Abstract
Elevated plasma clot lysis time (CLT) increases risk of venous and arterial thrombosis. It is unclear which fibrinolytic factors contribute to thrombosis risk. In 743 healthy control subjects we investigated determinants of CLT. By comparison with 770 thrombosis patients, we assessed plasma levels of fibrinolytic proteins as risk factors for a first thrombosis. Plasminogen activator inhibitor-1 (PAI-1) levels were the main determinants of CLT, followed by plasminogen, thrombin-activatable fibrinolysis inhibitor (TAFI), prothrombin, and α2-antiplasmin. Fibrinogen, factor VII, X, and XI contributed minimally. These proteins explained 77% of variation in CLT. Levels of the fibrinolytic factors were associated with thrombosis risk (odds ratios, highest quartile vs lowest, adjusted for age, sex, and body mass index: 1.6 for plasminogen, 1.2 for α2-antiplasmin, 1.6 for TAFI, 1.6 for PAI-1, and 1.8 for tissue plasminogen activator [t-PA]). Adjusting for acute-phase proteins attenuated the risk associated with elevated plasminogen levels. The risk associated with increased t-PA nearly disappeared after adjusting for acute-phase proteins and endothelial activation. TAFI and PAI-1 remained associated with thrombosis after extensive adjustment. In conclusion, CLT reflects levels of all fibrinolytic factors except t-PA. Plasminogen, TAFI, PAI-1, and t-PA are associated with venous thrombosis. However, plasminogen and t-PA levels may reflect underlying risk factors.
Introduction
Decreased fibrinolytic potential, as measured with a plasma-based assay, has consistently been shown to be a risk factor for venous and arterial thrombosis.1-4 Furthermore, we recently showed that the combination of hypofibrinolysis and risk factors associated with hypercoagulation resulted in a substantially greater risk than expected on the basis of the individual risks conferred by these factors.1 The clot lysis assay used in these studies determines time to half-maximal lysis of a plasma clot initiated with tissue factor. To induce fibrinolysis, tissue plasminogen activator (tPA) is added to the plasma before clot formation. The clot lysis time (CLT) calculated from the turbidity profile of the clot formation and clot lysis is thought to represent overall plasma fibrinolytic capacity. In in vitro experiments, we previously investigated the effect of changes in levels of a single fibrinolytic factor on CLT. CLT increased with the addition of active plasminogen activator inhibitor-1 (PAI-1) to pooled normal plasma and with the addition of pooled normal plasma to plasma of a patient with severe α2-antiplasmin deficiency. Furthermore, CLT increased when thrombin-activatable fibrinolysis inhibitor (TAFI) was added to immunodepleted normal plasma. When purified plasminogen was added to immunodepleted plasminogen-deficient plasma, CLT decreased.5 In these experiments, a linear relation between TAFI and α2-antiplasmin levels and CLT was found, whereas the relation between plasminogen and CLT was biphasic and between PAI-1 and CLT S-shaped. The relative contribution of the plasma concentration of different fibrinolytic factors to CLT or the association between these factors and CLT in the general population is, however, not known. The procoagulant capacity of plasma has been shown to contribute to some extent to CLT. For example, persons with the prothrombin G20210A mutation have longer CLTs as a result of increased thrombin generation, resulting in increased TAFI activation.6
On the basis of the experiments with purified proteins, we surmised CLT will be explained by a combination of plasma levels of plasminogen, α2-antiplasmin, TAFI, and PAI-1. Because a high concentration of t-PA is added to test plasma to induce fibrinolysis, the role of plasma t-PA levels in CLT is probably minor. In addition, we hypothesized that low levels of plasminogen and high levels of α2-antiplasmin, TAFI, and PAI-1 constitute a risk factor for venous thrombosis and explain the association between CLT and risk of venous thrombosis.
Studies on the association between plasma levels of individual fibrinolytic factors and risk of venous thrombosis are either lacking or provided inconclusive results. Surprisingly, the association between plasma levels of plasminogen and risk of venous thrombosis has not yet been investigated. Studies on plasminogen-deficient persons, however, have not provided any evidence for a causal role of plasminogen in venous thrombosis risk but were of limited power to detect an effect.7,8 Studies in which the authors investigated levels of α2-antiplasmin and the risk of venous thrombosis also are scarce and have failed to show an association, possibly because of low patient numbers.9,10 Increased TAFI levels were more consistently associated with risk of venous thrombosis11-13 although not in thrombophilic families.14
The role of PAI-1 and t-PA in venous thrombosis is controversial. In the Longitudinal Investigation of Thromboembolism Etiology study, a large population-based prospective study on venous thrombosis in middle-aged and elderly patients, no association was found between levels of PAI-1 or t-PA/PAI-1 complex and the risk of venous thrombosis.15 Although the authors of several other studies also failed to show an association between t-PA and PAI-1 and the risk of venous thrombosis, others did find an association (extensively reviewed by Prins and Hirsh16 ). In this review, it was concluded that there is evidence that increased plasma levels of t-PA and PAI-1 are associated with postoperative thrombosis.
The aim of the present study was to investigate the determinants of CLT in the general population. Furthermore, the association between plasma levels of plasminogen, α2-antiplasmin, TAFI, PAI-1, and t-PA and the risk of venous thrombosis was studied.
