Abstract
We evaluated human CD8+ T-cell responses generated by targeting antigens to dendritic cells (DCs) through various lectin receptors. We found the immunoreceptor tyrosine-based inhibitory motif-containing DC immunoreceptor (DCIR) to mediate potent cross-presentation. A single exposure to a low dose of anti-DCIR–antigen conjugate initiated antigen-specific CD8+ T-cell immunity by all human DC subsets including ex vivo–generated DCs, skin-isolated Langerhans cells, and blood myeloid DCs and plasmacytoid DCs. The delivery of influenza matrix protein (FluMP) through DCIR resulted in expansion of FluMP-specific memory CD8+ T cells. Enhanced specific CD8+ T-cell responses were observed when an antigen was delivered to the DCs via DCIR, compared with those induced by a free antigen, or antigen conjugated to a control monoclonal antibody or delivered via DC-SIGN, another lectin receptor. DCIR targeting also induced primary CD8+ T-cell responses against self (MART-1) and viral (HIV gag) antigens. Addition of Toll-like receptor (TLR) 7/8 agonist enhanced DCIR-mediated cross-presentation as well as cross-priming, particularly when combined with a CD40 signal. TLR7/8 activation was associated with increased expansion of the primed CD8+ T cells, high production of interferon-γ and tumor necrosis factor-α, and reduced levels of type 2–associated cytokines. Thus, antigen targeting via the human DCIR receptor allows activation of specific CD8+ T-cell immunity.
Introduction
Dendritic cells (DCs) play a key role in initiating and controlling the magnitude and the quality of adaptive immune responses.1,2 DCs decode and integrate signals received from their environment and ferry this information to cells of the adaptive immune system. The existence of subsets, which possess specialized as well as shared phenotype and functions, brings out another level of complexity to the DC system of antigen-presenting cells (APCs).3-5 Microbes can directly activate DCs through a variety of pattern recognition receptors, such as Toll-like receptors (TLRs),6 cell surface C-type lectin receptors (CLRs),7 and intracytoplasmic NOD-like receptors.8,9 In humans, certain CLRs distinguish DC subsets, with plasmacytoid DCs (pDCs) expressing BDCA2,10 Langerhans cells (LCs) expressing Langerin,11 and interstitial DCs expressing DC-SIGN.12 Other C-type lectins are expressed on other cell types, including endothelial cells and neutrophils. CLRs, such as DC–specific intracellular adhesion molecule-3-grabbing non-integrin (SIGN),7 can act as anchors for a large number of microbes and allow their internalization. Furthermore, CLRs also act as adhesion molecules between DCs and other cell types, including endothelial cells, T cells, and neutrophils.12,13 DEC-205/CD205, a lectin of unknown function, has been extensively studied in the mouse for its ability to endocytose ligands. Targeting antigens to mouse DCs through DEC-205 in the absence of DC activation results in tolerance induction.14,15 In contrast, targeting antigens in the presence of DC activation (CD40 and TLR3 agonists) results in the generation of immunity against a variety of antigens.14,16 Most studies demonstrating induction of CD4+ T-cell responses or primary CD8+ T-cell response against antigens delivered via DEC-205 have been limited to the transgenic mouse OT-I/II system.
Antigens have been targeted to mouse DCs through other surface molecules, including LOX-1 (a type II C-type lectin receptor that binds to HSP7017 ), mannose receptor,18 Dectin-1,19 Dectin-2,20 CD40,21 Langerin,22 Gb3 (a receptor for Shiga toxin23 ), DEC-205,24 and CLEC9A, which was recently reported to prime naive CD8+ T cells in mice.25-27 The targeting of antigens through receptors expressed on different murine DC subsets results in different functional outcomes.28,29 Targeting antigens to human DCs using conjugates of anti–DC-SIGN with keyhole limpet hemocyanin (KLH),30 anti–DEC-205 with HIV gag,31 and anti-mannose receptor with human chorionic gonadotropin hormone32 has been shown to be presented/cross-presented to blood CD4+ and CD8+ T cells, respectively, or to T-cell clones.
We have turned our attention to the lectin DCIR,33 which intriguingly is widely expressed on different types DCs, including DCs from blood. Indeed, DCIR was initially described as expressed on blood monocytes, B cells, neutrophils, granulocytes, and dermal DCs, but not LCs, and was also recently found to be expressed on pDCs.34 Functionally, it can serve as a receptor for HIV.35 The human genome encodes only a single DCIR gene, whereas the mouse genome presents 4 DCIR-like genes: DCIR2, DCIR3, DCIR4, and DCAR1. DCIR and DCAR share substantial sequence homology in their extracellular domains, However, DCAR associates with the immunoreceptor family tyrosine-based activation motif (ITAM)–bearing FcRγ chain, whereas DCIR contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) that recruits the SHP-1 and SHP-2 phosphatases.36 A human homolog of the mouse DCAR has not been identified thus far.
Here we report that anti-DCIR conjugate monoclonal antibody (mAb) delivers antigens to a wide range of DC subsets, allowing cross-presentation and cross-priming of human CD8+ T cells.
Methods
DC subsets
CD34+-derived DCs were generated in vitro from CD34+ hematopoietic progenitor cells (HPCs) isolated from the blood of healthy volunteers given granulocyte colony-stimulating factor to mobilize precursor cells. HPCs were cultured at 0.5 × 106 cells/mL in Yssel medium (Irvine Scientific) supplemented with 5% autologous serum, 50μM β-mercaptoethanol, 1% l-glutamine, 1% penicillin/streptomycin, granulocyte-macrophage colony-stimulating factor (GM-CSF, 50 ng/mL; Berlex), Fms-like tyrosine kinase 3 ligand (100 ng/mL; R&D Systems), and tumor necrosis factor-α (TNF-α, 10 ng/mL; R&D Systems) for 9 days. Media and cytokines were refreshed at day 5 of culture. Subsets of DCs, CD1a+CD14− LCs, and CD1a−CD14+ DCs were then sorted, yielding a purity of 95% to 99%. Monocyte-derived DCs were generated by culturing monocytes in RPMI supplemented with 10% fetal bovine serum with GM-CSF (100 ng/mL; Berlex) and interleukin-4 (IL-4, 25 ng/mL; R&D Systems) for 5 days, or with GM-CSF (100 ng/mL; Berlex) and interferon-α2b (IFN-α2b; 500 U/mL; Intron A; Schering-Plough) for 3 days. Myeloid DCs (mDCs) and pDCs were sorted from fresh peripheral blood mononuclear cells as Lin−HLA-DR+CD11c+CD123− and Lin−HLA-DR+CD11c−CD123+, respectively.
Epidermal LCs, dermal CD1a+ DCs, and dermal CD14+ DCs were purified from normal human skin specimens. Specimens were incubated in bacterial protease dispase type 2 (Roche Diagnostics) for 18 hours at 4°C, and then for 2 hours at 37°C. Epidermal and dermal sheets were then separated, cut into small pieces (∼ 1-10 mm), and placed in RPMI 1640 supplemented with 10% fetal bovine serum. After 2 days, the cells that migrated into the medium were collected and further enriched using a Ficoll-diatrizoate in a density of 1.077 g/dL. DCs were purified by cell sorting after staining with anti-CD1a fluorescein isothiocyanate (Dako) and anti-CD14 APC mAbs (Invitrogen). All protocols were reviewed and approved by the Baylor Research Institute Institutional Review Board.
