Abstract
Lupus anticoagulants (LACs) are associated with thromboembolic complications (TECs). LACs can be detected by their anticoagulant properties in thrombin generation assays, by the peak height (PH) and lag time (LT). To assess the thrombotic risk in LAC-positive patients, we have expressed the LAC activity quantitatively by PH/LT calibration curves, constructed for mixtures of monoclonal antibodies against β2-glycoprotein I (β2GPI) and prothrombin, spiked in normal plasma. PH/LT was determined in LAC patients, with (n = 38) and without (n = 21) TECs and converted into arbitrary LAC units. LAC titers ranged from 0 to 200 AU/mL, with 5 of 59 patients being negative. In the positive LAC titer population (54 of 59), LAC and anti-β2GPI immunoglobulin G (IgG) titers correlated with TECs, with odds ratios of 3.54 (95% CI, 1.0-1.7) and 10.0 (95% CI, 1.98-50.6), respectively. In patients with single or combined low titers, useful predictions on thrombosis could be made only after additional measurements of soluble P-selectin and factor VII. This layered strategy yielded positive and negative predictive values, sensitivity, and specificity values approximately 90% in this subgroup. Hence, LAC and anti-β2GPI IgG titers, when combined with selected markers of the hypercoagulable state, allow a relevant thrombotic risk assessment in nearly all patients with LACs.
Introduction
The antiphospholipid syndrome (APS) is an autoimmune disease characterized by the persistent presence of antiphospholipid (aPL) antibodies and recurrent occurrence of thrombosis or pregnancy complications. Laboratory criteria include lupus anticoagulants (LACs), measured in functional phospholipid-dependent coagulation assays, anticardiolipin antibodies (aCLs) and β2-glycoprotein I (β2GPI) antibodies, analyzed by enzyme-linked immunosorbent assays (ELISAs).1 Detection of aPL antibodies in the blood of patients with a history of thrombosis or pregnancy complications is an essential step in the diagnosis and management of the APS.2 Because rates of morbidity and mortality are high in the APS, awareness of the need for optimal prognostic markers in the prediction of complications of the APS is growing.3
The laboratory diagnosis assigns patients with a common event (thrombosis) to a group with a high risk of recurrence, a prerequisite for long-term oral anticoagulant therapy. Assays for the detection of aPL antibodies, therefore, must be sufficiently sensitive to identify patients correctly as being APS positive. They also need to be specific, because false-positive results have an effect on clinical decisions; patients with thrombosis and aPL antibodies possibly being given oral anticoagulant treatment indefinitely,4 potentially exposing them to a high risk of bleeding. Yet, a recent epidemiologic study could not identify clinical or immunologic predictors of thrombotic events or pregnancy rate of morbidity or mortality,5 and the current set of diagnostic tests for the APS still awaits tools to correctly identify patients at risk of clinical manifestations.
Despite this, an association between aPL antibodies and thrombosis has consistently been shown. Anti-β2GPI antibodies correlate with thrombosis.6-11 In contrast, a clear association between aCL antibodies and thrombosis is lacking, albeit that a role for aCL antibodies in pregnancy morbidity seems possible.12 aPL antibody positivity in multiple tests seems to reflect a more severe course of the disease. As a consequence, the determination of antibody profiles and subclassification of patients, according to the number and the type of tests with a positive score, are encouraged.1,13,14
Because LACs show the strongest correlation with thrombosis and pregnancy morbidity, LAC assays are preferred for the detection of functional aPL antibodies.7,12,15 Yet, the laboratory measurement of LACs is still a major challenge for the clinical laboratory. One specific test for the detection of LACs would be preferable, but such a test is not evident, in view of the multitude of mechanisms implicated in APS-associated clinical events. Despite refined procedures over the years,14 the diagnosis of APS still relies on a combination of tests, because no single test has sufficient specificity and sensitivity. Furthermore, LAC quantification in clinical samples has not been achieved.
The revival of global coagulation tests such as thrombography may help in the diagnosis and follow-up of the APS.16,17 We recently studied thrombin generation (TG) by calibrated automated thrombography in the diagnosis of LACs18 and have identified the peak height (PH), the lag time (LT), and their ratio PH/LT to be reliable parameters in the diagnosis of the APS. We have presently further investigated whether TG is suited for the quantitative expression of the LAC activity. By constructing monoclonal antibody–based calibration curves, we have shown, in a small series of patients with LACs with and without thromboembolic complications (TECs), that the PH/LT ratio can readily be converted in arbitrary units (AUs), thus enabling quantification of LAC activity. The further study of these LAC titers and anti-β2GPI immunoglobulin G (IgG) titers has allowed us to equate the titer relationship between clinical APS symptoms and aPL antibody potency. With the measurement of additional prothrombotic markers, the present study represents the first analytical effort to quantitatively assess the thrombotic risk in patients with LACs.
Methods
Subject selection and plasma collection
The control population in the pilot study comprised 25 healthy adult volunteers. Patients were selected from persons referred to our thrombophilia centers or referred for autoimmune disease testing. Plasma samples were collected according to the updated guidelines for LAC detection.1,14 After LAC analysis, all samples were kept at −80°C, until further analysis. Permission was given by the Ethical Committees of the Ghent and Leuven University Hospitals to store patient plasma samples for further analysis. In the pilot study, patients were divided in 3 groups, depending on presence or absence of LACs in their plasma. Group 1 (n = 8) consisted of patients persistently positive for LACs, without history of TECs. Group 2 (n = 8) also consisted of patients persistently positive for LACs, with a history of TECs. Group 3 (n = 21) consisted of patients manifesting thrombosis but without evidence of plasma LACs.
For the main study, plasma samples were selected from patients with confirmed LACs, diagnosed as described in the next subsection, that is, following established criteria (n = 59). Thrombotic episodes or pregnancy morbidity were retrospectively identified on consultation of medical records. Our initial selection included 38 patients with and 21 patients without thrombosis or pregnancy complications. Of these patients, 37 of 38 had evidence of thrombosis (mostly deep venous thromboembolism and pulmonary embolism), 3 in association with pregnancy complications; 1 patient of 38 presented with pregnancy morbidity only. Patients on oral anticoagulants (OACs; 11 of 59; International Normalized Ratio [INR] ranging from 0.97 to 2.44) were equally included, because we showed before that thrombography was possible in 1:1 mixtures of patient and normal plasma samples.18 Of these, only 3 of 11 patients had an INR greater than 1.5 at the moment of sample collection for aPL antibody testing. Therefore, LAC titers were determined in all samples (n = 59), but plasma volumes were sufficient to measure anti-β2GPI IgG titers in only 57 samples. Additional testing of a series of prothrombotic parameters (see “Additional analyses”) could be done in only 56 of 59 samples, also because of insufficient plasma for 3 patients. In this group of 56, 2 more patients (with TECs) in the subgroup on OACs (n = 11) were excluded: these patients had an INR of 1.9 and 2.44, respectively, in combination with reduced levels of factor VII (FVII; 52% and 34%), that is, below the cutoff, determined by receiver operating characteristic (ROC) analysis (see “Results”). Hence, additional thrombophilic parameters were performed on 54 of 59 patients (including 1 patient with INR > 1.5 but high FVII).
