely corresponding to cytosolic galectin-5 trapped within intraluminal vesicles. Moreover, when purified endosomal vesicles were treated with trypsin, a pool of galectin-5 was found to be resistant to trypsin, unless detergent was added (Figure 2C), thus demonstrating its presence within the endosome lumen.
Galectin-5 secretion is associated with the endosomal pathway. Endosomal vesicles were prepared and loaded on a linear sucrose gradient, as described in “Methods.” (A) Fractions 1 to 9 were collected from the top of the gradient and after TCA/acetone precipitation, proteins were separated by SDS-PAGE and analyzed by Western blot for the presence of TfR and Gal-5. (B) Fractions were collected and densities (g/mL) were obtained by refractometry. Pooled fractions corresponding to cytosol (Cyt), multivesicular endosomes (MVE), and endocytic vesicles (EV) were processed by SDS-PAGE and analyzed by Western blot for the presence of TfR and Gal-5. (C) Endosomal vesicles (100 μg protein) were subjected to trypsin digestion (150 μg/mL for 1 hour at RT) in the absence or presence of Triton X-100 (1.5%), then loaded on SDS-PAGE gels and analyzed by Western blot for Gal-5. A nontrypsinized sample was used as control.
Galectin-5 secretion is associated with the endosomal pathway. Endosomal vesicles were prepared and loaded on a linear sucrose gradient, as described in “Methods.” (A) Fractions 1 to 9 were collected from the top of the gradient and after TCA/acetone precipitation, proteins were separated by SDS-PAGE and analyzed by Western blot for the presence of TfR and Gal-5. (B) Fractions were collected and densities (g/mL) were obtained by refractometry. Pooled fractions corresponding to cytosol (Cyt), multivesicular endosomes (MVE), and endocytic vesicles (EV) were processed by SDS-PAGE and analyzed by Western blot for the presence of TfR and Gal-5. (C) Endosomal vesicles (100 μg protein) were subjected to trypsin digestion (150 μg/mL for 1 hour at RT) in the absence or presence of Triton X-100 (1.5%), then loaded on SDS-PAGE gels and analyzed by Western blot for Gal-5. A nontrypsinized sample was used as control.
When increasing amounts of collected exosomes were loaded on SDS-PAGE gel, galectin-5 was found to increase proportionally as assessed by Western blotting with the use of Tfr as a control (Figure 3A). Moreover, lengthening the in vitro maturation period enhanced the amount of galectin-5 secreted by the cells (Figure 3B). To assess the involvement of the endocytic pathway in galectin-5 secretion, we used various maturation conditions known to interfere with endocytosis and exosome release. Reticulocyte maturation at 4°C, in the presence of sodium fluoride or chloroquine, decreased the amount of exosomes secreted, as revealed by the amount of TfR released; and at the same time, proportionally diminished the pool of secreted galectin-5 (Figure 3C left). We then tested the effect of monensin, an ionophore that alters transferrin receptor recycling43 and increases exosome production in K562 cells44,45 and reticulocytes (M.V., unpublished data). Monensin treatment greatly increased the amount of TfR released from immature red cells via exosomes (Figure 3C right), in agreement with our previous data. Similarly, monensin also increased the amount of galectin-5 secreted with exosomes. Overall, these data suggest that the endosomal compartment might be the site at which galectin-5 translocates from the cytosol into the endosomal lumen, topologically equivalent to the cell exterior.
Galectin-5 is secreted through the exosomal pathway. Exosomes were collected by differential centrifugation from the culture medium of in vitro reticulocyte maturation in various conditions. (A) Increasing amounts of exosomes (10-80 μg of protein) were processed by SDS-PAGE and analyzed by Western blot for the presence of TfR and Gal-5 by the use of appropriate antibodies. (B) Exosomes released during 2, 6, and 24 hours of reticulocyte (100 μL of packed cell volume) in vitro maturation were isolated from the culture medium and compared for their TfR and Gal-5 content. (C) Exosomes collected from the medium after in vitro maturation (24 hours, 37°C) of reticulocytes in the absence (Ctrl) or presence of sodium fluoride (20mM), chloroquine (10μM), monensin (1μM), or after reticulocyte maturation at 4°C were analyzed.