Methods
Study design, study population, and data collection
For this study, patients and control subjects of the Multiple Environmental and Genetic Assessment of risk factors for venous thrombosis (MEGA) study were used. The design of the MEGA study has been described extensively.1 Between March 1999 and May 2002, consecutive unselected patients aged 18 to 70 years, with a first deep vein thrombosis of the leg or a first pulmonary embolism, were identified at 6 anticoagulation clinics in The Netherlands. Information on the diagnostic procedure was obtained from hospital records and general practitioners. A deep venous thrombosis was confirmed with Doppler ultrasonography. A pulmonary embolism was confirmed by a ventilation perfusion lung scan, spiral computed tomography, or angiogram. From January 2002 to September 2004, control subjects were selected from the same geographical area as the patients by random-digit dialing (RDD) with use of the Mitofsky-Waksberg method17 and were frequency matched for sex and age to the patients. This matching was performed on group level in which random control subjects were selected in numbers proportional to the number of patients within strata of sex and 5-year age groups. Patients or control subjects with severe psychiatric problems or those who could not speak Dutch were excluded. Participation rate was 83% among patients and 69% among control subjects. All participants signed an informed consent form in accordance with the Declaration of Helsinki. Approval for this study was obtained from the Medical Ethics Committee of the Leiden University Medical Center (Leiden, The Netherlands).
All participants were asked to complete a standardized questionnaire on acquired risk factors for venous thrombosis. Body mass index (BMI in kg/m2) was calculated from self-reported weight and height. All items in the questionnaire referred to the period before the index date, which was for the patients the date of diagnosis of thrombosis and for the controls the date they filled in the questionnaire. When participants were unable to fill in the questionnaire, questions were asked by telephone with the use of a standardized mini-questionnaire. Three months after discontinuation of the anticoagulant therapy, patients were invited to the anticoagulation clinic for a blood sample. Of patients who received prolonged anticoagulant therapy (> 1 year), blood was drawn under treatment with vitamin K antagonists. The control subjects were invited to the clinic for a blood draw after returning their questionnaire. All participants were interviewed regarding present anticoagulant use.
For the current study we analyzed all patients included in the MEGA study who were presented at the anticoagulation clinic between March 1, 2001, and May 31, 2002 (n = 770), and all RDD controls invited for participation from January 1, 2002, until December 31, 2003 (n = 743) who provided a blood sample, which can be considered random samples of all patients and RDD controls of whom blood samples were available.
As validation population to study determinants of CLT in, the control group of the Study of Myocardial Infarctions Leiden (SMILE) was used, which included 630 men between 18 and 70 years of age. Of these participants, measurements of all fibrinolytic variables were available. These men were without a history of myocardial infarction, without renal disease or severe (neuro)psychiatric problems, with a life expectancy of more than a year, and who had not taken any oral anticoagulants in the 6-month period before participation in the study.4
Blood collection and laboratory analysis
Blood samples were primarily drawn in the morning (median, 9:40 am; 95% before 11 am), without a systematic difference between patients and control subjects. Time between thrombosis and blood draw ranged from 95 to 877 days with a median of 299 days, and 74% of all patients provided a blood sample between 6 and 12 months after the thrombosis. Blood samples were drawn into vacuum tubes containing 0.106M trisodium citrate. Plasma was obtained by centrifugation at 2000g for 10 minutes at room temperature and stored in aliquots at −80°C. Lysis of a tissue factor–induced clot by exogenous tissue-type plasminogen activator (t-PA) was studied by monitoring changes in turbidity during clot formation and subsequent lysis as described previously.5 In short, 50 μL of plasma was pipetted in a 96-well microtiter plate. Subsequently, a 50-μL mixture containing phospholipid vesicles, t-PA (final concentration 56 ng/mL), tissue factor, and CaCl2 diluted in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) was added by the use of a multichannel pipette. In a kinetic microplate the optical density at 405 nm was monitored every 20 seconds, resulting in a clot-lysis turbidity profile. The CLT was derived from this clot-lysis profile and defined as the time from the midpoint of the clear to maximum turbid transition, representing clot formation, to the midpoint of the maximum turbid to clear transition, representing the lysis of the clot.
α2-Antiplasmin and plasminogen activity were measured by the use of chromogenic assays (STA Stachrom α2-antiplasmin and STA Stachrom plasminogen from Diagnostica Stago) and were performed on a STA-R coagulation analyzer with the use of a commercial calibration standard (Diagnostica Stago). PAI-1 antigen levels were measured with a Technozym PAI-1 enzyme-linked immunosorbent assay (ELISA) reagent kit (Kordia; Biopool). Plasma TAFI activity levels were determined with a chromogenic assay (Pefakit TAFI; Pentapharm LTD) by converting TAFI into its active form using a reagent containing thrombin-thrombomodulin and subsequently measuring the carboxypeptidase activity. Measurements were run on a BCS coagulation analyzer (Dade Behring Inc). Antigen levels of t-PA were assessed by ELISA by the use of a commercially available mouse anti-t-PA antibody (Nuclilab BV) as capture, and a biotin-labeled rabbit anti–human t-PA antibody (Nuclilab BV) as detecting antibody. Bound detecting antibody was visualized by the use of horseradish peroxidase–labeled streptavidin, followed by tetramethylbenzidine staining. A calibration curve was constructed with purified t-PA (Nuclilab BV). The interassay coefficients of variation were 6.6% for CLT, 1.6% for plasminogen, 4.6% for α2-antiplasmin, 5.8% for TAFI, 7.2% for PAI-1, and 8.1% for t-PA.