LAC detection
Persistent LACs were defined, according to the revised criteria as LAC positivity on at least 2 occasions and at least 12 weeks apart.1 LAC assays were performed according to the recommendations of the International Society on Thrombosis and Haemostasis with the use of screening, mixing and confirmation tests.14,20 Samples with prolonged screening tests were further analyzed by mixing and confirmation tests. As screening assays, a sensitive activated partial thromboplastin time (aPTT; PTT-LA; Diagnostica Stago), in combination with the diluted Russell viper venom time (dRVVT; LA-screen; Gradipore Ltd), or a dilute prothrombin time (dPT; Dade Innovin; Dade Behring) in combination with a dRVVT (LAC-screen, Instrumentation Laboratories) and silica clotting time (Instrumentation Laboratories) were used. Mixing studies were done when screening tests were prolonged. Reference values were calculated as described.14 Confirmatory tests were done by aPTT (Staclot-LA; Diagnostica Stago), dRVVT (LA-confirm; Gradipore Ltd), dPT, and silica clotting time, as described.19 Samples with a positive screening test, a mixing test result compatible with the presence of an inhibitor, and a positive confirmation test in at least 1 test system are reported as positive in this study.
Automated measurement of TG
The automated measurement of TG was performed in 1:1 mixtures of patient plasma and NPP as recently described.18,21 TG was triggered in platelet-poor plasma in the presence of an intermediate concentration of tissue factor (5pM) and a low concentration of phospholipids (1μM) to raise the phospholipid dependency of the test. Results are expressed as PH/LT ratio, as described before.18 TG parameters were normalized by division of the results for the 1:1 plasma mixtures and those measured for the NPP.
Calibration plasmas in TG measurements were prepared by spiking NPP with the well-characterized antiphospholipid monoclonal antibodies (Mabs) 23H9 and 28F4, raised against human β2GPI and prothrombin, respectively.22,23 These antibodies were added in a fixed ratio of 5:2 in NPP, starting at a concentration of 100 μg/mL for 23H9 and 40 μg/mL for 28F4, progressively diluted in NPP, to 2.5 μg/mL and 1 μg/mL, respectively. TG was measured in native NPP and Mab-spiked NPP samples, and calibration curves were constructed for the PH/LT ratio, as a function of the antibody concentration. One arbitrary unit per milliliter for the quantification of LACs was defined as the effect of the combined concentration of 23H9 (100 μg/mL) and 28F4 (40 μg/mL). Calibration curves were constructed for each run. For the patients with LACs, the PH/LT ratio was determined for multiple dilutions in NPP (1:2 to 1:20), and the PH/LT ratio was quantitatively expressed in AU/mL, after transformation on the corresponding 23H9/28F4 calibration line.
Additional analyses
All further parameters were analyzed in platelet-free plasma samples (9 volumes of venous blood into 1 volume of 0.109M sodium citrate) according to the manufacturer's instructions. Antibodies (IgG) against β2GPI and prothrombin were measured with ELISA Autoimmune EIA (Bio-Rad). Results are expressed in IgG arbitrary units (GAU)/mL. Human soluble P-selectin (sP-selectin; in ng/mL) and soluble E-selectin (in ng/mL) were measured by ELISA (Bender Medsystems). The FVII concentration (%) was measured in a 1-stage clotting assay with thromboplastin reagent (Neoplastin CI plus; Diagnostica Stago) and FVII-deficient plasma (Diagnostica Stago). Thrombin–antithrombin complex (TAT; in μg/L) was measured with Enzygnost TAT micro (Dade Behring).
Statistical analysis
Data were analyzed with MedCalc Version 7.1.0.0 (MedCalc Software). Comparison of results and correlation analysis was done with Pearson correlation and linear regression analysis. Results were presented as box-and-whisker plots or histograms. Results were compared with a Mann-Whitney test. P values (2-sided) less than .05 were considered statistically significant. ROC curve analysis was performed to discriminate diseased cases from healthy cases and to find a particular cutoff point or criterion for parameters used in this study. Fisher exact test was used to calculate an exact 2-sided P value for 2 × 2 frequency tables with small number of expected frequencies. Odds ratios (ORs) with 95% confidence interval (CI) were calculated to compare the outcome in patient groups with and without thrombosis.
Results
TG in patients and normal plasma samples
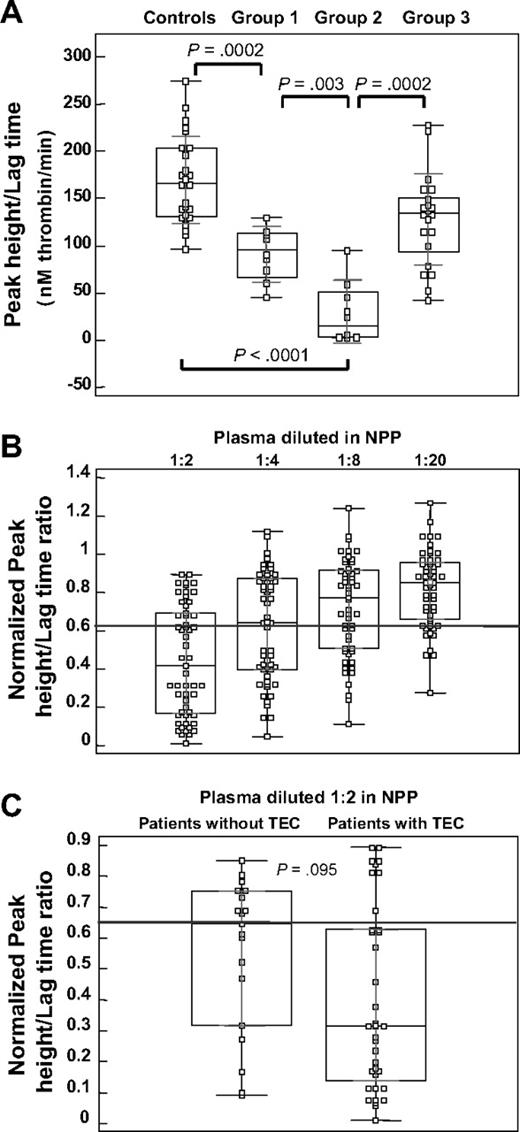
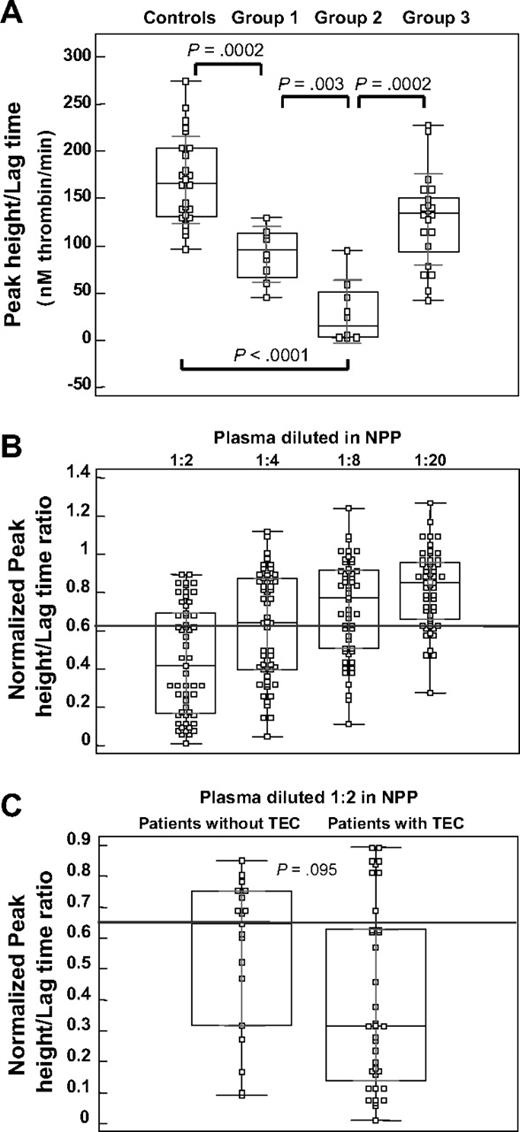