Galectin-5 is secreted through the exosomal pathway. Exosomes were collected by differential centrifugation from the culture medium of in vitro reticulocyte maturation in various conditions. (A) Increasing amounts of exosomes (10-80 μg of protein) were processed by SDS-PAGE and analyzed by Western blot for the presence of TfR and Gal-5 by the use of appropriate antibodies. (B) Exosomes released during 2, 6, and 24 hours of reticulocyte (100 μL of packed cell volume) in vitro maturation were isolated from the culture medium and compared for their TfR and Gal-5 content. (C) Exosomes collected from the medium after in vitro maturation (24 hours, 37°C) of reticulocytes in the absence (Ctrl) or presence of sodium fluoride (20mM), chloroquine (10μM), monensin (1μM), or after reticulocyte maturation at 4°C were analyzed.
Galectin-5 is present on the exosome surface
To further analyze the secretion of galectin-5 through the exosomal pathway, exosomes collected after reticulocyte maturation were fractionated by flotation on sucrose gradients and analyzed for the presence of TfR and galectin-5 by Western blot. Exosomes, as revealed by TfR detection (Figure 4A bottom), were distributed in fractions 7 to 10, corresponding to densities between 1.08 and 1.18 g/mL, as previously described.3 Exosomal galectin-5 was mainly detected in the same fractions, showing that the lectin is associated with vesicles (Figure 4A, middle). When the purified lectin was loaded alone on the gradient, galectin-5 was principally recovered on top of the gradient (Figure 4A top). Because galectins can be localized in the cytosol and also associated with the extracellular surface of the plasma membrane, it was important to determine how galectin-5 was spatially distributed in exosomes. Hence, exosomes collected during maturation of rat reticulocytes were stripped by carbonate treatment,37 and analyzed by Western blotting for the presence of galectin-5. The amount of galectin-5 associated with exosomes was decreased by the carbonate wash, whereas the TfR content was unchanged in the stripped vesicles (Figure 4B), suggesting that galectin-5 was partly associated with the extracellular side of the exosomal membrane.
Galectin-5 is located on the exosome surface. (A) Exosomes (400 μg of protein; bottom and middle) or purified Gal-5 (3 μg; top) were carefully loaded on a linear sucrose gradient. Fractions were collected from the top of the gradient, processed by SDS-PAGE, and analyzed by Western blot for the presence of TfR (bottom) and Gal-5 (middle and top) by the use of specific antibodies. Densities (g/mL) were obtained for each fraction by refractometry. (B) Exosome surface–associated proteins were released by a carbonate wash. Untreated exosomes and stripped vesicles were processed by SDS-PAGE and analyzed by Western blot for TfR and Gal-5. (C) Exosome material was coated on latex beads and analyzed by flow cytometry for Gal-5 by the use of anti–galectin-5 serum (solid line) or a preimmune serum (tinted pattern) and Alexa Fluor 488 donkey anti–rabbit IgG (left) or by the use of purified anti–galectin-5 IgGs and Alexa Fluor 488 donkey anti–rabbit IgG (solid line; right). As a control BSA-coated beads (dashed line) were treated similarly. Tinted patterns indicate the absence of primary antibody on exosome-coated beads. (D) Exosomes preincubated with purified anti–galectin-5 antibody (2 μg, 1 hour, RT; top and middle) or Gal-5 antibody (2 μg; bottom) were loaded on a linear sucrose gradient. Fractions were collected and analyzed for the presence of TfR (top) and Gal-5 antibody (middle and bottom) by the use of specific antibodies.
Galectin-5 is located on the exosome surface. (A) Exosomes (400 μg of protein; bottom and middle) or purified Gal-5 (3 μg; top) were carefully loaded on a linear sucrose gradient. Fractions were collected from the top of the gradient, processed by SDS-PAGE, and analyzed by Western blot for the presence of TfR (bottom) and Gal-5 (middle and top) by the use of specific antibodies. Densities (g/mL) were obtained for each fraction by refractometry. (B) Exosome surface–associated proteins were released by a carbonate wash. Untreated exosomes and stripped vesicles were processed by SDS-PAGE and analyzed by Western blot for TfR and Gal-5. (C) Exosome material was coated on latex beads and analyzed by flow cytometry for Gal-5 by the use of anti–galectin-5 serum (solid line) or a preimmune serum (tinted pattern) and Alexa Fluor 488 donkey anti–rabbit IgG (left) or by the use of purified anti–galectin-5 IgGs and Alexa Fluor 488 donkey anti–rabbit IgG (solid line; right). As a control BSA-coated beads (dashed line) were treated similarly. Tinted patterns indicate the absence of primary antibody on exosome-coated beads. (D) Exosomes preincubated with purified anti–galectin-5 antibody (2 μg, 1 hour, RT; top and middle) or Gal-5 antibody (2 μg; bottom) were loaded on a linear sucrose gradient. Fractions were collected and analyzed for the presence of TfR (top) and Gal-5 antibody (middle and bottom) by the use of specific antibodies.