Measurements of antithrombin and protein C levels were performed with a chromogenic assay and prothrombin (factor II) activity, factor VII activity, factor VIII activity, factor X activity, and factor XI activity were measured with a mechanical clot detection method on a STA-R coagulation analyzer following the instructions of the manufacturer (Diagnostica Stago). Total protein S levels and levels of factor IX antigen were determined by ELISA (Diagnostica Stago). Fibrinogen activity was measured on the STA-R analyzer according to methods of Clauss.18 von Willebrand factor (VWF) antigen was measured with the immunoturbidimetric method by use of STA Liatest kit, following the instructions of the manufacturer (Diagnostica Stago). Total homocysteine, total cysteine, and methionine (as sum methionine and methioninesulfoxide) were measured by the use of liquid chromatography–mass spectrometry.
The aforementioned parameters were all expressed in percentages relative to pooled normal plasma, except t-PA and PAI-1 levels, which were expressed in nanograms per milliliter; fibrinogen levels, which were expressed in grams per liter; and the sulfur amino acids, which were expressed in micromoles per liter. All laboratory measurements were performed without knowledge of whether the sample was from a patient or a control subject.
Statistical analysis
To study determinants of CLT in the general population, simple and multiple linear regression with CLT as dependent variable were performed in the control group. Because the use of vitamin K antagonists influences CLT, these analyses were restricted to subjects not taking oral anticoagulants in whom all investigated parameters (plasminogen, α2-antiplasmin, TAFI, PAI-1, t-PA, prothrombin, factor VII, factor VIII, factor IX, factor X, factor XI, protein S, protein C, antithrombin, fibrinogen, VWF, and CLT) were measured (n = 733). Nine subjects had missing values for the sulfur-containing amino acids (homocysteine, cysteine, and methionine) and were excluded from the analyses, including these 3 factors. Because the distribution of CLT is skewed, CLT was 10 log-transformed. t-PA, PAI-1, factor VIII and factor XI, homocysteine, and fibrinogen also had skewed distributions and were entered after a 10 log-transformation for a better fit of the model. The R2 was used as a measure of explained variance.
To compare the relative strength of the various determinants within the model, all variables (CLT and other plasma factors) were standardized by calculating Z-scores. The Z-score for an observation of a subject is calculated by subtracting the mean from the observed value and dividing the residual by the SD. Simple or multiple linear regression analysis was performed with the standardized variables. The resulting standardized regression coefficient (β) for a factor indicates the increase in SDs of log-CLT, when that particular factor increases with 1 SD and all other variables in the model are unchanged. The fit of the resulting regression models was examined by plotting the observed CLT versus the predicted CTL and versus the independent variables in the model. If this plot showed a nonlinear relationship for any of the independent variables, higher order terms were added to the model.
We estimated the unexplained variance (σ2unknown) by subtracting an estimate of variance as the result of the measurement error of CLT (σ2measurement error) from the residual variance of the regression analysis. The variance caused by the measurement error of CLT was estimated from the inter assay variation. Ninety normal pooled plasma samples were measured on 96-well plates (n = 90), yielding a SD of the 10 log-transformed CLT measurement of 0.028.
To study the effect of fibrinolytic factors and CLT on risk of venous thrombosis, levels of plasminogen, α2-antiplasmin, TAFI, PAI-1, t-PA, and CLT were grouped into quartiles on the basis of the distribution among the control subjects. Odds ratios (ORs) with 95% confidence intervals (95% CIs) were calculated, taking the lowest quartile as the reference group for the OR. Unconditional logistic regression was performed to adjust for age, sex, and other potential confounders. In the logistic regression model BMI, levels of factor VIII, fibrinogen, VWF, and fibrinolytic factors when used as covariates were included as continuous variables. VWF, fibrinogen, t-PA, and PAI-1 were included in the model after 10 log-transformation. Entering these factors as categorical variables did not change the results. Participants on oral anticoagulants (7 control subjects and 92 patients) were excluded in the analyses concerning CLT. SPSS 16.0 (SPSS) was used for statistical analyses.
Results
Determinants of CLT
To examine determinants of CLT in the general population, 733 control subjects of the MEGA study were studied, including 374 men and 359 women with a mean age of 46 years (range, 18-70 years). Mean CLT was 65.3 minutes (median, 61.7 minutes; range, 35.0-204.7 minutes).
Linear regression was performed to investigate the association between factors of the coagulation and fibrinolytic system and CLT by use of the log-transformed CLT as dependent variable. In Table 1 the standardized regression coefficients (β values) of the analyses, including plasminogen, α2-antiplasmin, TAFI, PAI-1, and t-PA, are shown. In simple linear regression analyses, all fibrinolytic factors except plasminogen were associated with CLT. The strongest association was found between PAI-1 and CLT, with a regression coefficient of 0.63 (95% CI 0.58-0.69), indicating that with every SD increase in PAI-1, CLT increases with 0.63 SD (PAI-1 and CLT log-transformed). This model had an R2 of 0.632 = 0.40, denoting that with PAI-1 as independent factor, 40% of the variation in CLT was explained. Including all fibrinolytic factors (plasminogen, α2-antiplasmin, TAFI, PAI-1, and t-PA) in a multiple regression model increased the explained variance to 53% (Table 1).