Expansion of antigen-specific T cells in DC/T-cell coculture
To assess the function of DCs in presenting FluMP- or MART-1–derived antigens, we used DCs from HLA-A201+ donors. Cells were cultured with conjugate mAbs at the indicated concentration. Syngeneic purified CD8+ T cells were cultured with the antigen-pulsed DCs at a DC/T ratio 1:20. CD40L (100 ng/mL; R&D Systems) was added to the culture after 24 hours to enhance cross-presentation by DCs.37 The cocultures were incubated at 37°C for 8 to 10 days. IL-2 was added at 10 U/mL at day 3. Where indicated, DCs were activated with TLR agonists: lipopolysaccharide (LPS; 10, 50, or 200 ng/mL), polyriboinosinic acid/polyribocytidylic acid (poly I:C; 5, 10, or 25 μg/mL), or thiazoloquinoline compound CL075 (0.2, 1, or 2 μg/mL; all from Invivogen). The expansion of FluMP-, MART-1–, and HIV gag p24-specific CD8+ T cells was evaluated using HLA-A201–FluMP(58-66) peptide (GILGFVFTL), HLA-A201–MART-1(26-35) peptide (ELAGIGILTV), and HLA-A201–p24(151-159) peptide (TLNAWVKVV) tetramers, respectively (Beckman Coulter). For the assessment of cross-priming to multiple CD8+ T cell–specific epitopes, CD34+-derived DCs were incubated with anti–DCIR-p24 or IgG4-p24 fusion mAbs and cultured with carboxyfluorescein succinimidyl ester (CFSE)–labeled CD8+ T cells at a DC/T ratio of 1:30. Antigen-pulsed DCs were activated with CD40L (100 ng/mL). After 2 consecutive stimulations, the CFSElow proliferating cells were sorted and restimulated for 24 hours with fresh DCs loaded with HIV gag p24 protein (2 μg/mL). The secreted IFN-γ was measured in the culture supernatants by Luminex. Alternatively, mDCs or IFN-α DCs were targeted with anti–DCIR-MART-1 or IgG4-MART-1 fusion proteins, activated as indicated, and cultured with naive CD8+ T cells for 10 days. Production of intracellular IFN-γ, as well as mobilization of CD107a (BD Biosciences) where indicated, was measured after 5 hours of restimulation with fresh autologous DCs that were loaded with 15-amino acid overlapping peptides derived from the MART-1 protein (2.5μM), or with the corresponding fusion protein in the presence of the protein transport inhibitor monensin (GolgiStop; BD Biosciences) and 0.25 μg/mL anti-CD28/anti-CD49d. Secretion of IFN-γ, TNF-α, IL-12p40, IL-4, IL-5, and IL-13 was measured in the supernatant after 40 hours by Luminex.
Results
DCIR is expressed by monocytes, B cells, and all DC subsets
Two monoclonal anti-DCIR clones were used throughout the studies: 9E8 and 24A5. These proved to be of high affinity (∼ 850pM and ∼ 560pM, respectively) as assessed by surface plasmon resonance analysis. They showed comparable staining of peripheral blood mononuclear cells (Figure 1A) and yielded comparable functional results throughout our study.
Cellular distribution of DCIR. (A) Flow cytometry analysis of DCIR expression on peripheral blood mononuclear cells. Circulating mononuclear cells were stained with 10 μg/mL anti-DCIR mAb followed by phycoerythrin-conjugated goat anti–mouse IgG. Cells were incubated with anti-CD19, anti-CD4, anti-CD8 (for lymphocytes), anti-CD16, anti-CD56 (for NK cells) not shown, anti-CD14 mAb (for monocytes), or with anti-CD11c, anti–HLA-DR, and anti-CD123 mAb (for pDCs or mDCs) and analyzed by flow cytometry. Data are representative of 3 independent experiments performed on 3 different donors. (B) Expression analysis of DCIR by flow cytometry on skin-derived DC subsets: epidermal LCs, dermal CD1a+ DCs, and dermal CD14+ DCs. (C) Human epidermal sheets, stained with anti-DCIR and analyzed by fluorescence microscopy, revealed the expression of DCIR on HLA-DR+ LCs. Image was captured using an Olympus BX51 microscope with Planapo 40×/0.95 dry objective, Photometrics Coolsnap HQ camera, and Metamorph software Version 6.2r6. Channel separation was done in Adobe Photoshop CS. (D) Expression analysis of DCIR by flow cytometry on CD34+-derived DC subsets CD1a+ LCs and CD14+ DCs.
Cellular distribution of DCIR. (A) Flow cytometry analysis of DCIR expression on peripheral blood mononuclear cells. Circulating mononuclear cells were stained with 10 μg/mL anti-DCIR mAb followed by phycoerythrin-conjugated goat anti–mouse IgG. Cells were incubated with anti-CD19, anti-CD4, anti-CD8 (for lymphocytes), anti-CD16, anti-CD56 (for NK cells) not shown, anti-CD14 mAb (for monocytes), or with anti-CD11c, anti–HLA-DR, and anti-CD123 mAb (for pDCs or mDCs) and analyzed by flow cytometry. Data are representative of 3 independent experiments performed on 3 different donors. (B) Expression analysis of DCIR by flow cytometry on skin-derived DC subsets: epidermal LCs, dermal CD1a+ DCs, and dermal CD14+ DCs. (C) Human epidermal sheets, stained with anti-DCIR and analyzed by fluorescence microscopy, revealed the expression of DCIR on HLA-DR+ LCs. Image was captured using an Olympus BX51 microscope with Planapo 40×/0.95 dry objective, Photometrics Coolsnap HQ camera, and Metamorph software Version 6.2r6. Channel separation was done in Adobe Photoshop CS. (D) Expression analysis of DCIR by flow cytometry on CD34+-derived DC subsets CD1a+ LCs and CD14+ DCs.
DCIR was found to be expressed by all circulating APCs as indicated by HLA-DR expression. These APCs include the CD14+ monocytes (both CD14+CD16− and CD14+CD16+ subsets), LIN−HLA-DR+CD11c+ blood mDCs, LIN−HLA-DR+CD11c−CD123+ pDCs and on CD19+ B lymphocytes. DCIR was not detected on CD3+ T cells (Figure 1A) or CD16+ and CD56+ NK cells (not shown). DCIR was expressed on purified epidermal LCs, dermal CD14−CD1a+, and dermal CD14+CD1a− DCs (Figure 1B). Immunofluorescence analysis of epidermal sheets further confirmed the expression of DCIR on HLA-DR+ LCs in situ (Figure 1C). DCIR is expressed on CD1a+ LCs and CD14+ interstitial DCs generated in vitro by culturing CD34+ HPCs with a combination of GM-CSF, Fms-like tyrosine kinase 3 ligand, and TNF-α for 9 days38 (Figure 1D), as well as on monocyte-derived DCs cultured with GM-CSF and IL-4, or with GM-CSF and type I IFN (not shown).
Cross-presentation of FluMP protein by anti-DCIR conjugates
Pilot experiments using a FluMP protein chemically coupled to anti-DCIR antibody (Figure 2AI) demonstrated that, when linked to an antigen, DCIR allows cross-presentation of the immunodominant HLA-A201–restricted FluMP(58-66) peptide (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This led us to construct fusion proteins based on recombinant anti-DCIR (or control IgG4 antibodies) and FluMP, but these failed to be efficiently secreted from transfected HEK293F cells. We therefore designed a strategy based on the high-affinity interaction (∼ 30pM) between cohesin and dockerin, 2 proteins of the cellulosome from Clostridium thermocellum (A.-L.F., S.Z., L. Ni, E.K., J. Quinn, S. Oh, J.B., G.Z., manuscript in preparation). The mAb.Dockerin fusion protein mAb.doc (Figure 2AII; supplemental Figure 2A) was readily secreted by transfected mammalian cells and purified on a protein A affinity column. The control hIgG4H and recombinant anti-DCIR antibodies each carry S229P and L236E substitutions, which stabilize a disulphide bond and abrogate residual FcR interaction.40 FluMP was produced in Escherichia coli as a soluble Cohesin fusion protein (coh.FluMP; Figure 2AII; supplemental Figure 2A). Targeting conjugates were generated by incubating equimolar amounts of mAb.doc and coh.FluMP for 15 minutes before being delivered to DCs. The recombinant anti–DCIR.doc-coh.FluMP complex mAb (supplemental Figure 2B filled arrow) bound to the surface of human monocyte–derived DCs, whereas the control conjugate IgG4-FluMP (supplemental Figure 2B empty arrow) did not bind the cells (supplemental Figure 2B).