To confirm the presence of galectin-5 on the vesicle surface, exosomes were adsorbed on latex beads and analyzed for the presence of galectin-5 by flow cytometry.3 When anti–galectin-5 serum was used to detect galectin-5, a small but reproducible shift in fluorescence intensity was obtained compared with the rabbit preimmune serum (Figure 4C left). Affinity purification of the anti–galectin-5 IgG by the use of GST–Gal-5 immobilized on a column refined this result, demonstrating the presence of galectin-5 on the surface of exosomes (Figure 4C). Sucrose-gradient analysis was then performed to verify the flow cytometric data. When loaded alone, affinity purified anti–galectin-5 antibody was recovered floating on top of the sucrose gradient after fractionation (Figure 4D bottom). However, a different distribution pattern was obtained when the antibody was incubated with exosomes before centrifugation, shifting its position to higher-density fractions within the gradient (Figure 4D middle), corresponding to the exosomal TfR detection (Figure 4D top). We concluded from these data that galectin-5 secreted by the exosomal pathway was partly bound to the surface of vesicles.
Lamp2 as a potential counterreceptor for galectin-5
Because the endolysosomal compartment disappears during reticulocyte maturation, we assessed the fate of Lamp2, a highly glycosylated membrane protein predominantly localized in endosomes during this time period. Its complex-type N-glycans fit the pattern of galectin-5 reactivity. Essentially pure populations of age-synchronized reticulocytes from the anemic rat were separated by the use of Percoll density gradients.5 Interestingly, the amount of Lamp2 decreased during reticulocyte maturation (F1 to F4) in a pattern very similar to that of the TfR (Figure 5A). In contrast the cellular content of actin did not change during reticulocytes maturation. Moreover Lamp2, which is mainly localized in the endosomal sub-fraction, was retrieved, associated with exosomes released during the maturation process (Figure 5B).
Lamp2 is lost during reticulocyte maturation. (A) Different populations of age-synchronized reticulocytes were obtained by Percoll density gradient centrifugation using blood of anemic rats as described in “Methods.” (Top) Analysis of the Lamp2, TfR, and actin content of the reticulocyte subpopulations (0.5 μL of packed cell volume) was carried out by Western blot after SDS-PAGE and transfer on membrane. F1 to F4 correspond to the Percoll fractions from lower to higher density (ie, from younger to older reticulocyte stages). “Retic” stands for the red cells collected before Percoll gradient, “Erythr.” stands for red cells collected from an untreated animal. (Bottom) Coomassie staining of the PVDF membrane before Western blot. (B) Rat reticulocytes were fractionated as described in “Methods.” The different fractions obtained (cytosol, plasma membrane, endosomes, and exosomes; 18 μg of protein) were loaded on a 10% SDS-PAGE, transferred onto PVDF membrane, and stained by Coomassie blue (bottom), then immunoblotted for the proteins indicated on the right (top).
Lamp2 is lost during reticulocyte maturation. (A) Different populations of age-synchronized reticulocytes were obtained by Percoll density gradient centrifugation using blood of anemic rats as described in “Methods.” (Top) Analysis of the Lamp2, TfR, and actin content of the reticulocyte subpopulations (0.5 μL of packed cell volume) was carried out by Western blot after SDS-PAGE and transfer on membrane. F1 to F4 correspond to the Percoll fractions from lower to higher density (ie, from younger to older reticulocyte stages). “Retic” stands for the red cells collected before Percoll gradient, “Erythr.” stands for red cells collected from an untreated animal. (Bottom) Coomassie staining of the PVDF membrane before Western blot. (B) Rat reticulocytes were fractionated as described in “Methods.” The different fractions obtained (cytosol, plasma membrane, endosomes, and exosomes; 18 μg of protein) were loaded on a 10% SDS-PAGE, transferred onto PVDF membrane, and stained by Coomassie blue (bottom), then immunoblotted for the proteins indicated on the right (top).