Next, we used a separate model without fibrinolytic variables but that included all coagulation factors and sulfur-containing amino acids (prothrombin; factor VII, VIII, IX, X, and XI; protein C; protein S; antithrombin; fibrinogen; VWF; cysteine; methionine; and homocysteine), which resulted in an R2 of 0.29 (regression coefficients not shown). When we combined all coagulation and fibrinolytic factors in one overall multiple model, it yielded a R2 of 0.58 (regression coefficients not shown). Table 2 (model A) shows a reduced model from a backward selection procedure with a similar R2 (0.58), which included the variables that remained significant at α = 0.10. PAI-1 remained the strongest determinant of CLT (β = 0.49; 95% CI 0.43-0.54).
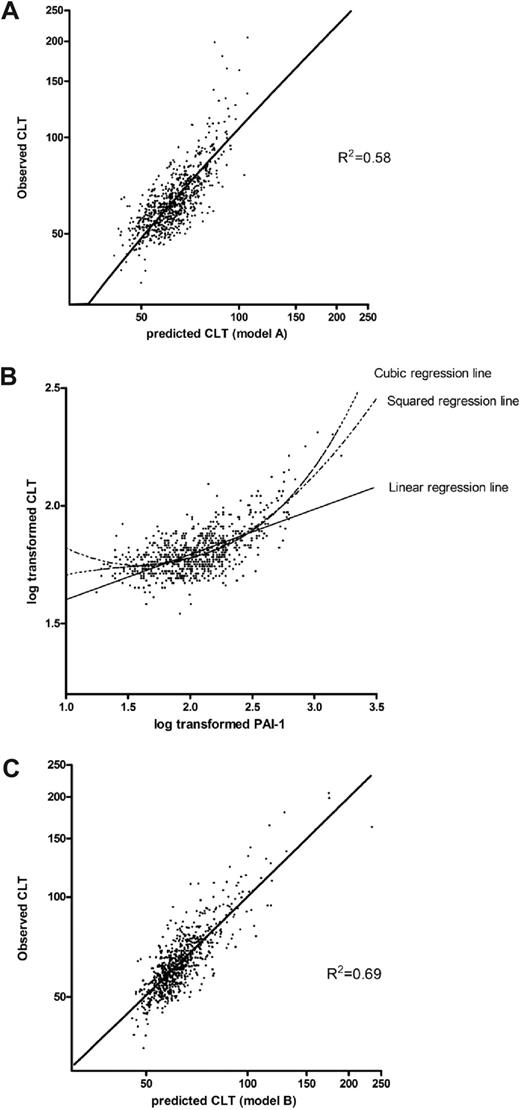
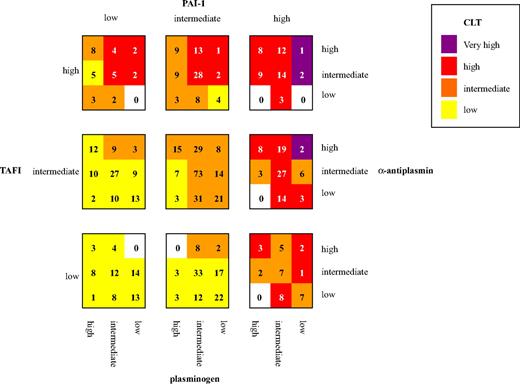
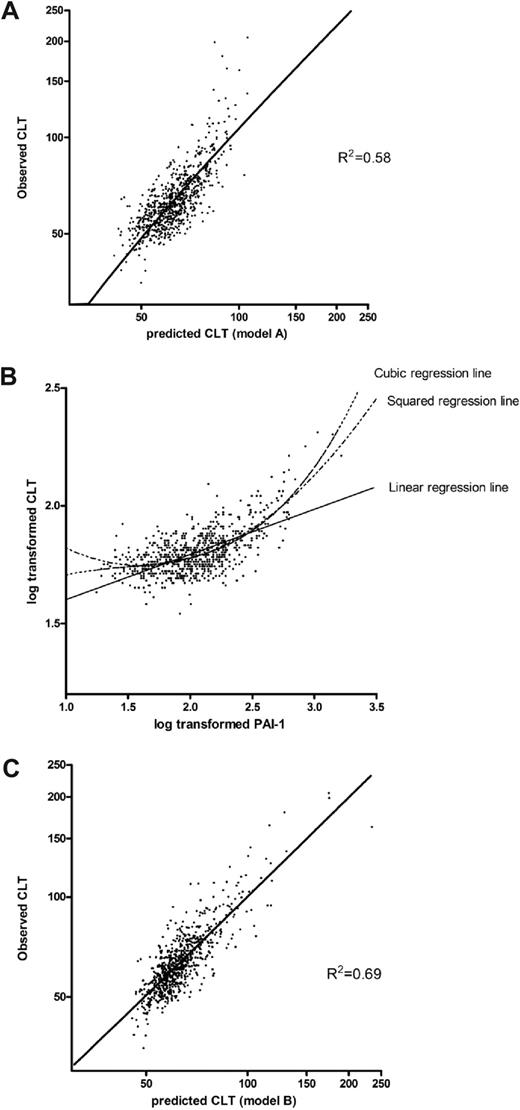
To obtain an estimation of the accuracy of the model, the predicted log-CLT Z-score was calculated for each study participant by the use of model A of Table 2 and back transformed to the original CLT scale. Figure 1A shows the observed CLT plotted against the predicted CLT. The fit of the model seems adequate for shorter CLT, but the longest CLTs are underestimated with this linear model. The reason for this is the nonlinear or cubic association between PAI-1 and CLT as shown in Figure 1B. Therefore, to better fit the model, a squared and a cubic term for PAI-1 were added to the overall model, including all coagulation and fibrinolytic factors. Table 2, model B, shows the reduced model from a backward selection procedure. CLT predicted with regression coefficients from a multiple regression model including the variables of model B plotted against the observed CLT is shown in Figure 1C.