In our pilot study, patients with LACs were divided into 2 groups: one without historical evidence of TECs (group 1; n = 8) and one with evidence of TECs (group 2; n = 8). The LT and PH were measured for these groups and for a reference group, consisting of control plasma samples (controls; n = 25) and an independent patient group, with evidence of TECs but negative for LACs (group 3; n = 21). Figure 1A shows the box plot representation for the distribution of their PH/LT ratio. This figure shows that group 1 (90.7 ± 29.7nM/minute) and group 2 (29.8 ± 33.6nM/minute) are different and divergent from the control group (169.6 ± 46.4nM/minute) and group 3 (128.1 ± 48.5nM/minute). Group 2 differs significantly from all other groups. Even though the PH/LT ratio in group 3 was mildly reduced, compared with the controls, the PH/LT ratios for patients with LACs and TECs were more reduced than for the LAC-negative TEC samples.
TG parameters in patients with LACs. (A) Box-and-whisker plots for the PH/LT ratio, measured in healthy controls (n = 25) and in patients with persistent LACs, without (group 1, n = 8) or with (group 2, n = 8) evidence of thrombosis, compared with patients with LAC negative for thrombosis (group 3, n = 21). The central box represents the 25th and 75th percentiles and the middle line represents the median. The vertical black line extends from the minimum to the maximum value, outliers being displayed as separate points. The light lines represent the 1 SD group intervals. P values, calculated with the Mann-Whitney test, are represented for comparisons between experimental groups. (B) Box-and-whisker plots for normalized PH/LT ratio, for patients with persistent LACs (n = 54) with reduced PH/LT ratio, in multiple dilutions with NPP (1:2 to 1:20). The gray line represents the cutoff value for the PH/LT ratio (0.9). (C) Box-and-whisker plots for normalized PH/LT ratio, measured for patients with persistent LACs (n = 54) with reduced PH/LT ratio, with or without TECs; patient plasma is diluted 1:2 with NPP. The gray line represents the cutoff value for the PH/LT ratio (0.9). P value, calculated with the Mann-Whitney test, represents the comparisons between the 2 patient groups.
TG parameters in patients with LACs. (A) Box-and-whisker plots for the PH/LT ratio, measured in healthy controls (n = 25) and in patients with persistent LACs, without (group 1, n = 8) or with (group 2, n = 8) evidence of thrombosis, compared with patients with LAC negative for thrombosis (group 3, n = 21). The central box represents the 25th and 75th percentiles and the middle line represents the median. The vertical black line extends from the minimum to the maximum value, outliers being displayed as separate points. The light lines represent the 1 SD group intervals. P values, calculated with the Mann-Whitney test, are represented for comparisons between experimental groups. (B) Box-and-whisker plots for normalized PH/LT ratio, for patients with persistent LACs (n = 54) with reduced PH/LT ratio, in multiple dilutions with NPP (1:2 to 1:20). The gray line represents the cutoff value for the PH/LT ratio (0.9). (C) Box-and-whisker plots for normalized PH/LT ratio, measured for patients with persistent LACs (n = 54) with reduced PH/LT ratio, with or without TECs; patient plasma is diluted 1:2 with NPP. The gray line represents the cutoff value for the PH/LT ratio (0.9). P value, calculated with the Mann-Whitney test, represents the comparisons between the 2 patient groups.
We have reported before that in 1:1 plasma mixtures, weak LACs may not be detected in TG.18 Correspondingly, in our present patient selection, we found no anticoagulant activity in TG for 5 of 59 plasmas, although these patients were weakly positive in dPT, dRVVT. or aPTT assays, that is, by classic criteria (not shown). Therefore, subsequent calculations of the LAC titer (see next subsection) was restricted to 54 of 59 patients with LACs. Figure 1B shows the PH/LT ratio for these 54 patients with LACs; results are normalized.18 The PH/LT ratio in this cohort was measured (double-blinded) in multiple dilutions with NPP (1:2 to 1:20). Repetitive analysis of NPP in each run (n = 8) resulted in a coefficient of variation of 9.8% for the PH/LT ratio. Consequently, the corresponding cutoff value was set at 0.9, showing a reduced PH/LT ratio in all patients positive for LACs diluted 1:2 in NPP, progressively lost with further dilution.
The normalized PH/LT ratio, for these patients (n = 54) showed a trend toward a different distribution pattern in patients with and without TECs (P = .095) in the 2-fold diluted plasma (Figure 1C), however characterized by an extended distribution.