Galectin-5 modulates exosome uptake by macrophages
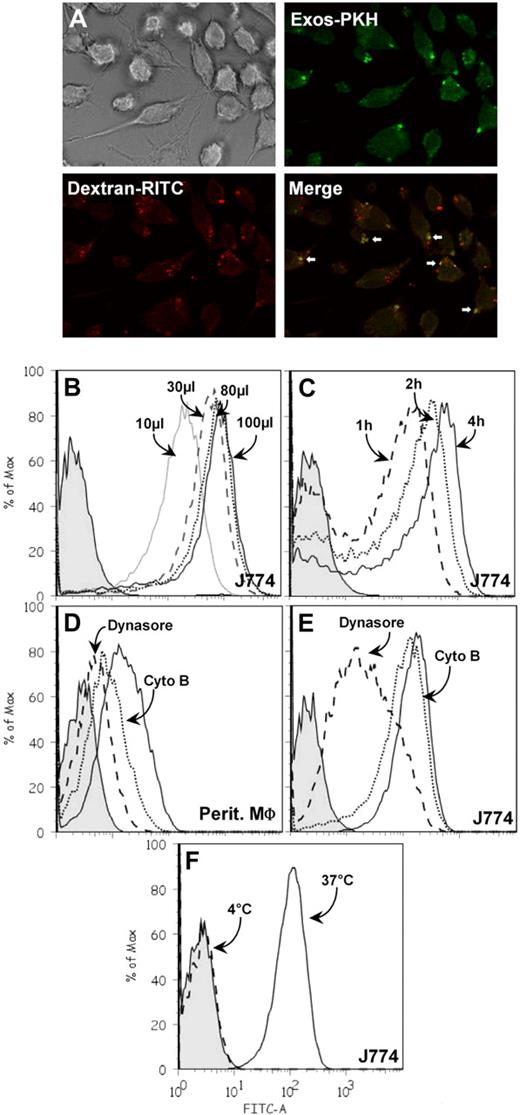
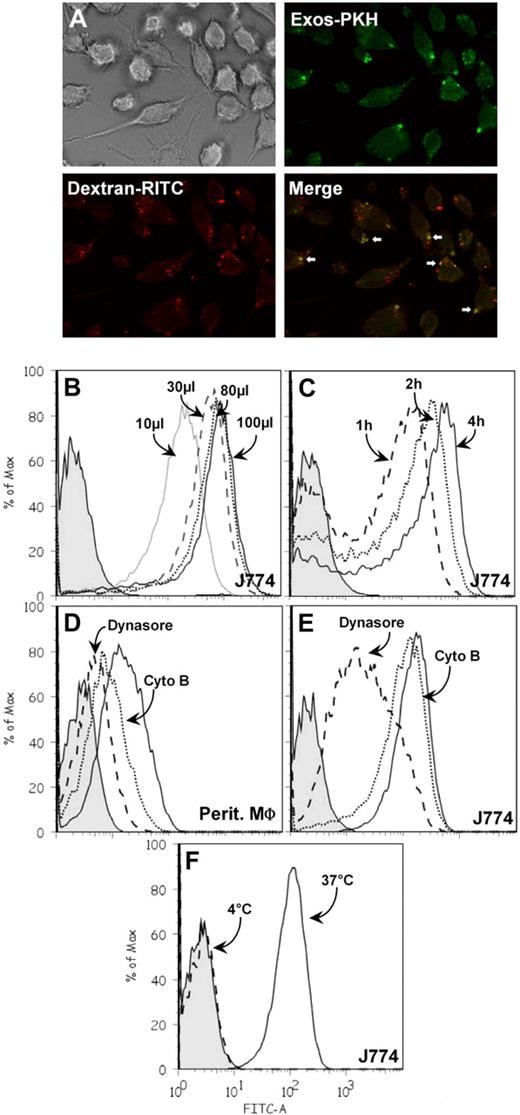
We next tested whether the presence of galectin-5 on the exosome surface could affect their uptake by phagocytic cells. Rat reticulocyte exosomes were labeled with PKH67, a fluorescent lipid intercalator, and then incubated with rat peritoneal macrophages or the J774 macrophage mouse cell line. The macrophages were subsequently analyzed by fluorescence microscopy and flow cytometry for exosome uptake. A punctated fluorescent pattern was obtained after incubation of cells with PKH-labeled exosomes (Figure 6A). Note that TRITC-dextran gave a very similar pattern with some colocalization of fluorescent markers, suggesting vesicle endocytosis. Cell-associated PKH67, as measured by the shift in peak fluorescence intensity, was dependent on both the amount of exosomes added (Figure 6B) and the incubation time (Figure 6C). Moreover, exosome uptake by both types of macrophages was very efficiently inhibited by incubation with dynasore, a cell-permeable inhibitor of dynamin,46 and to a lesser extent by incubation with cytochalasin B, an inhibitor of actin polymerization (Figure 6D-E). These data convincingly show that exosomes bearing galectin-5 on their surface could be internalized by macrophages. This process should be slowed down when exosomes are incubated with macrophages at 4°C, and, indeed, this was the case (Figure 6F).