Scatterplots of the association between the observed CLT with the predicted CLT, using 2 different models, and with PAI-1. (A) Observed CLT plotted against the predicted CLT with the use of regression coefficients derived from a linear regression model, including levels of prothrombin, factor VII, factor IX, factor X, protein C, antithrombin, fibrinogen, plasminogen, α2-antiplasmin, TAFI, and PAI-1 (Table 2; model A). (B) Association between PAI-1 and CLT. (C) Observed CLT plotted against predicted CLT with the use of regression coefficients derived from a linear regression including levels of prothrombin, factor VII, factor X, factor XI, fibrinogen, plasminogen, α2-antiplasmin, TAFI, PAI-1, and a squared and cubic term for PAI-1 (Table 2; model B).
Scatterplots of the association between the observed CLT with the predicted CLT, using 2 different models, and with PAI-1. (A) Observed CLT plotted against the predicted CLT with the use of regression coefficients derived from a linear regression model, including levels of prothrombin, factor VII, factor IX, factor X, protein C, antithrombin, fibrinogen, plasminogen, α2-antiplasmin, TAFI, and PAI-1 (Table 2; model A). (B) Association between PAI-1 and CLT. (C) Observed CLT plotted against predicted CLT with the use of regression coefficients derived from a linear regression including levels of prothrombin, factor VII, factor X, factor XI, fibrinogen, plasminogen, α2-antiplasmin, TAFI, PAI-1, and a squared and cubic term for PAI-1 (Table 2; model B).
The R2 of this model increased to 0.69, meaning that the residual variation in log-CLT was 31%. The estimated residual variance was equal to 0.0028. The estimated variance caused by measurement error was 0.0282 = 0.00077, because our interassay SD was equal to 0.028. This is equal to 27% of the residual variance. Consequently, 8% (27% of 31%) of the total variation in CLT is caused by measurement error and 23% (73% of 31%) of the variation in CLT remains unexplained. Accordingly, 77% of the variation in CLT could be explained.
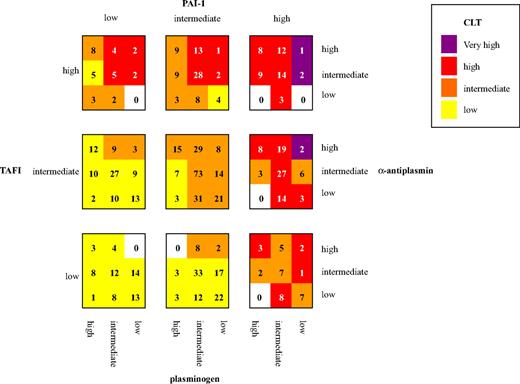
Figure 2 illustrates the effect of the combination of levels of fibrinolytic factors (plasminogen, α2-antiplasmin, TAFI, and PAI-1) on CLT in control subjects. Each fibrinolytic factor was divided in 3 groups (low, intermediate, and high level, by use of the mean ± two-thirds × SD as arbitrary cutoff points), resulting in 81 categories. Mean CLT for each category was calculated. The mean CLTs were classified as low (less than one-third × SD), intermediate (less than one-third × SD to one-third × SD), high (one-third × SD to 2 × SD) and very high (> 2 × SD). Groups of similar mean CLT are indicated with the same color, with the more intense colors representing longer mean CLT. Thus, confirming data from the analyses in Tables 1 and 2, the figure shows a clear increase in CLT with increasing plasma levels of PAI-1, TAFI, and α2-antiplasmin and decreasing levels of plasminogen. Remarkable is the control subject in the upper right corner of the figure with low levels of plasminogen and high levels of α2-antiplasmin, TAFI, and PAI-1 with a CLT 3.5 × SD above mean CLT.
CLT for different combinations of levels of fibrinolytic factors. Numbers in the squares indicate the number of control subjects included in each category. Cutoffs for fibrinolytic factors (μ = population mean; SD = standard deviation): low: less than μ minus two-thirds SD; intermediate: μ minus two-thirds SD to μ plus two-thirds SD; high: more than μ plus two-thirds SD. Cutoff for CLT low: less than μ minus one-third SD; intermediate: μ minus one-third SD to μ plus one-third SD; high: μ plus one-third SD to μ plus 2SD; very high: more than μ plus 2SD. Cutoffs for CLT and PAI-1 were determined by 10 log-transformed values.
CLT for different combinations of levels of fibrinolytic factors. Numbers in the squares indicate the number of control subjects included in each category. Cutoffs for fibrinolytic factors (μ = population mean; SD = standard deviation): low: less than μ minus two-thirds SD; intermediate: μ minus two-thirds SD to μ plus two-thirds SD; high: more than μ plus two-thirds SD. Cutoff for CLT low: less than μ minus one-third SD; intermediate: μ minus one-third SD to μ plus one-third SD; high: μ plus one-third SD to μ plus 2SD; very high: more than μ plus 2SD. Cutoffs for CLT and PAI-1 were determined by 10 log-transformed values.