Quantification of a LAC titer and determination of the anti-β2GPI IgG titer
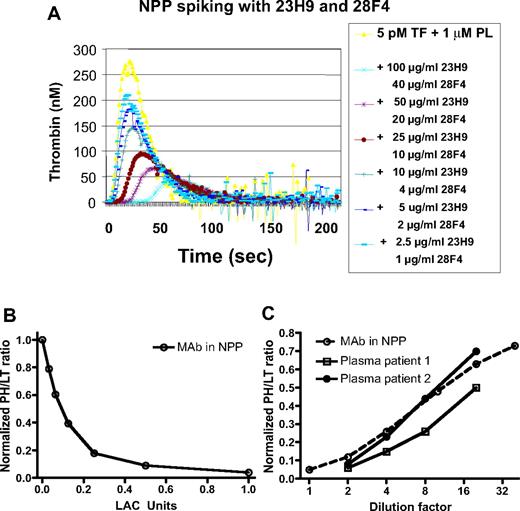
TG in the calibration plasmas spiked with 23H9 and 28F4 showed a reduced PH and a prolonged LT, in a concentration-dependent manner as previously described18 (Figure 2A). A 7-point point-to-point calibration curve was, therefore, constructed for the normalized PH/LT ratio (Figure 2B). The normalized PH/LT measured in NPP (1 on y-axis) was defined as the zero point on the x-axis of the calibration curve. To compare the anticoagulant responsiveness of the PH and the LT for each antibody separately, NPP was spiked with increasing concentrations of 23H9 and 28F4, not combined. Each antibody inhibited the PH and LT in a concentration-dependent manner (data not shown), but 28F4 was a more potent anticoagulant. It was therefore used at a maximal concentration of 40 μg/mL.
TG: calibration of the anticoagulant effect. (A) TG as a function of the indicated concentrations of 23H9 and 28F4 added to NPP, initiated by 5pM tissue factor (TF) and 1μM phospholipids (PLs). (B) Calibration curve with 7 calibration plasmas (NPP spiked with decreasing concentrations of 23H9 and 28F4) constructed for the normalized PH/LT ratio as a function of antibody concentration, defined as LAC arbitrary units. One LAC AU/mL was defined as the PH/LT of the calibration plasma spiked with the combination of 23H9 (100 μg/mL) + 28F4 (40 μg/mL). The normalized PH/LT measured in the native NPP (1 on y-axis) has no LAC activity. (C) Normalized PH/LT ratio measured for NPP spiked with 23H9 anti-β2GPI IgG (100 μg/mL) and 28F4 (40 μg/mL; Mab in NPP), and for 2 representative patient plasma samples, as a function of the indicated dilution factor.
TG: calibration of the anticoagulant effect. (A) TG as a function of the indicated concentrations of 23H9 and 28F4 added to NPP, initiated by 5pM tissue factor (TF) and 1μM phospholipids (PLs). (B) Calibration curve with 7 calibration plasmas (NPP spiked with decreasing concentrations of 23H9 and 28F4) constructed for the normalized PH/LT ratio as a function of antibody concentration, defined as LAC arbitrary units. One LAC AU/mL was defined as the PH/LT of the calibration plasma spiked with the combination of 23H9 (100 μg/mL) + 28F4 (40 μg/mL). The normalized PH/LT measured in the native NPP (1 on y-axis) has no LAC activity. (C) Normalized PH/LT ratio measured for NPP spiked with 23H9 anti-β2GPI IgG (100 μg/mL) and 28F4 (40 μg/mL; Mab in NPP), and for 2 representative patient plasma samples, as a function of the indicated dilution factor.
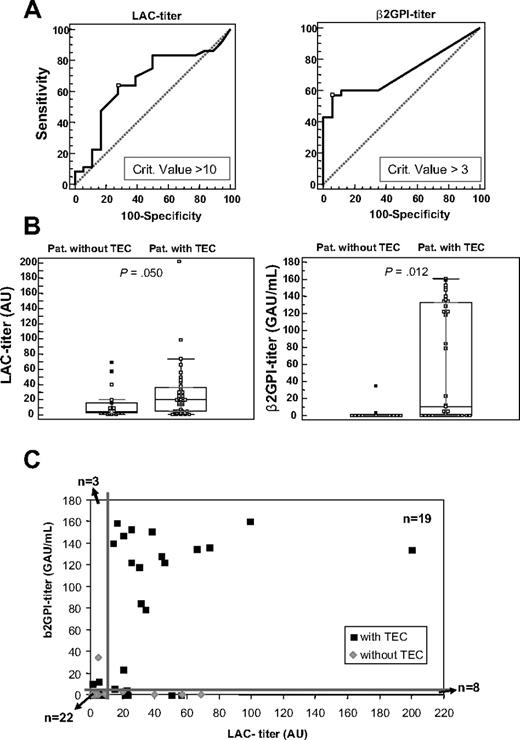
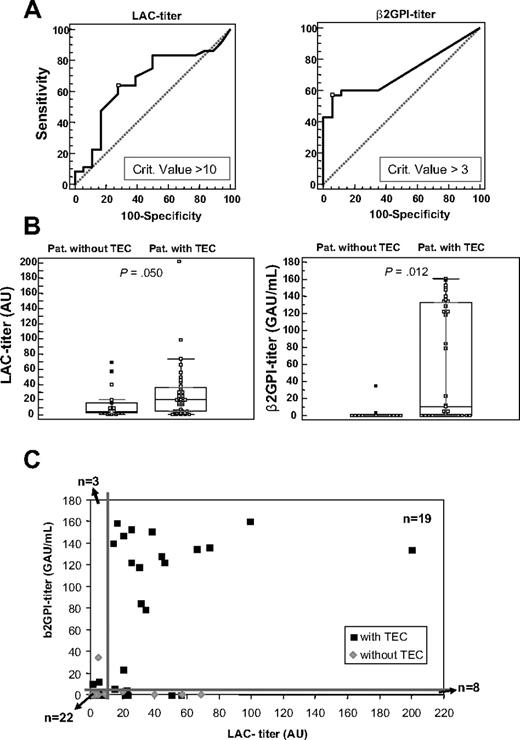
In parallel dilutions, the PH/LT ratio for NPP spiked with 23H9/28F4 and for patient plasma samples showed comparable dependence on the plasma dilution factor (Figure 2C). Therefore, the PH/LT ratio of the 54 patients positive for LACs in TG was converted into LAC arbitrary units per milliliter (AU/mL), with these calibration lines. We evaluated 3 methods for the calculation of the titer, which resulted in comparable LAC titers (see “Calculation of LAC titer” in supplemental Data, available on the Blood website; see the Supplemental Materials link at the top of the online article). However, the normalized PH/LT ratio measured in 2-fold diluted patient plasma resulted in the best ROC analysis, indicating a titer of 10 AU/mL as the best discriminator for disease (Figure 3A). Therefore, all samples were measured in 1:1 plasma mixtures, and titer values were multiplied by 100. Hence, calculated titers ranged from 1 to 200 AU/mL. LAC titers in patients with and without TECs were slightly differently distributed, with weakly elevated values in the TEC group (P = .050; Figure 3B). Of the patients positive for TECs, 23 of 36 had more than 10 AU/mL and 12 of 18 patients negative for TECs were below 10 AU/mL. This resulted in an OR for the association with TEC of 3.54 (95% CI, 1.07-11.67). The positive and negative predictive values (PPV and NPV) for this LAC titer assay were 79% and 48%, respectively; sensitivity and specificity were 64% and 67%, respectively (Figure 4).