Internalization of PKH67-labeled exosomes by macrophages. (A) PKH67-labeled exosomes (20 μg) and dextran-TRITC (0.1 mg/mL) were incubated with J774 macrophages (30 minutes, 37°C) grown on glass coverslips and processed for immunofluorescence, as described. The cells were then monitored by fluorescence microscopy and transmission as indicated in the figure. PKH67-labeled exosomes were incubated with macrophages under the conditions indicated. After washing, cells were trypsinized and the fluorescence intensity was measured by flow cytometry. Tinted patterns always indicate cell autofluorescence. (B) PKH67-labeled exosomes, 10 μL (gray solid line), 30 μL (dashed line), 80 μL (dotted line), 100 μL (solid line) were incubated with J774 macrophages for 4 hours at 37°C. (C) PKH67-labeled exosomes (30 μL) were incubated with J774 macrophages for 1 hour (dashed line), 2 hours (dotted line), or 4 hours (solid line) at 37°C. (D-E) PKH67-labeled exosomes (30 μL) were incubated with peritoneal or J774 macrophages for 4 hours at 37°C with 80μM dynasore (dashed line), 5 μg/mL cytochalasin B (dotted line), or with the carrier dimethyl sulfoxide (solid line). (F) PKH67-labeled exosomes (30 μL) were incubated with J774 macrophages for 4 hours at 4°C (dashed line) or 37°C (solid line).
Internalization of PKH67-labeled exosomes by macrophages. (A) PKH67-labeled exosomes (20 μg) and dextran-TRITC (0.1 mg/mL) were incubated with J774 macrophages (30 minutes, 37°C) grown on glass coverslips and processed for immunofluorescence, as described. The cells were then monitored by fluorescence microscopy and transmission as indicated in the figure. PKH67-labeled exosomes were incubated with macrophages under the conditions indicated. After washing, cells were trypsinized and the fluorescence intensity was measured by flow cytometry. Tinted patterns always indicate cell autofluorescence. (B) PKH67-labeled exosomes, 10 μL (gray solid line), 30 μL (dashed line), 80 μL (dotted line), 100 μL (solid line) were incubated with J774 macrophages for 4 hours at 37°C. (C) PKH67-labeled exosomes (30 μL) were incubated with J774 macrophages for 1 hour (dashed line), 2 hours (dotted line), or 4 hours (solid line) at 37°C. (D-E) PKH67-labeled exosomes (30 μL) were incubated with peritoneal or J774 macrophages for 4 hours at 37°C with 80μM dynasore (dashed line), 5 μg/mL cytochalasin B (dotted line), or with the carrier dimethyl sulfoxide (solid line). (F) PKH67-labeled exosomes (30 μL) were incubated with J774 macrophages for 4 hours at 4°C (dashed line) or 37°C (solid line).