To validate the model, we performed an additional regression analysis in the control group of SMILE (n = 630) by using the variables of the model of Table 1. Prothrombin levels were not measured in SMILE, and we were thus not able to use the model as presented in Table 2. Regression coefficients in this analysis were similar to those found in MEGA: −0.01 for plasminogen, 0.23 for α2-antiplasmin, 0.17 for TAFI, and 0.53 for PAI-1. The R2 of this model was 0.39.
Fibrinolytic proteins and risk of venous thrombosis
Next, the association between plasma levels of the individual fibrinolytic components and risk of venous thrombosis was investigated. In these analyses, 770 patients and 743 control subjects were included. Mean age at time of blood draw of patients was 49 years (range, 19-71 years) and mean age of control participants was 46 years (range, 18-70 years). In the control group, 379 participants (51%) were men, and in the patient group, 347 participants (45%) were men. Of all patients 215 (28%) were diagnosed with an isolated pulmonary embolism, 475 (62%) with an isolated deep vein thrombosis of the leg, and 80 (10%) with both a pulmonary embolism and a deep vein thrombosis of the leg.
Increased levels of each of the individual fibrinolytic proteins were associated with an increased risk of venous thrombosis after adjustment for age and sex (Table 3). Because levels of fibrinolytic factors increase with increasing BMI,19,20 risks were further adjusted for BMI. After adjusting for BMI, the ORs were 1.6 (95% CI 1.2-2.2) for plasminogen, 1.6 (95% CI 1.2-2.1) for TAFI, 1.6 (95% CI 1.1-2.1) for PAI-1, and 1.8 (95% CI 1.3-2.6) for t-PA for the highest quartile compared with the lowest. α2-Antiplasmin was no longer associated with risk of venous thrombosis (OR 1.2; 95% CI 0.9-1.7).
Previously, it has been shown that plasminogen, PAI-1, and t-PA are markers of inflammation.20,21 Indeed the risks of thrombosis somewhat decreased after adjustment for markers of inflammation. The risk of venous thrombosis, adjusted for age, sex, BMI, and levels of the acute-phase proteins fibrinogen and factor VIII, decreased to 1.3 (95% CI 0.9-1.8) for the highest quartile of plasminogen. After adjusting for the same factors the risk for the highest quartile of PAI-1 was still 1.7-fold increased (95% CI 1.2-2.3) and for the highest levels of t-PA, the adjusted OR decreased to 1.5 (95% CI 1.0-2.1), all compared with the first quartile.
t-PA also can be seen as a marker of endothelial activation,22 so we further adjusted for plasma levels of VWF. The risk of venous thrombosis for subjects with the highest levels of t-PA reduced to 1.3 (95% CI 0.9-1.9) after adjusting for age, sex, BMI, fibrinogen, factor VIII, and VWF. Similar adjustments in the analyses of the other fibrinolytic factors did not considerably change the results. Adjusting for age, sex, fibrinogen, factor VIII, and VWF resulted in ORs for the 4th quartile of 1.4 (95% CI 0.9-2.0) for plasminogen, 1.0 (95% CI 0.7-1.5) for α2-antiplasmin, 1.4 (95% CI 1.0-2.0) for TAFI, and 1.6 (95% CI 1.1-2.2) for PAI-1. Finally, we adjusted the risks for each fibrinolytic variable additionally for the other fibrinolytic factors. The risk for the 4th quartile of plasminogen adjusted for age, sex, BMI, factor VIII, fibrinogen, VWF, and for plasma levels of α2-antiplasmin, PAI-1, t-PA, and TAFI was 1.3 (95% CI 0.9-2.0). Similar analysis for the other fibrinolytic factors gave an OR of 1.0 (95% CI 0.7-1.4) for α2-antiplasmin, 1.4 (95% CI 1.0-2.0) for TAFI, 1.5 (95% CI 1.0-2.1) for PAI-1, and 1.1 (95% CI 0.7-1.6) for t-PA. Hence PAI-1 and TAFI were still associated with venous thrombosis in these models.
To investigate whether CLT increases the risk of venous thrombosis also independently of the fibrinolytic factors, risk of venous thrombosis for quartiles of CLT was estimated after adjustment for age and sex and fibrinolytic factors. Age- and sex-adjusted ORs were 1.5 (95% CI 1.1-2.2) for subjects in the 2nd quartile of CLT, 2.7 (95% CI 1.9-3.7) for the 3rd and 3.4 (95% CI 2.5-4.9) for the 4th quartile, all compared with the lowest. After further adjustment for plasma levels of PAI-1 the risk of venous thrombosis remained 1.5-fold (95% CI 1.1-2.2), 2.5-fold (95% CI 1.8-3.6), and 3.0-fold (95% CI 2.1-4.5) increased for the 2nd, 3rd, and 4th quartile, respectively. Entering squared and cubic terms for PAI-1 into the model did not change these results (data not shown). Further adjustment for levels of plasminogen, α2-antiplasmin, TAFI, t-PA, and prothrombin produced ORs of 1.4 (95% CI 1.0-2.0) for the 2nd, 2.6 (95% CI 1.8-3.8) for the 3rd and 3.2 (95% CI 2.0-5.2) for the 4th quartile of CLT.