Engineering and characterization of targeted proteins into DCIR mAb. (A) Five constructs are shown. (I) Diagram of mouse IgG1 cross-linked to the target antigen FluMP. (II-III) Diagram of chimeric mAbs (IgG4).doc conjugated to coh.antigen (FluMP [II] or MART-1 [III]). (IV-V) Diagram of chimeric fusion mAb IgG4-antigen (MART-1 [IV] or HIV gag p24 [V]). (B) Staining of HLA-A201–FluMP complexes on CD34+-derived DCs unpulsed (control DCs, gray histogram), or pulsed with 50nM DCIR-targeted FluMP. Cells were activated with 5 μg/mL anti-CD40 mAb (12E12, Baylor Research Institute; BIIR) and stained after 24 hours with phycoerythrin-labeled tetramerized anti–HLA-A201–FluMP Fab (M1D12).39 (C) Cross-presentation of FluMP to CD8+ T cells by autologous HLA-A201+CD34+–derived LCs that were cultured with 8nM (top panel) or 0.8nM (bottom panel) of anti–DCIR.doc-coh.FluMP or IgG4.doc-coh.FluMP conjugate mAbs. Dot plots show the proportions of HLA-A201–FluMP(58-66) peptide tetramer-positive CD8+ T cells after 10 days. (D) Proportions of HLA-A201–FluMP(58-66) tetramer-positive CD8+ T cells induced by DCs that were pulsed for 18 hours with 8nM anti–DCIR.doc-coh.FluMP or control IgG2a.doc-coh.FluMP conjugate mAbs, washed and cultured with autologous CD8+ T cells for 10 days. Graphs show the proportions of HLA-A201–FluMP(58-66) tetramer-positive CD8+ T cells, mean ± SD; n = 3.
Engineering and characterization of targeted proteins into DCIR mAb. (A) Five constructs are shown. (I) Diagram of mouse IgG1 cross-linked to the target antigen FluMP. (II-III) Diagram of chimeric mAbs (IgG4).doc conjugated to coh.antigen (FluMP [II] or MART-1 [III]). (IV-V) Diagram of chimeric fusion mAb IgG4-antigen (MART-1 [IV] or HIV gag p24 [V]). (B) Staining of HLA-A201–FluMP complexes on CD34+-derived DCs unpulsed (control DCs, gray histogram), or pulsed with 50nM DCIR-targeted FluMP. Cells were activated with 5 μg/mL anti-CD40 mAb (12E12, Baylor Research Institute; BIIR) and stained after 24 hours with phycoerythrin-labeled tetramerized anti–HLA-A201–FluMP Fab (M1D12).39 (C) Cross-presentation of FluMP to CD8+ T cells by autologous HLA-A201+CD34+–derived LCs that were cultured with 8nM (top panel) or 0.8nM (bottom panel) of anti–DCIR.doc-coh.FluMP or IgG4.doc-coh.FluMP conjugate mAbs. Dot plots show the proportions of HLA-A201–FluMP(58-66) peptide tetramer-positive CD8+ T cells after 10 days. (D) Proportions of HLA-A201–FluMP(58-66) tetramer-positive CD8+ T cells induced by DCs that were pulsed for 18 hours with 8nM anti–DCIR.doc-coh.FluMP or control IgG2a.doc-coh.FluMP conjugate mAbs, washed and cultured with autologous CD8+ T cells for 10 days. Graphs show the proportions of HLA-A201–FluMP(58-66) tetramer-positive CD8+ T cells, mean ± SD; n = 3.
To determine whether the recombinant anti–DCIR.doc-coh.FluMP complex mAb was processed and presented by DCs, DCs from an HLA-A201+ donor were cultured for 24 hours with 50nM conjugate mAb and stained with the monoclonal antibody (M1D12) that detects FluMP(58-66) peptide bound to HLA-A201. DCs exposed to anti–DCIR-FluMP conjugate mAb display HLA-A201–FluMP(58-66) peptide complexes on their surface (Figure 2B black histogram).
To assess presentation of antigen to purified CD8+ T cells, the recombinant conjugate mAbs were offered at 2 concentrations (8nM and 0.8nM) to CD34+-HPC–derived LCs. Anti–DCIR.doc-coh.FluMP, at 8nM, was more potent in inducing the expansion of FluMP-specific CD8+ T cells than the IgG4.doc-coh.FluMP (10.5% tetramer-positive cells vs 0.9%; Figure 2C upper panel). The potency of targeting via DCIR was confirmed at a lower conjugate mAb concentration (0.8nM) where the control conjugate mAb was barely cross-presented (2.8% vs 0.2% positive cells; Figure 2C bottom panel). The ability of DCIR to target antigen to DCs was further illustrated when the DCs were exposed for only 18 hours to the conjugate mAbs (8nM) and washed before culturing with CD8+ T cells (4.12% ± 2.13% vs 0.05% ± 0.02% tetramer-positive cells; Figure 2D).
Thus, targeted delivery of antigen to ex vivo–generated DCs via DCIR allows efficient cross-presentation of proteins to CD8+ T cells.
Anti-DCIR conjugates allow cross-presentation of proteins by skin Langerhans cells blood mDCs and blood pDCs
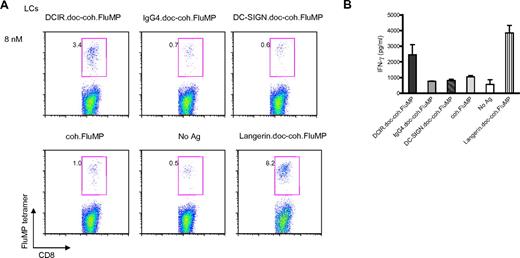
Because these fusion proteins are intended to be used as vaccines, we assessed whether the constructs would be cross-presented by human DC subsets isolated from either skin or blood. Thus, 8nM recombinant anti–DCIR.doc-coh.FluMP complex was added to cultures of 5 × 103 sorted epidermal HLA-A201+ LCs and 1 × 105 purified blood CD8+ T cells for 10 days (Figure 3). This resulted in expansion of FluMP-specific CD8+ T cells by DCIR-targeted LCs compared with the IgG4 control complex mAb (3.4% vs 0.7%). Free coh.FluMP (1%) or a complex against a lectin, which is not expressed by LCs (ie, DC-SIGN.doc-coh.FluMP, 0.6%; Figure 3A), was very weakly cross-presented, if at all. An antibody-antigen complex against Langerin, a LC-specific lectin, induced expansion of FluMP-specific CD8+ T cells by LCs (8.2%). The expansion of tetramer-specific CD8+ T cells (Figure 3A) correlated with the levels of IFN-γ measured in the culture supernatant (Figure 3B).
DCIR allows cross-presentation of proteins by LCs. (A) Skin-derived LCs from an HLA-A201+ donor were targeted with 8nM each of anti-DC.doc-coh.FluMP, IgG4.doc-coh.FluMP conjugate mAbs, or free FluMP matured with CD40L, and cocultured with autologous CD8+ T cells. Ten days later, CD8+ T-cell expansion was evaluated by specific HLA-A201–FluMP(58-66) tetramer staining. Data are representative of 2 independent experiments performed with cells from 2 different donors. (B) IFN-γ levels as measured by Luminex in the culture supernatant of CD8+ T cells expanded for 10 days by autologous skin LCs targeted with anti–DCIR.doc-coh.FluMP or IgG4.doc-coh.FluMP conjugate mAbs. Graph represents mean ± SD; n = 3.
DCIR allows cross-presentation of proteins by LCs. (A) Skin-derived LCs from an HLA-A201+ donor were targeted with 8nM each of anti-DC.doc-coh.FluMP, IgG4.doc-coh.FluMP conjugate mAbs, or free FluMP matured with CD40L, and cocultured with autologous CD8+ T cells. Ten days later, CD8+ T-cell expansion was evaluated by specific HLA-A201–FluMP(58-66) tetramer staining. Data are representative of 2 independent experiments performed with cells from 2 different donors. (B) IFN-γ levels as measured by Luminex in the culture supernatant of CD8+ T cells expanded for 10 days by autologous skin LCs targeted with anti–DCIR.doc-coh.FluMP or IgG4.doc-coh.FluMP conjugate mAbs. Graph represents mean ± SD; n = 3.
Both subsets of blood DCs, CD11c+ mDCs and BDCA2+ pDCs, express DCIR (Figure 1A). Thus, mDCs and pDCs purified from the same cytapheresis samples41 were tested for their ability to cross-present FluMP delivered via DCIR. A total of 5 × 103 DCs were cultured with 1 × 105 autologous CD8+ T cells and decreasing concentrations of either free coh.FluMP, or IgG4.doc-coh.FluMP conjugate or the anti–DCIR.doc-coh.FluMP conjugate.