LAC titer and anti-β2GPI IgG titer in patients with LAC. (A) ROC analysis for the LAC titer (n = 54) and anti-β2GPI IgG titer (n = 52), with indication of the criterion value suggested as the best discriminator for disease, measured in plasma of patients with LACs with LAC titer more than 0 AU. (B) Box-and-whisker plots for LAC titer (n = 54) in arbitrary units (AU/mL) and anti-β2GPI-titer (n = 52) in IgG arbitrary units (GAU/mL) in patients (pat.) without or with TECs (n = 54). P value, calculated with the Mann-Whitney test, represents the comparisons between the 2 patient groups. (C) Dot plot of the LAC titer versus the anti-β2GPI IgG titer in plasma of patients positive for LACs with LAC titer more than 0 (n = 52). The thick lines represent the cutoff value for high LAC titers (≥ 10 AU) and anti-β2GPI titers (≥ 3 GAU/mL).
LAC titer and anti-β2GPI IgG titer in patients with LAC. (A) ROC analysis for the LAC titer (n = 54) and anti-β2GPI IgG titer (n = 52), with indication of the criterion value suggested as the best discriminator for disease, measured in plasma of patients with LACs with LAC titer more than 0 AU. (B) Box-and-whisker plots for LAC titer (n = 54) in arbitrary units (AU/mL) and anti-β2GPI-titer (n = 52) in IgG arbitrary units (GAU/mL) in patients (pat.) without or with TECs (n = 54). P value, calculated with the Mann-Whitney test, represents the comparisons between the 2 patient groups. (C) Dot plot of the LAC titer versus the anti-β2GPI IgG titer in plasma of patients positive for LACs with LAC titer more than 0 (n = 52). The thick lines represent the cutoff value for high LAC titers (≥ 10 AU) and anti-β2GPI titers (≥ 3 GAU/mL).
Predictive value of LAC titer, anti-β2GPI IgG titer, and sP-selectin and FVII levels in the overall LAC-positive population. (A) Histograms (percentage) of sensitivity, specificity, PPV, NPV, as indicated, for the LAC titer (AU), the anti-β2GPI IgG titer (GAU/mL), the combination of LAC- and anti-β2GPI IgG titer for the overall LAC-positive population with LAC titer more than 0 AU (n = 52-54); sP-selectin (ng/mL), FVII (%), and the combination of sP-selectin and FVII, for the overall (INR/FVII corrected) LAC-positive population (n = 54). (B) 2 × 2 Contingency tables for the LAC titer, the anti-β2GPI IgG titer, the combination of LAC titer and anti-β2GPI IgG titer, sP-selectin, FVII, and the combination of sP-selectin and FVII in the patient populations, subdivided as indicated. P values calculated with Fisher exact test are indicated for each 2 × 2 table.
Predictive value of LAC titer, anti-β2GPI IgG titer, and sP-selectin and FVII levels in the overall LAC-positive population. (A) Histograms (percentage) of sensitivity, specificity, PPV, NPV, as indicated, for the LAC titer (AU), the anti-β2GPI IgG titer (GAU/mL), the combination of LAC- and anti-β2GPI IgG titer for the overall LAC-positive population with LAC titer more than 0 AU (n = 52-54); sP-selectin (ng/mL), FVII (%), and the combination of sP-selectin and FVII, for the overall (INR/FVII corrected) LAC-positive population (n = 54). (B) 2 × 2 Contingency tables for the LAC titer, the anti-β2GPI IgG titer, the combination of LAC titer and anti-β2GPI IgG titer, sP-selectin, FVII, and the combination of sP-selectin and FVII in the patient populations, subdivided as indicated. P values calculated with Fisher exact test are indicated for each 2 × 2 table.
In the same patient cohort (n = 52), the calculated cutoff value for the anti-β2GPI IgG titer by the 99th percentile of 50 healthy volunteers was 1.8 GAU/mL. ROC analysis showed an anti-β2GPI IgG titer of 3 GAU/mL as the best discriminator for disease (Figure 3A). Anti-β2GPI IgG titers in patients with and without TECs were significantly different (P = .012; Figure 3B), but spread out over a broad range. Thus, 20 of 35 patients with TECs had more than 3 GAU/mL and 15 of 17 patients without TECs were below 3 GAU/mL. This resulted in an OR for the association with TEC of 10.0 (95% CI, 1.98-50.6). The sensitivity and specificity for the antibody titer analysis were 57% and 88%, respectively, PPV and NPV were 91% and 50%, respectively (Figure 4).
When the LAC titer and the anti-β2GPI IgG titer were plotted against each other for this cohort (n = 52; Figure 3C), the dot plot shows that 18 of 19 patients with LACs with simultaneous high titer for LACs (≥ 10 AU/mL) and anti-β2GPI IgG (≥ 3 GAU/mL) were patients with TECs (including both patients on OACs with INR > 1.5). The one patient in this group without TECs had a LAC titer of 20 AU/mL and a borderline anti-β2GPI IgG titer of 3 GAU/mL. This analysis further showed that 22 of 52 patients with LACs had both low titers for LACs and for anti-β2GPI IgG. Of those, nevertheless, 11 of 22 presented with TECs, 11 of 22 without. Or, although double high titers in 19 of 52 patients were strongly associated with thrombosis, double low titers were no guarantee for absent TECs, which was still 50% in this group. In patients with singularly high titer aPL antibodies, ie with either high LACs (≥ 10 AU/mL; n = 8) or high anti-β2GPI IgG (≥ 3 GAU/mL; n = 3), patients with and without TECs were found. In the high LAC titer group with low anti-β2GPI IgG titer (n = 8), only 2 of 8 patients had positive antiprothrombin antibody titers (5.9 and 10.2 GAU/mL; cutoff is 4.8 GAU/mL); both were positive for thrombosis. In the overall population, antiprothrombin antibodies were less abundant (in 13 of 57 patients, with value above the cutoff of 4.8 GAU/mL) than anti-β2GPI antibodies (in 23 of 57 patients, with value above the cutoff of 1.8 GAU/mL). Table 1, listing the detailed information for the 13 patients, shows that 10 of 13 patients with antiprothrombin antibodies were already identified as having combined high LAC and anti-β2GPI IgG titers. Furthermore, although Table 1 shows that 11 of 13 patients with antiprothrombin antibodies had TECs, 2 × 2 contingency table analysis did not show an association between antiprotrombin antibodies and TECs in the entire cohort (P = .178; n = 52).