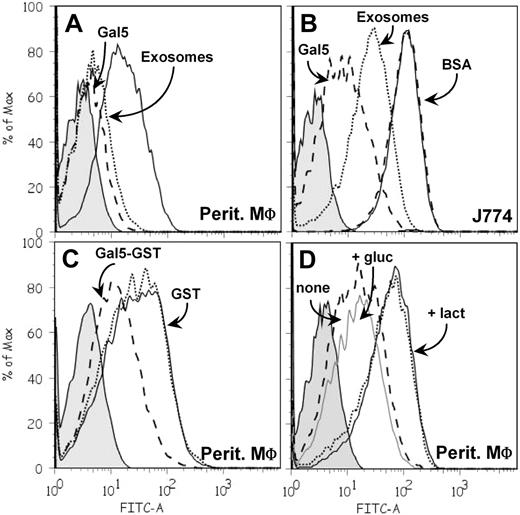
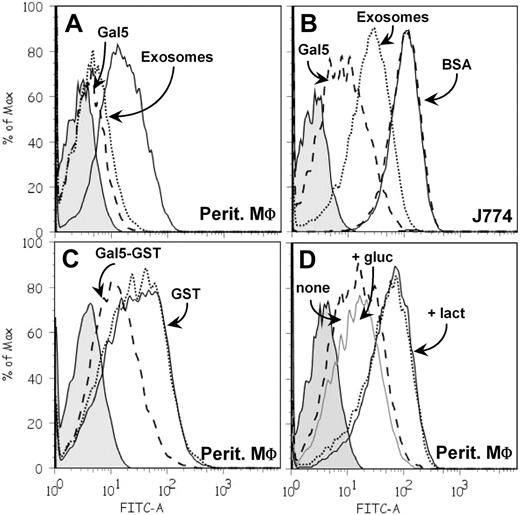
To gain further insight into the molecular aspects of internalization of PKH67-labeled exosomes by macrophages, we added an excess of either unlabeled exosomes or purified galectin-5 during incubation, with the aim of inhibiting vesicle uptake. The presence of unlabeled exosomes markedly decreased the amount of PKH67-exosomes internalized by peritoneal macrophages and J774 cells, whereas the addition of purified galectin-5 led to an even greater decrease for both types of macrophage (Figure 7A-B). Similarly, recombinant GST–Gal-5 was able to decrease PKH67-labeled exosome uptake, which was not the case for GST alone (Figure 7C). As a control, the addition of BSA did not affect PKH67-exosome uptake (Figure 7B). To assess the carbohydrate dependence of this process, preincubation of galectin-5 (purified or recombinant) with lactose was found to impair its capacity to inhibit exosome uptake, in contrast to preincubation of galectin with the osmolarity control, glucose (Figure 7D). In view of these results, we hypothesized that galectin-5 inhibited exosome uptake by masking β-galactosides on the surface of exosomes. Fittingly, when asialofetuin, a glycoprotein with strong ligand properties for galectin-5,22 was added together with PKH67-labeled vesicles during the assay, the amount of exosomes internalized was efficiently diminished (data not shown), thus suggesting competition of N-glycan chains of asialofetuin for galectin-5–reactive determinants on the macrophage surface.
Galectin-5 modulates exosome uptake by macrophages. PKH67-labeled exosomes (60 μg protein/mL) were incubated with macrophages for 3 hours at 37°C. After washing, cell fluorescence intensity was measured by flow cytometry. Tinted patterns always indicate cell autofluorescence. Data obtained after incubation of PKH67-labeled exosomes alone with macrophages are represented by a solid black line. PKH67-labeled exosomes were incubated with peritoneal (A) or J774 (B) macrophages in the presence of purified Gal-5 (50μM; dashed line), unlabeled exosomes (300 μg protein/mL; dotted line), or BSA (50μM; dashed line). (C) PKH67-labeled exosomes were incubated with peritoneal macrophages in the presence of GST–Gal-5 (50μM; dashed line) or GST (50μM; dotted line). (D) PKH67-labeled exosomes and GST–Gal-5 (50μM) were incubated with peritoneal macrophages after preincubation (1 hour, RT) of GST–Gal-5 in 150 mM lactose (dotted line) or glucose (gray solid line) or medium alone (dashed line).