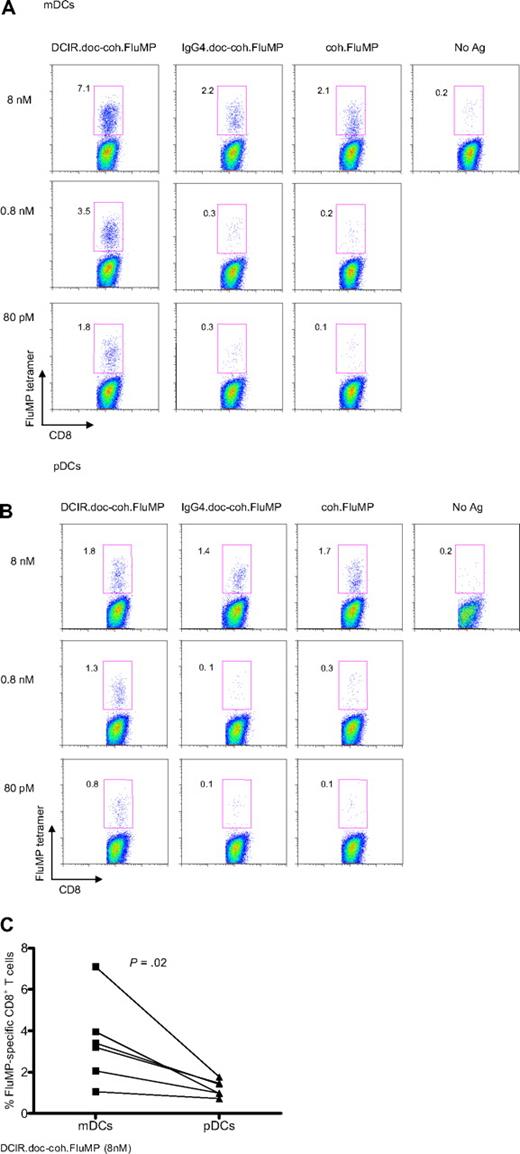
The anti–DCIR.doc-coh.FluMP complex mAb (Figure 4A) efficiently targeted FluMP to mDCs because concentrations as low as 80pM yielded 1.8% tetramer-positive cells. Coh.FluMP itself and the control IgG4.doc-coh.FluMP conjugate were able to induce expansion of antigen-specific CD8+ T cells only at 8nM.
DCIR is a global target for all blood DC subsets. (A) Blood-derived mDCs from an HLA-A201+ donor are targeted with 8nM, 0.8nM, or 80pM each anti–DCIR.doc-coh.FluMP (clone 24A5), IgG4.doc-coh.FluMP conjugate mAbs, or free coh.FluMP, matured with CD40L and cocultured with autologous CD8+ T cells. Ten days later, CD8+ T-cell expansion was evaluated by specific HLA-A201–FluMP(58-66) tetramer staining. Data are representative of 3 independent experiments. (B) Blood-derived pDCs from an HLA-A201+ donor were targeted with 8nM, 0.8nM, or 80pM each anti–DCIR.doc-coh.FluMP (clone 24A5), IgG4.doc-coh.FluMP, or free coh.FluMP, matured with CD40L and cocultured with autologous CD8+ T cells. Ten days later, T-cell expansion was evaluated by specific HLA-A201–FluMP(58-66) tetramer staining. Data are representative of 3 independent experiments. (C) Percentage of FluMP-specific CD8+ T cells induced by 8nM DCIR.doc-coh.FluMP complex mAb-targeted mDCs or pDCs. Graph represents results of 3 independent experiments using 2 different clones of DCIR mAb. P = .02.
DCIR is a global target for all blood DC subsets. (A) Blood-derived mDCs from an HLA-A201+ donor are targeted with 8nM, 0.8nM, or 80pM each anti–DCIR.doc-coh.FluMP (clone 24A5), IgG4.doc-coh.FluMP conjugate mAbs, or free coh.FluMP, matured with CD40L and cocultured with autologous CD8+ T cells. Ten days later, CD8+ T-cell expansion was evaluated by specific HLA-A201–FluMP(58-66) tetramer staining. Data are representative of 3 independent experiments. (B) Blood-derived pDCs from an HLA-A201+ donor were targeted with 8nM, 0.8nM, or 80pM each anti–DCIR.doc-coh.FluMP (clone 24A5), IgG4.doc-coh.FluMP, or free coh.FluMP, matured with CD40L and cocultured with autologous CD8+ T cells. Ten days later, T-cell expansion was evaluated by specific HLA-A201–FluMP(58-66) tetramer staining. Data are representative of 3 independent experiments. (C) Percentage of FluMP-specific CD8+ T cells induced by 8nM DCIR.doc-coh.FluMP complex mAb-targeted mDCs or pDCs. Graph represents results of 3 independent experiments using 2 different clones of DCIR mAb. P = .02.
pDCs were also able to cross-present the 3 forms of recombinant FluMP at a concentration of 8nM. At 0.8nM and 80pM, anti–DCIR.doc-coh.FluMP complex mAb allowed cross-presentation of the FluMP antigen, although free coh.FluMP, or IgG4.doc-coh.FluMP conjugates were not cross-presented (Figure 4B). Compared with pDCs, mDCs targeted with 8nM of anti–DCIR.doc-coh.FluMP complex mAb were able to induce a more robust expansion of FluMP-specific CD8+ T cells (as measured with a specific HLA-A201 tetramer, Figure 4C; P = .02).
Taken together, these data indicate that anti-DCIR mAb potently targets proteins for cross-presentation by skin LCs, blood mDCs, and pDCs.
Cross-priming of MART-1 and HIV gag proteins by anti-DCIR conjugates
We then tested whether DCIR would permit the cross-priming of naive CD8+ T cells using: (1) anti-DCIR.dockerin and cohesin fused to the 10-mer MART-1(26-35) HLA-A201–restricted peptide (EAAGIGILTV; Figure 2AIII); and (2) anti-DCIR directly fused to MART-1 recombinant protein (Figure 2AIV) or to HIV gag p24 protein (Figure 2AV). Epidermal HLA-A201+ LCs were cultured with autologous T cells with 30nM anti–DCIR.doc-coh.MART-1 or IgG4.doc-coh.MART-1 complex mAbs. After 10 days, the binding of MART-1(26-35)–HLA-A201+ tetramer indicated that anti–DCIR.doc-coh.MART-1 complex mAb allowed skin-derived LCs to prime CD8+ T cells and expand MART-1–specific CD8+ T cells (Figure 5A).
Cross-priming of Mart-1 and HIV gag p24 protein by anti-DCIR fusion mAb. (A) Skin-derived LCs from an HLA-A201+ donor were purified and cultured for 10 days with autologous purified T cells in the presence of 30nM anti–DCIR.doc-coh.MART-1 or IgG4.doc-coh.MART-1 conjugate mAbs. DCs were activated with CD40L. MART-1–specific CD8+ T-cell expansion was measured with a specific HLA-A201-MART-1(26-35) tetramer. (B) Anti–DCIR-MART-1 or IgG4-MART-1 (25nM) fusion proteins were used to target monocyte-derived IFN-α DCs. DCs were activated with CD40L and cultured with naive autologous CD8+ T cells. After 10 days, cells were restimulated for 24 hours with fresh DCs loaded with peptides derived from MART-1 protein or with unloaded DCs as a control. Plot shows the percentage of primed CD8+ T cells coexpressing IFN-γ and CD107a in response to a specific MART-1 peptide cluster. (C) CD34+-derived LCs were targeted with DCIR-MART-1 or control IgG4-MART-1 fusion proteins and cultured with naive CD8+ T cells for 9 days. Graph represents the percentage of cells coexpressing Granzyme B and perforin as analyzed at the end of the culture by flow cytometry. Values in the graph are the average of triplicates ± SD. Data are representative of 2 independent experiments. (D) Anti–DCIR-p24 or control IgG4-p24 (25nM) fusion proteins were used to target CD34+-derived LCs. DCs were activated with CD40L and cultured with naive autologous CD8+ T cells. After 2 consecutive stimulations, the proliferated cells were sorted and restimulated for 24 hours with fresh LCs and HIV gag p24 protein to evaluate IFN-γ secretion by Luminex. Cells with no protein served as a control. Values are average of duplicates. Data are representative of 2 independent experiments.