Combining the LAC titer and the anti-β2GPI titer into a single approach was not beneficial. This analysis (1 high value vs 2 low values) results in an OR of 4.0 (95% CI, 1.18-13.6), that is, intermediate between that for the LAC and anti-β2GPI titer. Correspondingly, the combined assay had 69% sensitivity, 65% specificity, 80% PPV, and 50% NPV (Figure 4). These analyses underscored that for 33 (63%) of 52 patients with LACs with a low LAC or a low anti-β2GPI IgG titer (or combined low titers), quantitative LAC and/or anti-β2GPI IgG (and antiprotrombin) titer assays are insufficient to predict their thrombotic risk. The 5 patients with LACs with LAC titer of 0 AU/mL also had an anti-β2GPI IgG titer of 0 GAU/mL. Adding these patients to the cohort (n = 52 + 5) did not influence this conclusion; for the combined analysis of LAC and anti-β2GPI IgG titers in this group (n = 57), a comparable OR (4.3) and sensitivity (65%), specificity (70%), PPV (80%), and NPV (52%) were found as for the 52 patients with a positive LAC titer, just described.
Procoagulant and vascular parameters
TAT levels were not different between patients with and without TECs and proved to be useless in the further discrimination of patients with LACs. ROC analysis for soluble E-selectin measurements gave an area under the curve (AUC) of 0.673 (P = .037), but the correlation with TECs was inverse and not significant. Patients with TECs had lower titers than patients without TECs (P = .160).
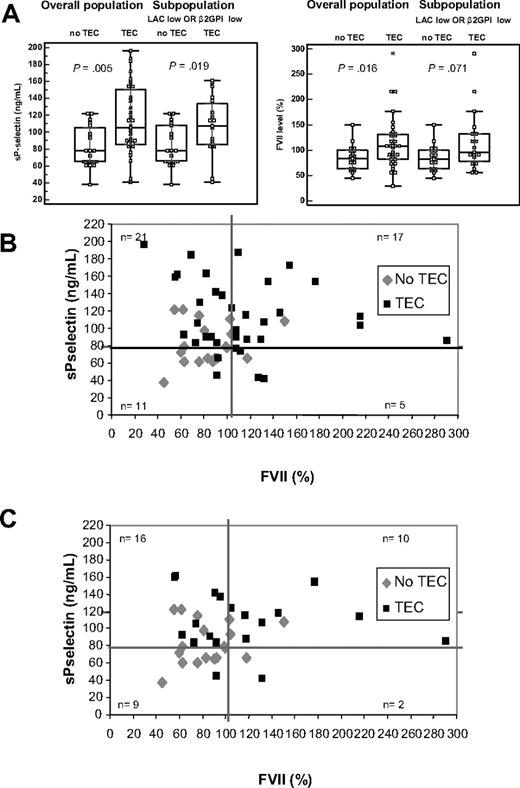
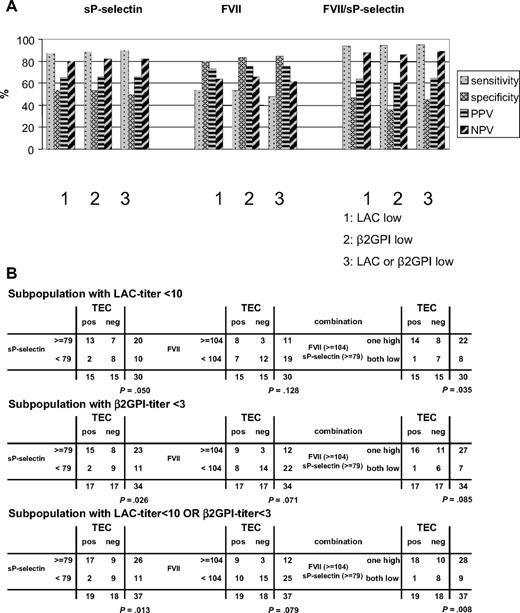
sP-selectin titers were significantly higher (P = .005) in the patient population positive for TECs (n = 35) compared with the patients without TECs (n = 19). sP-selectin in the patient subpopulation with either low LAC titer or low anti-β2GPI IgG titer (n = 37) gave comparable results (P = .019), when analyzed as a function of the presence of thrombosis (Figure 5A). FVII levels also showed a significant higher level in patients with (n = 35) and without (n = 19) TECs in the overall population (P = .016) as well as a trend toward a different distribution pattern in the subpopulation with low LAC or low anti-β2GPI IgG titer (P = .071; Figure 5A).
sP-selectin titer and FVII levels in patients with LACs. (A) Box-and-whisker plots for sP-selectin (ng/mL) and FVII (%) in patients without or with TECs in the (INR/FVII corrected) total patient population (n = 54),and in the subpopulation with low LAC titer or anti-β2GPI IgG titer (n = 37), as indicated. P value, calculated with the Mann-Whitney test, represents the comparisons between the experimental groups with and without TECs. (B) Dot plot of the sP-selectin titer (ng/mL) versus FVII (%) for these patients (n = 54). (C) Dot plot of the sP-selectin titer (ng/mL) versus FVII (%) for the subpopulation of patients positive for LACs with low LAC titer or low anti-β2GPI IgG titer (n = 37). In both cases, the thick lines represent the cutoff value for high sP-selectin titers (≥ 79 ng/mL) and FVII titers (≥ 104%).
sP-selectin titer and FVII levels in patients with LACs. (A) Box-and-whisker plots for sP-selectin (ng/mL) and FVII (%) in patients without or with TECs in the (INR/FVII corrected) total patient population (n = 54),and in the subpopulation with low LAC titer or anti-β2GPI IgG titer (n = 37), as indicated. P value, calculated with the Mann-Whitney test, represents the comparisons between the experimental groups with and without TECs. (B) Dot plot of the sP-selectin titer (ng/mL) versus FVII (%) for these patients (n = 54). (C) Dot plot of the sP-selectin titer (ng/mL) versus FVII (%) for the subpopulation of patients positive for LACs with low LAC titer or low anti-β2GPI IgG titer (n = 37). In both cases, the thick lines represent the cutoff value for high sP-selectin titers (≥ 79 ng/mL) and FVII titers (≥ 104%).