Discussion
Increased CLT as measured with an overall plasma-based assay is associated with an increased risk of venous and arterial thrombosis.1-4,23 However, the factors influencing CLT in the general population were unknown, which prompted us to look for determinants of CLT in a large group of healthy control subjects derived from the MEGA study. In this study we could explain 77% of the variation in CLT. Plasma levels of PAI-1 explained the majority of the variance in CLT, followed by TAFI levels, which is in line with the observation from the present study that elevated levels of PAI-1 and TAFI are independent risk factors for venous thrombosis. Plasma levels of α2-antiplasmin and plasminogen were associated with CLT to similar extent as TAFI. α2-Antiplasmin was not associated with venous thrombosis. Surprisingly, plasminogen was positively associated with venous thrombosis. Levels of t-PA did not determine CLT but were associated with risk of venous thrombosis. Plasminogen and t-PA, however, may be just markers of other risk factors such as inflammation and endothelial activation. Besides fibrinolytic factors, prothrombin is an important determinant of CLT. Fibrinogen, factor VII, X, and XI contributed to the variation in CLT to a lesser extent.
Determinants of CLT
Our results are in agreement with previous in vitro experiments in which CLT increased with increasing levels of α2-antiplasmin, TAFI, and PAI-1, and with decreasing levels of plasminogen.5 Moreover, we could replicate the associations found in the MEGA study to a large extent in the control group of the SMILE, an independent study population. In our CLT assay t-PA is added to initiate fibrinolysis, mimicking the in vivo release of endothelial t-PA after stimulation.22 Consequently, plasma levels of t-PA do not influence the CLT. Elevated prothrombin levels increase CLT, probably via increased TAFI activation, which is in line with other researchers who showed that subjects with the prothrombin 20210A mutation had longer CLTs than those without the mutation, and with researchers who demonstrated that the lysis time of prothrombin-enriched plasma clots was prolonged proportionally to the amount of added prothrombin in a TAFI-dependent manner.1,6 Finally, levels of fibrinogen or the sulfur-containing amino acids that also have been found to be associated with stiffer or denser clots that are more resistant to fibrinolysis24 were minimally or not associated with CLT.
With a statistical model including all factors associated with CLT, we could explain 77% of the variation in CLT. The 23% unexplained variation may be caused by factors not measured in our study but known to influence fibrinolysis or by yet-unknown factors. Possible candidates are coagulation factor XIII and tissue factor pathway inhibitor, which were, although not strongly, previously shown to be associated with CLT when an age-adjusted model in the control group of the LETS (LEiden Thrombophilia Study) was used.2 Other proteins known to influence fibrinolysis are, for instance, lipoprotein(a),25 which competes with plasminogen for binding to fibrinogen; vitronectin,26 which binds to PAI-1 and stabilizes it; histidine-rich glycoprotein, which binds to plasminogen and modulates plasminogen bioavailability for plasmin generation27 ; and the plasmin inhibitors α2-macroglobulin, α1-antitrypsin, and C1 inhibitor.28
We are currently examining possible genetic factors influencing CLT through quantitative trait loci analysis in an extended thrombophilic pedigree.23 Part of the unexplained variation may be the result of lack of fit of the model as the linear regression model assumes a linear relation between the proteins and CLT, which may not always be completely accurate. In addition, only plasma antigen or activity levels of the fibrinolytic factors are included. Consequently, the functionality or stability of the factors and the interplay between the factors in the coagulation and fibrinolytic cascade are not taken into account. Plasma levels of plasminogen and α2-antiplasmin are presumably not the limiting factors in fibrinolysis because they circulate at high concentrations in healthy subjects. The amount of plasmin formed and subsequent lysis of the clot may therefore depend more on regulating steps before final activation of plasminogen than on the total amount of plasminogen or α2-antiplasmin present in plasma.29
Although CLT could largely be explained by the levels of the fibrinolytic factors and prothrombin, the association between CLT and venous thrombosis remained after adjusting for the fibrinolytic factors measured. This finding suggests that indeed CLT is not fully explained by these factors and that additional proteins are involved. Alternatively, a complex interplay between fibrinolytic factors and prothrombin, not fully accounted for by the statistical models is responsible for the association between elevated CLT and venous thrombosis.
Fibrinolytic proteins and risk of venous thrombosis
To our knowledge, this is the first large study on levels of plasminogen and α2-antiplasmin and risk of venous thrombosis. Although plasminogen levels were negatively associated with CLT, unexpectedly plasminogen was positively associated with risk of venous thrombosis. Plasma levels of plasminogen are strongly linked to plasma levels of other coagulation and fibrinolytic factors (see supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), which may mask a protective effect of elevated plasminogen levels on venous thrombosis. The role of plasminogen in thrombosis risk, however, has always been unclear. Although plasmin is thought to be the key enzyme responsible for fibrin degradation, plasminogen-deficient subjects do not appear to experience thrombotic events but suffer primarily from ligneous conjunctivitis, a rare form of chronic conjunctivitis characterized by the development of firm fibrin-rich lesions mainly on the tarsal conjunctivae (reviewed by Brandt30 ). In contrast, the authors of population-based studies have found increased plasminogen levels to be associated with an increased risk of arterial thrombosis.19,20 One explanation for this association is that plasminogen levels are increased by inflammatory processes.21 We have previously demonstrated that the positive association between plasma levels of plasminogen and myocardial infarction disappears after adjustment for markers of inflammation.31 In the current study, adjustment for the acute-phase proteins fibrinogen and factor VIII attenuated the association between plasminogen and venous thrombosis, indeed suggesting that plasminogen is a marker of inflammation. However, plasminogen could play a role in venous thrombosis through alternative pathways. Plasmin has other substrates besides fibrin, such as protease-activated receptor-1, the extracellular matrix, tissue factor pathway inhibitor, and factor V, and plasmin could induce endothelial damage, all potentially important in thrombosis risk.32-36
Although α2-antiplasmin was positively associated with CLT, no association was found between α2-antiplasmin and risk of venous thrombosis after adjusting for BMI. This finding is in agreement with 2 small studies in which levels of α2-antiplasmin were not associated with postoperative thrombosis.9,10 As stated previously, α2-antiplasmin normally circulates at high levels and may therefore not be a limiting factor.