Cross-priming of Mart-1 and HIV gag p24 protein by anti-DCIR fusion mAb. (A) Skin-derived LCs from an HLA-A201+ donor were purified and cultured for 10 days with autologous purified T cells in the presence of 30nM anti–DCIR.doc-coh.MART-1 or IgG4.doc-coh.MART-1 conjugate mAbs. DCs were activated with CD40L. MART-1–specific CD8+ T-cell expansion was measured with a specific HLA-A201-MART-1(26-35) tetramer. (B) Anti–DCIR-MART-1 or IgG4-MART-1 (25nM) fusion proteins were used to target monocyte-derived IFN-α DCs. DCs were activated with CD40L and cultured with naive autologous CD8+ T cells. After 10 days, cells were restimulated for 24 hours with fresh DCs loaded with peptides derived from MART-1 protein or with unloaded DCs as a control. Plot shows the percentage of primed CD8+ T cells coexpressing IFN-γ and CD107a in response to a specific MART-1 peptide cluster. (C) CD34+-derived LCs were targeted with DCIR-MART-1 or control IgG4-MART-1 fusion proteins and cultured with naive CD8+ T cells for 9 days. Graph represents the percentage of cells coexpressing Granzyme B and perforin as analyzed at the end of the culture by flow cytometry. Values in the graph are the average of triplicates ± SD. Data are representative of 2 independent experiments. (D) Anti–DCIR-p24 or control IgG4-p24 (25nM) fusion proteins were used to target CD34+-derived LCs. DCs were activated with CD40L and cultured with naive autologous CD8+ T cells. After 2 consecutive stimulations, the proliferated cells were sorted and restimulated for 24 hours with fresh LCs and HIV gag p24 protein to evaluate IFN-γ secretion by Luminex. Cells with no protein served as a control. Values are average of duplicates. Data are representative of 2 independent experiments.
The successful expression of anti–DCIR-MART-1 fusion protein (Figure 2AIV) allowed us to further assess cross-priming to other epitopes of the MART-1 protein. Thus, DCs were exposed to either anti–DCIR-MART-1 or IgG4-MART-1 fusion protein or to no protein, activated with CD40L and cultured with autologous purified naive CD8+ T cells. After 10 days, cells were restimulated for 5 hours with DCs loaded with clusters of individual peptides derived from the MART-1 protein or with unloaded DCs. Mobilization of CD107a, a marker for cytotoxic activity determination, to the cell surface and the expression of intracytoplasmic IFN-γ were measured to assess specific cytotoxic T lymphocyte responses. Anti–DCIR-MART-1 fusion protein induced expansion of MART-1–specific CD8+ T cells to peptides from clusters 1, 4, and 5 of the MART-1 protein (Figure 5B). Targeting DCs with anti–DCIR-MART-1 fusion protein induced expansion of CD8+ T cells expressing high levels of the effector molecules Granzyme B and perforin (Figure 5C).
Anti–DCIR-p24 and IgG4-p24 fusion proteins (Figure 2AV) were also well secreted from HEK293F cells. Thus, purified naive CD8+ T cells from healthy persons were labeled with CFSE and primed by 2 consecutive 7-day cultures with DCs and with either of these fusion proteins, or no protein. The proliferating CFSElowCD8+ T cells were sorted and rechallenged with HIV gag p24 (p24) protein-loaded DCs. CD8+ T cells primed with anti–DCIR-p24 fusion protein (Figure 5D black bar) were able to secrete IFN-γ in response to the p24 challenge, whereas control fusion proteins did not (Figure 5D gray bar). This indicates specific priming of naive CD8+ T cells by the anti–DCIR-p24 fusion protein. These results demonstrated that targeting antigens via DCIR allows priming of CD8+ T cells specific for both self- and non–self-antigens.
TLR7/8 agonist enhances DCIR-mediated cross-presentation
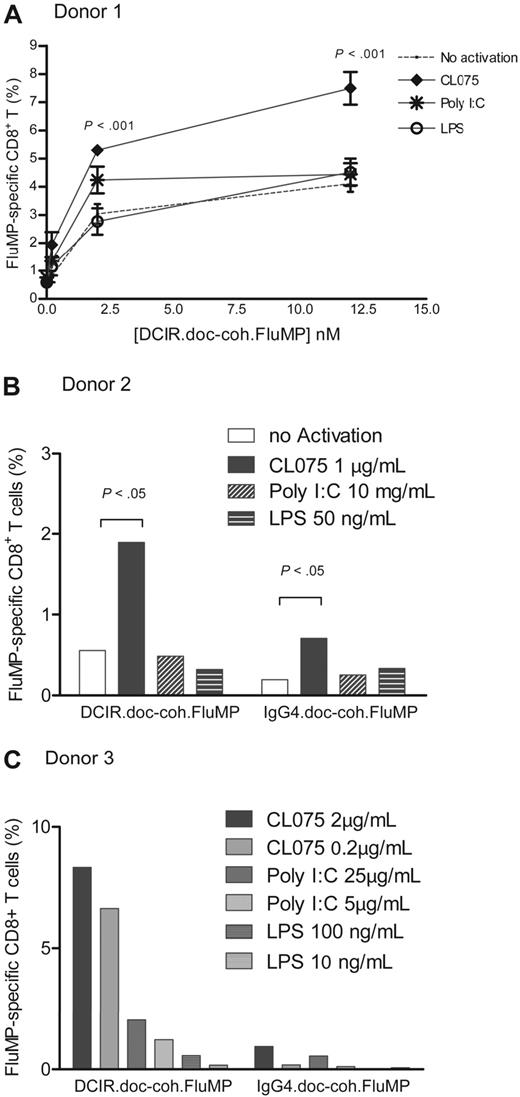
As TLR triggering activates DCs, we analyzed whether TLR ligands would enhance the antigen-specific CD8+ T-cell responses induced by mDCs targeted with anti-DCIR complexes. A total of 5 × 103 purified blood HLA-A201+ mDCs were cultured with increasing amounts of anti–DCIR.doc-coh.FluMP complex mAb and agonists for TLR3 (poly I:C; 5 μg/mL), TLR4 (LPS; 50 ng/mL), or TLR7/8 (CL075; 1 μg/mL), and 1 × 105 autologous purified CD8+ T cells. The specific FluMP CD8+ T-cell response was measured after 8 to 10 days using HLA-A201–FluMP(58-66) tetramer. The TLR3 agonist (poly I:C) enhanced the FluMP-specific responses at low concentration of the targeting complex (2nM and 0.2nM), whereas activation via TLR4 did not. The TLR7/8 agonist (CL075) was found to be the most potent in expanding FluMP-specific CD8+ T cells (Figure 6A). The CL075-enhanced response was observed for all tested concentrations of anti–DCIR.doc-coh.FluMP complex and was dependent on the presence of the mAb targeting complex (Figure 6A-B). Increasing the concentrations of poly I:C from 5 μg/mL to 25 μg/mL or LPS from 50 ng/mL to 200 ng/mL did not significantly enhance the expansion of the antigen-specific CD8+ T cells in response to the anti–DCIR.doc-coh.FluMP complex mAb. TLR3 activation, however, resulted in higher FluMP-specific response than TLR4 activation (Figure 6C). Low concentration of 0.2 μg/mL of the TLR7/8 agonist was sufficient to enhance the FluMP-specific response (Figure 6C). No significant synergistic effect was seen when soluble CD40L was added in addition to the TLR agonist (not shown).