ROC analysis showed an sP-selectin titer (AUC = 0.726; P = .002) of 79 ng/mL and FVII (AUC = 0.639; P = .079) of 104% as best discriminator for disease. In the overall (INR/FVII corrected) study population (n = 54), sP-selectin titers showed 83% sensitivity, 53% specificity, 76% PPV, and 63% NPV and differed strongly in both patient categories (Figure 4). Likewise, high and low FVII distributed differently in both patient categories (Figure 4). FVII levels showed sensitivity of 54% and specificity of 84%; PPV was 86% and NPV was 50%. The ORs for sP-selectin and FVII were 5.37 (95% CI,1.53-18.9) and 6.33 (95% CI,1.56-25.7), respectively. Correspondingly, the combination of sP-selectin with FVII levels showed a highly significant increase of sensitivity to 94% and NPV to 82% (Figure 4) with an OR of 14.9 (95% CI, 2.75-80.3). Figure 5B shows, when sP-selectin titers and FVII were combined, that the majority of patients with LACs free of TECs have low FVII levels, findings confirmed in the target subpopulation with low LAC or low anti-β2GPI IgG titer (n = 37; Figure 5C). In addition, of all patients in this subgroup, with high titers for both sP-selectin and FVII, 8 of 10 presented with TECs (PPV, 80%), whereas of all patients with double low parameters, 8 of 9 had no TECs (NPV, 88.9%).
Combination of LAC titer, anti-β2GPI IgG titer, sP-selectin and FVII levels
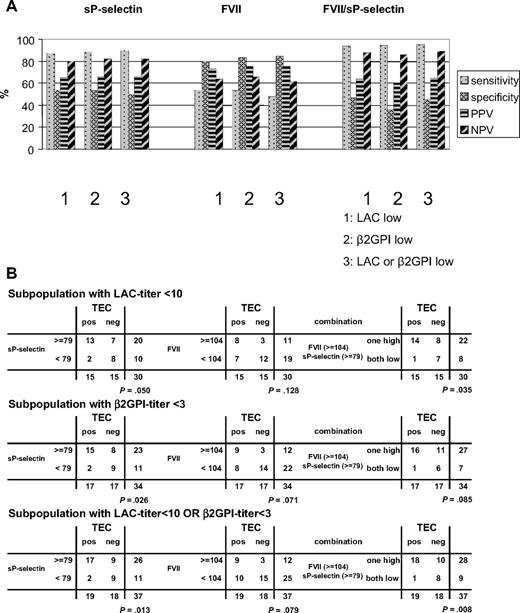
Because the combination of sP-selectin and FVII measurements proved informative in the (INR/FVII corrected) total patient population (Figure 4), we investigated whether these parameters would be informative for the thrombotic risk in the various subgroups of patients, either low in only LAC or anti-β2GPI titers, or corresponding to the target subpopulation, with “low LAC or low anti-β2GPI IgG titer” (including patients with LAC titer = 0 AU). The results are shown in Figure 6 for sP-selectin, FVII, or their combination.
Predictive value of sP-selectin and FVII in the LAC-positive subpopulations. (A) Histograms (percentage) of sensitivity, specificity, PPV, NPV for sP-selectin (ng/mL), FVII (%) and the combination of sP-selectin and FVII in the indicated subpopulations of patients positive for LACs (including those with LAC titer = 0 AU/mL). (B) 2 × 2 tables for sP-selectin (ng/mL) and FVIIa (%) and their combination in the indicated subpopulations with low LAC titer (AU < 10), low anti-β2GPI titer (< 3 GAU/mL) and the subpopulation with low LAC titer or low anti-β2GPI titer, as indicated (including those with LAC titer = 0 AU/mL). P values calculated with Fisher exact test are indicated for each 2 × 2 table.
Predictive value of sP-selectin and FVII in the LAC-positive subpopulations. (A) Histograms (percentage) of sensitivity, specificity, PPV, NPV for sP-selectin (ng/mL), FVII (%) and the combination of sP-selectin and FVII in the indicated subpopulations of patients positive for LACs (including those with LAC titer = 0 AU/mL). (B) 2 × 2 tables for sP-selectin (ng/mL) and FVIIa (%) and their combination in the indicated subpopulations with low LAC titer (AU < 10), low anti-β2GPI titer (< 3 GAU/mL) and the subpopulation with low LAC titer or low anti-β2GPI titer, as indicated (including those with LAC titer = 0 AU/mL). P values calculated with Fisher exact test are indicated for each 2 × 2 table.
This analysis shows that sP-selectin alone has a high sensitivity and NPV throughout the various subpopulations. FVII, in contrast, has good specificity and PPV. Combining both further improves sensitivity and NPV. The NPV for the combination of sP-selectin and FVII was already 82% in the total (n = 54) LAC patient population but increases further, especially in the subpopulation with low LAC titer or low anti-β2GPI IgG titer (n = 37), to 89%. Fisher exact test shows the lowest P value (P = .008) for this 2 × 2 table, showing that the significant relationship between the 2 classification factors, or the significance difference in the respective proportions, is highest by combining the 4 parameters in this manner. The OR for this parameter combination of 14.4 (95% CI,1.57-132) points to a strong association with thrombosis in the subpopulation with low LAC titer or low anti-β2GPI IgG titer. This analysis shows that sP-selectin and FVII can be used, alone or combined, to predict or exclude thrombosis in the target population with low LAC or anti-β2GPI IgG titer.
Discussion
APS is an important cause of acquired thrombophilia, and the laboratory recognition of this thrombotic phenotype would be of considerable clinical value. Yet the thrombogenicity assessment in patients with APS is still hampered by laboratory tests with poor predictive value for the thrombotic phenotype.1,3 One of the drawbacks of the current classic coagulation tests for identifying the presence or absence of LACs is that they provide only information about the presence of aPL antibodies but not the concentration of antibodies, complicating a correct assessment of LAC levels and their evolution. LACs may be falsely positive, especially if identified as of “mild potency,” found in elderly patients or if diagnosed for the first time.24 Therefore, LAC activity should be quantitatively expressed to indicate the strength and to make the differentiation possible between high and low titers in a numerical manner. In parallel with the quantification of aCL and anti-β2GPI antibodies, it will help to stratify patients into risk groups.
Quantification of LACs in clinical samples has been complicated by the lack of a suitable standard of activity. aPL antibodies are polyclonal, and their heterogeneity is difficult to translate into an international standard. Preparation, characteristics, and intended use of such a standard material were recently discussed in the Scientific and Standardization Committee during the XXIIth Congress of the International Society of Thrombosis and Hemostasis in July 2009. One of the proposals was to prepare mixtures of Mabs against the most frequent aPL antibody antigens, to approximate the complex spectrum of naturally occurring aPL antibodies in patients. Mabs to β2GPI and to prothrombin have already been developed in the past22,23,25 and can be used for this purpose. Le Querrec et al26 demonstrated with the current clotting assays that quantification is feasible, by calibrating plasmas. However, they observed large differences in LAC responsiveness between the selected assays. As an alternative, TG has the potential for quantitative LAC detection.18 The strongly reduced PH/LT ratio in our pilot study in the TEC patient group suggested that this ratio might be useful to identify patients with LACs at risk of TECs. Yet, in line with previous reports, TG parameters showed a large intraindividual variation in the control group16,21 even larger in the LAC patient populations, reflecting the heterogeneity of LACs.27 Nevertheless, by calibration curves in TG, constructed with increasing concentrations of mixtures of Mabs to β2GPI and to prothrombin and after measurement of a normalized PH/LT,18 LAC titers could presently be expressed quantitatively, in arbitrary units, because the PH/LT ratio in the standard and patient samples showed similar dilution curves. This nonlinear transformation also showed a broad variation in patients positive for LACs, with titers ranging from 0 to 200 U/mL. LAC titers (> 0 AU/mL) calculated from the PH/LT ratio discriminated well between patients with LACs with and without TECs (OR, 3.5; PPV, 79%), although the low NPV (48%) was clinically insufficient to discriminate between patients with LACs at risk for thrombosis or not.