Galectin-5 modulates exosome uptake by macrophages. PKH67-labeled exosomes (60 μg protein/mL) were incubated with macrophages for 3 hours at 37°C. After washing, cell fluorescence intensity was measured by flow cytometry. Tinted patterns always indicate cell autofluorescence. Data obtained after incubation of PKH67-labeled exosomes alone with macrophages are represented by a solid black line. PKH67-labeled exosomes were incubated with peritoneal (A) or J774 (B) macrophages in the presence of purified Gal-5 (50μM; dashed line), unlabeled exosomes (300 μg protein/mL; dotted line), or BSA (50μM; dashed line). (C) PKH67-labeled exosomes were incubated with peritoneal macrophages in the presence of GST–Gal-5 (50μM; dashed line) or GST (50μM; dotted line). (D) PKH67-labeled exosomes and GST–Gal-5 (50μM) were incubated with peritoneal macrophages after preincubation (1 hour, RT) of GST–Gal-5 in 150 mM lactose (dotted line) or glucose (gray solid line) or medium alone (dashed line).
Discussion
We have determined that galectin-5 is present on the surface of reticulocytes and erythrocytes. Our results strongly imply that distinct erythroid surface glycans are counter-receptors. Studies on other galectins have revealed a high level of target specificity for distinct glycoproteins such as CD7, α5β1-integrin, or laminin.16 For galectin-9 present in exosomes of patients with nasopharyngeal carcinoma, the cell-death–inducing receptor Tim-3 expressed by mature Th1 cells appears to be a functional ligand.47 In neuroblastoma cells, galectin-dependent growth regulation, for example, by p53-induced gene 1 (galectin-7), and between T-effector and T-regulatory cells, is mediated by ganglioside GM1 binding.48,49 Because galectin-5 also interacts with the ganglioside GM1 pentasaccharide chain,29 we considered the possibility that galectin-5 was associated with the surface of rat erythroid cells through GM1 binding. However, our experimental evidence did not support an association of galectin-5 with raft domains purified from erythroid plasma membranes. Because binding to multitriantennary N-glycans proved particularly strong,29 respective glycoproteins are likely candidates as physiologic counter-receptors.
Because galectin-5 was apparently not secreted under in vitro erythrocyte subculture conditions, we hypothesized that it might be secreted during reticulocyte maturation, a time during which the plasma membrane is significantly remodeled, in part, by secretion of exosomes. Typical membrane (glyco)proteins such as distinct receptors (eg, TfR),11 transporters (eg, nucleoside transporters),11 or adhesion proteins (eg, integrin α4β1)10 are subject to this process in reticulocytes. These membrane (glyco)proteins are lost via selective incorporation into invaginations of the endosomal membrane during MVE biogenesis and expulsion of these intralumenal vesicles into the extracellular space, by fusion of MVE with the plasma membrane. We show here that rat reticulocytes secrete galectin-5 in a temperature- and ATP-dependent manner. The amount of galectin-5 secreted into the extracellular medium is strongly correlated with the release of exosomes, in terms of kinetics and sensitivity to inhibitory conditions. Further, galectin-5 is released in association with exosomes, as demonstrated by its comigration with TfR and its density in a sucrose gradient.
Cytosolic proteins can also be secreted into the extracellular medium via the exosomal pathway; translationally controlled tumor protein is an example of such a protein.50 These exosomes are formed by membrane invagination toward the lumen of the endosomal compartment and thus contain cytosolic proteins. Fittingly, secreted translationally controlled tumor protein was found to be associated with rat reticulocyte exosomes (supplemental Figure 4). In addition, we demonstrated that galectin-5 was also present on the surface of exosomes. The fact that a pool of galectin-5 associated with endosomes was resistant to trypsin treatment and that galectin-5 was present on the surface of exosomes as previously noted for galectin-9,47 indicates that galectin-5 translocation could occur from the cytosol into the lumen of the endosomal compartment. The local concentration of galectin-5 would then favor its binding to glycans present on the surface of intraluminal vesicles and/or on the endosomal limiting membrane. At this point, by fusion of endosomes with the plasma membrane, bound galectin-5 would either reach the cell surface or be released in association with exosomes. Galectin-5 might thus be involved in selective segregation of glycoconjugates on the limiting endosomal membrane, favoring their sorting into intralumenal vesicles (secreted as exosomes) and thus their disappearance on the reticulocyte plasma membrane. However, galectin-5 recycled to the plasma membrane might be involved in favoring the sorting of specific glycoconjugates into exosomes after endocytosis, as we previously showed using an exogenously added lectin.4
Interestingly, we found that Lamp2, a highly glycosylated protein, is lost during reticulocyte maturation through the exosomal pathway. Lamp2 is enriched in the endo-lysosomal compartment and might thus be a counter-receptor for galectin-5 after its translocation from the cytosol. Moreover, Lamp2 possesses a tyrosine motif allowing its endocytosis through clathrin-coated pits. Of note, galectin-based sorting of distinct glycoproteins with complex-type N-glycans such as dipeptidyl peptidase IV, carcinoembryonic antigen, or mucin-like membrane MUC1 through the endocytic/recycling pathway has been recently described for galectin-4 in the apical biosynthetic pathway in enterocyte-like cells.51,52 It is therefore tempting to speculate that galectin-5 in rat or EDA in rabbit might facilitate down-regulation of the expression of specific target glycoconjugates during erythropoiesis. This would be in agreement with the progressive decrease in galactoside-containing components from the cell surface of rabbit erythroid cells during differentiation of erythroblasts into mature erythrocytes.15 Here again, by sorting membrane glycoproteins, exosomal galectin-5 would facilitate completion of a removal process already started during nuclear extrusion.