TLR7/8-signaling enhances DCIR-mediated secondary CD8+ T-cell response by mDCs. (A) Blood-derived mDCs from an HLA-A201+ donor were targeted with 12nM, 2nM, or 200pM of anti–DCIR.doc-coh.FluMP complex mAb, activated with either TLR3, TLR4, or TLR7/8 agonists (poly I:C, LPS, or CL075) and cocultured with autologous CD8+ T cells for 10 days. Graph represents the percentage of FluMP-specific CD8+ T cells measured with a specific HLA-A201–FluMP(58-66) tetramer for each amount of anti–DCIR.doc-coh.FluMP complex mAb and with each DC-activator tested. DCs with no activation were used as a control: no activation (—), TLR7/8 (♦), TLR3 (*), and TLR4 (○) agonists; CL075, poly I:C, and LPS, respectively. Data are representative of 4 independent experiments with 4 different donors. The graph represents mean ± SD; n = 3. (B) Blood-derived mDCs from an HLA-A201+ donor were targeted with 8nM anti–DCIR.doc-coh.FluMP or IgG4.doc-coh.FluMP complex mAb, activated with TLR7/8, TLR3, and TLR4 agonists (CL075, poly I:C, and LPS, respectively) and cocultured with autologous CD8+ T cells for 10 days. Graph represents the percentage of FluMP-specific CD8+ T cells as measured with a specific HLA-A201–FluMP(58-66) tetramer. Conditions indicated in the graph are as follows: no activation, CL075 1μg/mL; poly I:C, 10 μg/mL; and LPS, 50 ng/mL. The graph represents mean ± SD; n = 3. (C) Same experiment as in panel B. Graph represents the mean percentage of FluMP-specific CD8+ T cells as measured with a specific HLA-A201–FluMP(58-66) tetramer. Conditions indicated in the graph are as follows: no activation; CL075-0.2μg/mL and 2 μg/mL; poly I:C, 5 μg/mL and 25 μg/mL; LPS, 10 ng/mL and 100 ng/mL.
TLR7/8-signaling enhances DCIR-mediated secondary CD8+ T-cell response by mDCs. (A) Blood-derived mDCs from an HLA-A201+ donor were targeted with 12nM, 2nM, or 200pM of anti–DCIR.doc-coh.FluMP complex mAb, activated with either TLR3, TLR4, or TLR7/8 agonists (poly I:C, LPS, or CL075) and cocultured with autologous CD8+ T cells for 10 days. Graph represents the percentage of FluMP-specific CD8+ T cells measured with a specific HLA-A201–FluMP(58-66) tetramer for each amount of anti–DCIR.doc-coh.FluMP complex mAb and with each DC-activator tested. DCs with no activation were used as a control: no activation (—), TLR7/8 (♦), TLR3 (*), and TLR4 (○) agonists; CL075, poly I:C, and LPS, respectively. Data are representative of 4 independent experiments with 4 different donors. The graph represents mean ± SD; n = 3. (B) Blood-derived mDCs from an HLA-A201+ donor were targeted with 8nM anti–DCIR.doc-coh.FluMP or IgG4.doc-coh.FluMP complex mAb, activated with TLR7/8, TLR3, and TLR4 agonists (CL075, poly I:C, and LPS, respectively) and cocultured with autologous CD8+ T cells for 10 days. Graph represents the percentage of FluMP-specific CD8+ T cells as measured with a specific HLA-A201–FluMP(58-66) tetramer. Conditions indicated in the graph are as follows: no activation, CL075 1μg/mL; poly I:C, 10 μg/mL; and LPS, 50 ng/mL. The graph represents mean ± SD; n = 3. (C) Same experiment as in panel B. Graph represents the mean percentage of FluMP-specific CD8+ T cells as measured with a specific HLA-A201–FluMP(58-66) tetramer. Conditions indicated in the graph are as follows: no activation; CL075-0.2μg/mL and 2 μg/mL; poly I:C, 5 μg/mL and 25 μg/mL; LPS, 10 ng/mL and 100 ng/mL.
For every tested concentration or combination of activators tested, FluMP-specific responses to anti–DCIR.doc-coh.FluMP were always significantly higher than those induced by the control IgG4.doc-coh.FluMP (Figure 6B-C), or free coh.FluMP (not shown). Thus, TLR7/8 activation enhances DCIR-dependent cross-presentation of protein antigen by mDCs.
TLR7/8 agonist enhances DCIR-mediated cross-priming
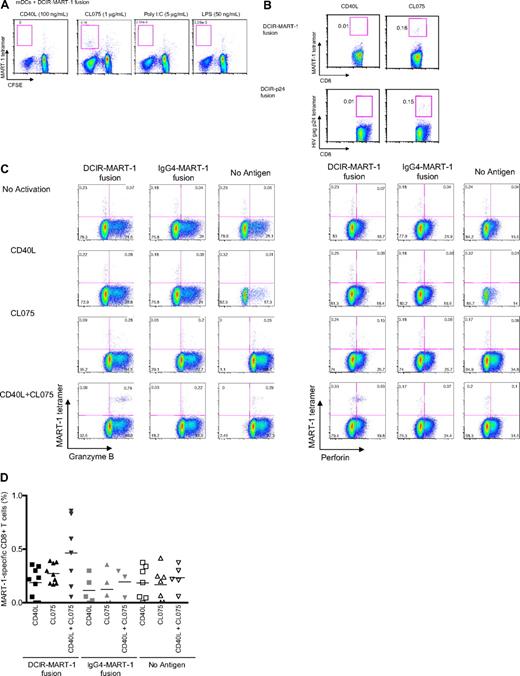
We next examined whether TLR7/8 ligand would also enhance DCIR-mediated primary CD8+ T-cell responses. Blood HLA-A201+ mDCs were cultured with either anti–DCIR-MART-1 or the IgG4-MART-1 fusion protein (Figure 2AIV). The DCs were activated with CD40L, TLR3-L, TLR4-L, or TLR7/8-L and cocultured with purified CFSE-labeled naive CD8+ T cells. The expansion of MART-1(26-35)–HLA-A2–restricted CFSElow CD8+ T cells was assessed after 10 days using a specific tetramer (Figure 7A). TLR7/8-activated DCs induced the highest expansion of MART-1–specific CD8+ T cells (0.18%; Figure 7A). In a second experiment using blood mDCs, a single dose of both anti–DCIR-MART-1 or anti–DCIR-p24 fusion protein (Figure 2AIV and 2AV, respectively) together with the TLR7/8 agonist, but not CD40L, induced expansion of MART-1 and HIV gag p24-HLA-A201-tetramer binding CD8+ T cells (0.18% vs 0.01% and 0.15% vs 0.01%; Figure 7B). Unlike secondary responses, however, cosignaling via both CD40- and TLR7/8 resulted in a synergistic effect and a larger expansion of tetramer-binding CD8+ T cells compared with CD40L or TLR7/8 agonist alone (0.3% vs 0.37% vs 0.85%; Figure 7C-D). Thus, TLR7/8 agonist enhances cross-priming and cross-presentation of antigen-specific CD8+ T cells.