Analogously, anti-β2GPI IgG titers were significantly higher in patients positive for LACs with thrombosis (OR, 10) and had a higher PPV (91%) but comparably low NPV (50%). Comparison of the LAC titer and the anti-β2GPI IgG titer, at first, suggested even that a quantitatively expressed LAC activity would not be more informative in TEC predictions than the measurement of anti-β2GPI IgG titers in patients with aPL antibody, as recommended.7
Yet our present findings confirmed that β2GPI is the main antigen in APS9,28 ; of 22 patients with high anti-β2GPI IgG titers, 20 manifested TECs. Correspondingly, of the 27 patients with a high LAC titer, 19 also had high anti-β2GPI titers, confirming that in 70% of the patients the potent anticoagulant effect measured in TG is related to the presence of anti-β2GPI IgG antibodies. Moreover, the quantitative coupling of β2GPI and LAC measurements showed that those patients with high titers in each assay almost perfectly (18 of 19, 95%) matched the patient category with TECs. Hence, high LAC titers, combined with high β2GPI-titers, were strongly informative for almost 40% (19 of 52) of patients with APS with regard to their thrombotic risk, substantiating the usefulness of LAC titer determinations, in addition to the measurement of anti-β2GPI IgG titers.7 However, β2GPI is not the only antigen implicated in the APS10,29 ; correspondingly, we found 8 patients with high LAC titer and low anti-β2GPI IgG titer, findings explained to some degree by the occurrence of antiprothrombin antibodies. Yet, in agreement with other studies, we found antiprothrombin antibodies to be considerably less abundant and to be poorly associated with thrombosis. Nonetheless, antibodies against phosphatidylserine-prothrombin complex rather than antibodies against prothrombin alone should be further explored as a candidate of one of the laboratory criteria for the classification of the APS.30 In addition, in a small subset of 3 patients, the anti-β2GPI antibodies were not functionally relevant: in this group, LAC titers were low, despite high anti-β2GPI titers.
Somewhat surprisingly, our analysis showed that in the population with either low LAC and/or low β2GPI-titers (more than 60% of patients), anti-β2GPI antibody detection and/or LAC titer determinations were insufficient to make predictions on thrombosis. Furthermore, we have presently analyzed 5 patients (5 of 59), who were positive for LACs, according to current guidelines,1,14 but without detectable LAC titer in TG and without measurable anti-β2GPI IgG antibodies, albeit that 2 of 5 presented with TECs. We saw no alternative but to include these patients in our overall analyses, be it in a 2-step approach. Indeed, thrombosis is a common event in the general population, and very low antibody titers may rather be an epiphenomenon than be causative: the dRVVT is a sensitive test for LAC detection.18 Therefore, the current serologic criteria may lead to overdiagnosis in patients with only 1 positive laboratory criterion.
It is, therefore, clear from the present study that additional procoagulant markers need to be analyzed for patients with weak LACs, to reach clinically useful information. Our present study has identified 2 such markers, that is, sP-selectin and FVII, be it that these are of limited value in patients on OACs. We only analyzed a limited number of potential activation markers and do not exclude the utility of additional ones in the APS. Yet recent studies have shown that P-selectin has procoagulant properties and reflects a prothrombotic state in human subjects. Elevated sP-selectin has been implicated as a risk factor for thromboembolism and as a predictive biomarker for recurrence of TECs in patients with a first episode of unprovoked thrombosis and in patients with LACs with a previous event.31,32 In our hands, in the overall patient population (corrected for anticoagulant effects), sP-selectin and FVII were associated with thrombosis, albeit with different sensitivity and specificity. OR analysis showed that their combined use was more informative; the PPV and NPV for this approach were high. On the basis of the determination of anti-β2GPI IgG titers, LAC titers, sP-selectin and FVII levels, we believe a layered strategy can be adopted, to stratify the thrombotic risk in patients with suspected APS. First, patients are analyzed quantitatively with respect to their LAC and β2GPI titers, which reduces the need for further assays in approximately 40% of patients. FVII measurements in the remainder of the samples can rule out thrombosis in patients not receiving anticoagulatants, with high specificity and PPV; in our hands only 3 (5.6%) of 54 patients were misjudged, when applying this sequential approach. When combined with sP-selectin, thrombosis could be identified with a high sensitivity and NPV; only 1 (1.9%) of 54 patients was misjudged. Or, low levels of FVII or sP-selectin can exclude TECs in 89% of cases, with high sensitivity (95%) and OR up to 14.4.
Our present analysis only covers a small patient population, and more extensive prospective investigations will be needed to validate layered approaches in thrombosis predictions in the APS. Such seems warranted, in view of the necessity to correctly assess the thrombotic risk in patients with APS, facing potentially long anticoagulant therapy.
In conclusion, our work has shown that a quantitative assessment of the anticoagulant activity in the APS is well within reach in a single assay, such as thrombography, which could constitute an alternative for the multitude of assays used today. However, even a quantitative LAC assay is only partially informative in the prediction/exclusion of thrombosis in patients with APS, and a layered strategy, involving additional hemostasis assays, may have to be adopted to accomplish this task.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank M. Van Russelt, A. De Saar, M. Luypaert, and S. Van kerckhoven for their technical assistance in the identification of APS patients and performance of TG and other measurements. We also thank Prof Em J. Vermylen for critically reading this manuscript.
The Center for Molecular and Vascular Biology is supported by the Excellentiefinanciering K.U.Leuven (EF/05/13).
Authorship
Contribution: K.D. and M.F.H. designed and performed the research, analyzed the data, and wrote the paper; and K.P. contributed to patient selection and identification.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katrien Devreese, Coagulation Laboratory, Laboratory for Clinical Biology, Ghent University Hospital, De Pintelaan, 185 (2P8), B-9000 Gent, Belgium; e-mail: katrien.devreese@uzgent.be.