Besides its possible role in segregation/sorting of suitable N-acetyllactosamine-bearing glycoconjugates into exosomes during reticulocyte maturation, we investigated the possibility that galectin-5 could affect exosome uptake by phagocytic cells. Our data, obtained by our use of PKH67-labeled exosomes and macrophages, suggest that this is indeed the case. We verified that PKH-67 cell labeling after exosome incubation was not caused by PKH67 transfer or vesicle fusion with the cell plasma membrane. On the contrary, macrophage labeling was dependent on temperature, incubation time, exosome concentration, and was inhibited by drugs affecting endocytosis and/or phagocytosis. Furthermore, we demonstrated that internalization of labeled exosomes was specifically decreased by exogenously adding an excess of exosomes or galectin-5. Preincubation of galectin-5 with lactose but not with glucose abolished its ability to inhibit vesicle internalization by macrophages. However, preincubation of PKH67-labeled exosomes with lactose did not decrease their uptake by macrophages (not shown), thus ruling out the possibility that oligomers of galectin-5 facilitate internalization of exosomes by bridging β-galactoside determinants on the surface of vesicles and macrophages. This evidence indicates that, in our experiments, the excess of added galectin-5 masked internalization determinants, thereby impairing exosome uptake by macrophages. Because uptake of PKH67-labeled exosomes was markedly decreased when incubation with macrophages was carried out in the presence of EDTA (supplemental Figure 5), binding of exosomes to macrophages could be mediated by their C-type lectin receptor.53
In the red-cell maturation setting, galectin-5 would, besides its putative exosomal segregation/sorting function, thus be involved in limiting interactions between glycoproteins presenting galactoside-containing determinants on the surface of red cells with C-type lectin receptors on phagocytic cells. The competition of 2 different lectins for the same epitopes would then attain functional relevance. However, the size of exosomes (60-80 nm) compared with the size of cells (6-8 μm) likely allows (1) a greater density of N-acetyllactosamine–bearing glycoconjugates per membrane surface unit and (2) the possibility of being taken up through endocytosis, the average size of clathrin coated pits being around 0.3 μm, eg via macrophage C-type asialoglycoprotein receptors.16,53 This study suggests that the secretion of galectin-5 has a direct impact on the biogenesis and fate of rat reticulocyte exosomes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank J. A. Chasis and B. Friday for critical reading of the manuscript. We are grateful to Adam Telerman and Robert Amson for providing TCTP antibodies.
This study was supported by research funding from CNRS, the French “Ministère de la Recherche,” the “Ligue Nationale contre le Cancer” (Comité de l'Hérault), the Verein zur Förderung des biologisch-technologischen Fortschritts in der Medizin e.V., and an EC Marie Curie Research Training Network (contract no. CT-2005-019561).
Authorship
Contribution: C.B. and L.B. performed the research; C.B., P.B.-B., S.A., R.M., H.-J.G., and M.V. analyzed results; and M.V. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for L.B. is Red Cell Physiology Lab, New York Blood Center, New York, NY.
Correspondence: Michel Vidal, UMR 5235, Univ. Montpellier II–cc107, 34095 Montpellier Cédex 05, France; e-mail: mvidal@univ-montp2.fr.