TAFI defines the molecular connection between the coagulation and fibrinolytic cascades.37,38 We find TAFI levels to be associated with an increased risk of venous thrombosis, which is in accordance with previous studies investigating the association between TAFI levels and first thrombosis, either in a general population11 or in factor V Leiden carriers,12 and with a study on TAFI and recurrent venous thrombosis.13 In these studies, TAFI levels greater than the 90th percentile of the control group were associated with a 2- to 4-fold increased risk of venous thrombosis.
The role of t-PA and PAI-1 in venous thrombosis is controversial. Although the authors of several studies have found a positive relation with venous thrombosis, others have not. In an extensive review, Prins and Hirsh16 concluded that PAI-1 and t-PA may be important in venous thrombosis, especially in patients undergoing surgery. In present study, elevated levels of PAI-1 and t-PA were associated with venous thrombosis. Adjustment for acute-phase proteins and VWF attenuated the increased risk found in subjects with high t-PA levels. Because most of the t-PA antigen in plasma is bound to PAI-1 and therefore inactive, it is plausible that t-PA is just a marker of underlying processes, such as inflammation and endothelial activation. This observation is in agreement with 2 studies investigating the association between t-PA and arterial thrombosis.20,31 Increased PAI-1 levels were still associated with venous thrombosis, even after extensive adjustment, which suggests that hypofibrinolysis caused by elevated PAI-1 levels indeed increases thrombosis risk.
It should be noted that the adjustments made for plasma proteins in all analyses presented must be considered with caution. Adjustments are justified for factors that are risk factors for venous thrombosis and that influence the fibrinolytic factor of interest but are not influenced by the fibrinolytic factor themselves.39 Because coagulation factors and fibrinolytic factors tend to cluster and correlate and possibly share genetic regulation, causal inference and justification of the adjustments is difficult.40-42
In conclusion, variation in CLT could be explained for 77%. PAI-1 was the principal determinant of CLT, followed by plasminogen, TAFI, prothrombin, and α2-antiplasmin. Increased plasma levels of plasminogen, TAFI, PAI-1, and t-PA were associated with an increased risk of venous thrombosis, although plasminogen and t-PA may be markers of other risk factors for venous thrombosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the directors of the Anticoagulation Clinics of Amersfoort (Mark H. H. Kramer, MD), Amsterdam (Mary Remkes, MD), Leiden (Felix J. M. van der Meer, MD), The Hague (Eric van Meegen, MD), Rotterdam (A. A. Harry Kasbergen, MD), and Utrecht (Hanneke de Vries-Goldschmeding, MD) who made the recruitment of patients possible, and the interviewers (José C. M. van den Berg, Birgit Berbee, Saskia van der Leden, Mieke Roosen, and Liesbeth C. Willems of Brilman) who performed the blood draws. We also thank Ingeborg de Jonge, MSc, Rebecca Roelofsen, MSc, Marion Streevelaar, Lucie M. J. Timmers, MSc, and Ank J. Schreijer for their administrative support and data management. The fellows Irene D. Bezemer, PhD, Jeanet W. Blom, MD, Astrid van Hylckama Vlieg, PhD, Elisabeth R. Pomp, PhD, Lidwine W. Tick, MD, and Karlijn J. van Stralen, PhD took part in every step of the data collection. Marian A. Weijne, Lucy M. Leverink, Annelies J. Hoenderdos, Wil F. Kopatz, Sultana Moschatsis, Jelle Adelmeijer, Carla J. M. van Dijk, Rob van Eck, Jeroen van der Meijden, Petra J. Noordijk, and Thea Visser performed the laboratory measurements. We express our gratitude to all participants of the MEGA study.
This research was supported by the European Hematology Association (2005/04), the Netherlands Heart Foundation (NHS 98.113 and 2005B060), the Dutch Cancer Foundation (RUL 99/1992), and the Netherlands Organization for Scientific Research (912-03-033 2003). The funding organizations did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript.
Authorship
Contribution: M.E.M. designed the present study, analyzed and interpreted the data, and drafted the manuscript; T.L. designed the present study, interpreted the data, critically reviewed the analyses, and participated in writing the manuscript. P.G.d.G., J.C.M.M., and S.l.C. interpreted the data and critically reviewed the analyses and the manuscript; C.J.M.D. designed the overall study, performed the data collection, interpreted the data, and critically reviewed the analyses and the manuscript; and F.R.R. designed the overall study, interpreted the data, and critically reviewed the analyses and the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Frits R. Rosendaal, Department of Clinical Epidemiology, C7-P, Leiden University Medical Center, P.O. Box 9600, 2300 RC Leiden, the Netherlands; e-mail: f.r.rosendaal@lumc.nl.