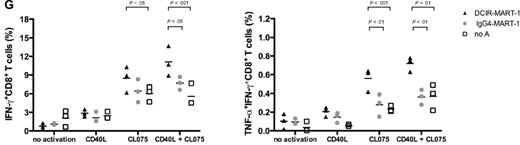
TLR7/8-signaling enhances DCIR-mediated primary CD8+ T-cell response by mDCs. (A) Blood-derived mDCs from an HLA-A201+ donor were targeted with 17nM of anti–DCIR-MART-1 or a control IgG4-MART-1 fusion proteins, activated with CD40L (100 ng/mL), CL075 (1 μg/mL), poly I:C (5 μg/mL), or LPS (50 ng/mL) and cocultured with autologous naive CD8+ T cells for 10 days. The expansion of MART-1–specific CD8+ T cells was measured with a specific HLA-A201-MART-1(26-35) tetramer. Data are of 2 independent experiments with 2 different donors. (B) Blood-derived mDCs from an HLA-A201+ donor were targeted with 30nM of anti–DCIR-MART-1 fusion protein or anti–DCIR-p24, activated with either CD40L or TLR7/8 agonists, and cocultured with autologous naive CD8+ T cells for 10 days. (Top panel) The proportions of HLA-A201-MART-1(26-35) peptide tetramer-positive CD8+ T cells expanded by purified blood mDCs cultured with anti–DCIR-MART-1 fusion protein and activated with either CD40L or TLR7/8 agonist. (Bottom panel) The proportions of HLA-A201-HIV gag p24(151-159) peptide tetramer-positive CD8+ T cells expanded by purified blood mDCs targeted with anti–DCIR-p24 fusion protein and activated with either CD40L or TLR7/8 agonist. Data are of 2 independent experiments with 2 different donors. (C) The expression of intracellular effector molecules Granzyme B and perforin was assessed by flow cytometry on CD8+ T cells primed by IFN-α DCs targeted with 10nM of anti–DCIR-MART-1 or IgG4-MART-1 fusion proteins and activated with CD40L, CL075, or a combination of CD40L and CL075. The expression on the antigen specific MART-1(26-35)-positive cells was analyzed by co staining with the corresponding HLA-A201-tetramer. Data are representative of 2 independent experiments. (D) The frequency of MART-1–specific CD8+ T cells, as measured with a specific HLA-A201-MART-1(26-35) tetramer, after expansion with anti–DCIR-MART-1–targeted DCs that were activated with CD40L, TLR7/8 ligand, or a combination of CD40L and TLR7/8 ligand. IgG4-MART-1 fusion protein or no antigen conditions served as controls. Each dot represents a single experiment. (E top panel) IFN-α DCs were targeted with 17nM of anti–DCIR-MART-1 or a control IgG4-MART-1 fusion proteins, activated with CD40L (100 ng/mL), CL075 (1 μg/mL), poly I:C (10 μg/mL), or LPS (50 ng/mL) and cocultured with autologous naive CD8+ T cells. Ten days later, cells were restimulated with fresh DCs that were loaded with 15mer overlapping peptides derived from the MART-1 protein. Plots show the level of intracytoplasmic IFN-γ by CD8+ T cells after 5-hour stimulation in the presence of monensin. Data are representative of 3 independent experiments. (Bottom panel) Anti–DCIR-p24 or a control IgG4-p24 fusion proteins were used as a model antigen. (F) IFN-α DCs were targeted with 113nM anti–DCIR-MART-1 fusion protein activated with either CD40L (100 ng/mL) or CL075 (1 μg/mL) and cocultured with autologous naive CD8+ T cells. Ten days later, cells were restimulated with fresh DCs that were loaded with 15mer overlapping peptides derived from the MART-1 protein. The levels of IL-4, IL-5, IL-13, IFN-γ, TNF-α, and IL-12p40 were measured by Luminex in the culture supernatant after 24 hours. The graph represents mean ± SD; n = 3. (G) IFN-α DCs were targeted with 10nM anti–DCIR-MART-1 (▴) or a control IgG4-MART-1 ( ) fusion proteins activated with either CD40L (100 ng/mL) or CL075 (1 μg/mL), or a combination of CD40L and CL075 and cocultured with autologous naive CD8+ T cells. Coculture in the absence of an antigen served as an additional control (□). Ten days later, cells were restimulated with fresh IFN-α DCs that were loaded with MART-1 fusion protein and analyzed by flow cytometry for their intracellular cytokine production. Graphs show the frequency of IFN-γ (left panel) and IFN-γ+TNF-α+ (right panel) producing CD8+ T cells primed by DCIR-targeted, or control IFN-α DCs after 5-hour restimulation in the presence of monensin and 0.25 μg/mL of anti-CD28/CD49d mAb (n = 3).
) fusion proteins activated with either CD40L (100 ng/mL) or CL075 (1 μg/mL), or a combination of CD40L and CL075 and cocultured with autologous naive CD8+ T cells. Coculture in the absence of an antigen served as an additional control (□). Ten days later, cells were restimulated with fresh IFN-α DCs that were loaded with MART-1 fusion protein and analyzed by flow cytometry for their intracellular cytokine production. Graphs show the frequency of IFN-γ (left panel) and IFN-γ+TNF-α+ (right panel) producing CD8+ T cells primed by DCIR-targeted, or control IFN-α DCs after 5-hour restimulation in the presence of monensin and 0.25 μg/mL of anti-CD28/CD49d mAb (n = 3).
TLR7/8-signaling enhances DCIR-mediated primary CD8+ T-cell response by mDCs. (A) Blood-derived mDCs from an HLA-A201+ donor were targeted with 17nM of anti–DCIR-MART-1 or a control IgG4-MART-1 fusion proteins, activated with CD40L (100 ng/mL), CL075 (1 μg/mL), poly I:C (5 μg/mL), or LPS (50 ng/mL) and cocultured with autologous naive CD8+ T cells for 10 days. The expansion of MART-1–specific CD8+ T cells was measured with a specific HLA-A201-MART-1(26-35) tetramer. Data are of 2 independent experiments with 2 different donors. (B) Blood-derived mDCs from an HLA-A201+ donor were targeted with 30nM of anti–DCIR-MART-1 fusion protein or anti–DCIR-p24, activated with either CD40L or TLR7/8 agonists, and cocultured with autologous naive CD8+ T cells for 10 days. (Top panel) The proportions of HLA-A201-MART-1(26-35) peptide tetramer-positive CD8+ T cells expanded by purified blood mDCs cultured with anti–DCIR-MART-1 fusion protein and activated with either CD40L or TLR7/8 agonist. (Bottom panel) The proportions of HLA-A201-HIV gag p24(151-159) peptide tetramer-positive CD8+ T cells expanded by purified blood mDCs targeted with anti–DCIR-p24 fusion protein and activated with either CD40L or TLR7/8 agonist. Data are of 2 independent experiments with 2 different donors. (C) The expression of intracellular effector molecules Granzyme B and perforin was assessed by flow cytometry on CD8+ T cells primed by IFN-α DCs targeted with 10nM of anti–DCIR-MART-1 or IgG4-MART-1 fusion proteins and activated with CD40L, CL075, or a combination of CD40L and CL075. The expression on the antigen specific MART-1(26-35)-positive cells was analyzed by co staining with the corresponding HLA-A201-tetramer. Data are representative of 2 independent experiments. (D) The frequency of MART-1–specific CD8+ T cells, as measured with a specific HLA-A201-MART-1(26-35) tetramer, after expansion with anti–DCIR-MART-1–targeted DCs that were activated with CD40L, TLR7/8 ligand, or a combination of CD40L and TLR7/8 ligand. IgG4-MART-1 fusion protein or no antigen conditions served as controls. Each dot represents a single experiment. (E top panel) IFN-α DCs were targeted with 17nM of anti–DCIR-MART-1 or a control IgG4-MART-1 fusion proteins, activated with CD40L (100 ng/mL), CL075 (1 μg/mL), poly I:C (10 μg/mL), or LPS (50 ng/mL) and cocultured with autologous naive CD8+ T cells. Ten days later, cells were restimulated with fresh DCs that were loaded with 15mer overlapping peptides derived from the MART-1 protein. Plots show the level of intracytoplasmic IFN-γ by CD8+ T cells after 5-hour stimulation in the presence of monensin. Data are representative of 3 independent experiments. (Bottom panel) Anti–DCIR-p24 or a control IgG4-p24 fusion proteins were used as a model antigen. (F) IFN-α DCs were targeted with 113nM anti–DCIR-MART-1 fusion protein activated with either CD40L (100 ng/mL) or CL075 (1 μg/mL) and cocultured with autologous naive CD8+ T cells. Ten days later, cells were restimulated with fresh DCs that were loaded with 15mer overlapping peptides derived from the MART-1 protein. The levels of IL-4, IL-5, IL-13, IFN-γ, TNF-α, and IL-12p40 were measured by Luminex in the culture supernatant after 24 hours. The graph represents mean ± SD; n = 3. (G) IFN-α DCs were targeted with 10nM anti–DCIR-MART-1 (▴) or a control IgG4-MART-1 ( ) fusion proteins activated with either CD40L (100 ng/mL) or CL075 (1 μg/mL), or a combination of CD40L and CL075 and cocultured with autologous naive CD8+ T cells. Coculture in the absence of an antigen served as an additional control (□). Ten days later, cells were restimulated with fresh IFN-α DCs that were loaded with MART-1 fusion protein and analyzed by flow cytometry for their intracellular cytokine production. Graphs show the frequency of IFN-γ (left panel) and IFN-γ+TNF-α+ (right panel) producing CD8+ T cells primed by DCIR-targeted, or control IFN-α DCs after 5-hour restimulation in the presence of monensin and 0.25 μg/mL of anti-CD28/CD49d mAb (n = 3).
) fusion proteins activated with either CD40L (100 ng/mL) or CL075 (1 μg/mL), or a combination of CD40L and CL075 and cocultured with autologous naive CD8+ T cells. Coculture in the absence of an antigen served as an additional control (□). Ten days later, cells were restimulated with fresh IFN-α DCs that were loaded with MART-1 fusion protein and analyzed by flow cytometry for their intracellular cytokine production. Graphs show the frequency of IFN-γ (left panel) and IFN-γ+TNF-α+ (right panel) producing CD8+ T cells primed by DCIR-targeted, or control IFN-α DCs after 5-hour restimulation in the presence of monensin and 0.25 μg/mL of anti-CD28/CD49d mAb (n = 3).
TLR7/8 ligand increases CTL effector molecules and decreases type 2 cytokine production
The next set of studies was designed to determine whether TLR7/8 triggering during DCIR targeting would alter the quality of the elicited responses. Thus, naive CD8+ T cells were cultured with autologous HLA-A201+ mDCs and anti–DCIR-MART-1 fusion protein without activation or with CD40L or CL075 alone, or CD40L + CL075. After 10 days, cells were stained with HLA-A201–MART-1(26-35) tetramer and Granzyme B or perforin-specific mAbs. Compared with each activator alone, the combination of CD40L and TLR7/8 agonist induced higher expression of the effector molecules Granzyme B (Figure 7C left panel) and perforin (Figure 7C right panel) by the expanded CD8+ T cells.
Compared with CD40L-, poly I:C-, or LPS-conditioned DCs, CD8+ T cells that were primed by DCs targeted with anti–DCIR-MART-1 fusion protein and TLR7/8 agonist expressed higher amounts of IFN-γ in response to a specific restimulation with autologous DCs loaded with peptides from the MART-1 protein (Figure 7E top panel). A second model antigen, HIV gag p24, allowed us to further demonstrate the effect of TLR7/8 ligand on the quality of the primed CD8+ T cells. Thus, CD8+ T cells primed by anti–DCIR-p24 fusion protein-targeted DCs and activated with TLR7/8 agonist, expressed higher amounts of IFN-γ compared with CD40L-, poly I:C-, or LPS-activated DCs in response to a specific restimulation with autologous DCs loaded with 15-amino acid–overlapping peptides from the HIV gag p24 protein (Figure 7E lower panel). Interestingly, DCIR-primed CD8+ T cells produced a different set of cytokines, in response to reactivation with MART-1 peptide-loaded DCs, according to whether they were initially exposed to either CD40L- or CL075-triggered DCs (Figure 7F). Whereas CD40L-matured IFN-α DCs induced naive CD8+ T cells to express high amounts of type 2 cytokines (IL-4, IL-5, and IL-13), TLR7/8-exposed DCs educated naive CD8+ T cells to preferentially secrete IFN-γ and TNF-α with markedly reduced amounts of IL-4, IL-5, and IL-13 (Figure 7F). Furthermore, compared with each activator alone, a combination of TLR7/8 and CD40L induced the higher expansion of IFN-γ–producing as well as IFN-γ and TNF-α double-cytokine–producing CD8+ T cells in response to fresh DCs loaded with the corresponding MART-1 fusion protein (Figure 7G). As expected, targeting MART-1 through DCIR resulted in higher expansion of IFN-γ as well as IFN-γ and TNF-α–producing CD8+ T cells compared with the nontargeted antigen-control IgG4 fusion protein, or no antigen (Figure 7E,G). Thus, TLR7/8 activation alters the quality of primary CD8+ T-cell responses by DCIR-targeted mDCs, by enhancing IFN-γ and TNF-α and reducing type 2 cytokine secretion.
Discussion
This study was initiated on the premise that ligation of DCIR, a surface lectin that expresses an ITIM motif, will result in deactivation or prevention of activation of DCs. As described earlier, DCIR is expressed at high density on blood monocytes and at lower levels on B cells.33 DCIR is also expressed at high density on purified dermal CD14+ DCs in accordance with earlier immunohistochemistry data.33 However, at variance with these data, DCIR was found to be expressed on epidermal LCs, after their purification, as well as on intact epidermal sheets. The discrepancy of the 2 studies regarding LCs is intriguing as DCIR expression is also observed with LCs generated in vitro by culturing CD34+ HPCs with GM-CSF and TNF-α.38 We, and others, also found DCIR to be expressed at high density on blood myeloid DCs42 and blood pDCs.34 Thus, DCIR is expressed by all human DC subsets of blood and skin DCs.
Engaging DCIR with 12 different anti-DCIR antibodies neither inhibited nor enhanced DC activation as measured by either expression of CD80, CD83, and CD86 or the secretion of cytokine (such as IL-6 and IL-12). DCIR cross-linking neither enhanced nor inhibited the DC-mediated proliferation of CD4+ and CD8+ T cells (data not shown). In addition, as assessed by microarray analysis, ligation of DCIR, as opposed to CD40, did not reveal an activation gene signature by isolated epidermal cells (data not shown). However, evidence for the inhibitory role of DCIR has been documented in dcir-deficient mice that showed an exacerbated response to collagen-induced arthritis, with increased numbers of activated DCs and activated CD4+ T cells.43 It should be noted, however, that mouse and human differ considerably at the level of the DCIR gene complex because the mouse genome encodes 4 DCIR-like molecules (DCIR-2, DCIR-3, DCIR-4, and DCAR-1), whereas the human genome encodes a single one. Alternative explanations include the possibility that the mAbs we have generated are unable to provide negative signals or that our antibodies cross-react with an as yet unidentified human counterpart of the mouse activating receptor DCAR. Another possibility might be that the inhibitory signal of DCIR is delivered in cells other than DCs (ie, monocytes or B cells).36 In the human, a recent study34 demonstrated a slight inhibition of TLR9-induced IFN-α production by pDCs, without affecting the expression of costimulatory molecules, and reduced IL-12 and TNF-α production by TLR8-activated mDCs.42 Finally, as demonstrated for other lectins, such as BDCA-210 and DCAL-2,44 depending on the cellular context, ITIMs can sometimes stimulate rather than repress cellular activation.45
Antigens delivered through the receptor DCIR were found to be efficiently cross-presented to memory T cells. A concentration of anti–DCIR.doc-coh.FluMP complex mAb as low as 80pM was sufficient to induce significant expansion of FluMP-specific CD8+ T cells. This represents an approximately 100-fold enhancement of the intrinsic antigen presentation capacity. Such an effect has been reported earlier in murine studies with fusion proteins of DEC-205.16 A remarkable finding is that all the tested DC subsets were found to be targeted by the DCIR fusion proteins and induce a specific CD8+ T-cell response. Indeed, at variance with previous studies,33,34 anti-DCIR was able to efficiently deliver antigens to blood pDCs as well as epidermal LCs and allowed development of specific CD8+ T-cell responses. Antigen delivery through DCIR not only allowed the expansion of memory FluMP-specific CD8+ T cells but also resulted in the priming of naive CD8+ T against the melanoma differentiation antigen MART-1 and the HIV gag p24 protein. Furthermore, DCIR-mediated response was broad and specific to multiple epitopes of MART-1 protein. Recently a monoclonal antibody to DCIR2 was found to preferentially target the CD8α−DCIR2+ DC subset in mice, resulting in preferential induction of major histocompatibility complex (MHC) class II–restricted reactivation of CD4+ T cells.28 Likewise, anti-DCIR was shown to target KLH to human pDCs, thereby allowing proliferation of a KLH-specific CD4+ T-cell line.34 Our study now demonstrates that DCIR is also a powerful means to establish and reactivate antigen-specific CD8+ T-cell responses. All DCs, including skin LCs, blood mDCs, and pDCs, were efficient at cross-presenting antigen delivered through DCIR. All together, these data indicate that antigen delivery through DCIR, like DEC-205, can result in the induction of both MHC class I– and MHC class II–restricted immune responses.
Because future vaccines will probably be composed of these targeted antigens together with an adjuvant, we have also addressed whether microbial (TLR) stimulation would improve DCIR-mediated antigen cross-presentation by mDCs. Among all the tested activators, TLR7/8 agonist proved most effective in this process and induced the highest proliferation of antigen-specific effector CD8+ T cells in both primary and secondary responses, particularly in the case of primary responses, when delivered together with a CD40 signal. In addition to amplifying the specific CD8+ T-cell response, TLR7/8 triggering also affected the quality of the induced T cells by promoting high expression of IFN-γ, and effector molecules, such as Granzyme A, Granzyme B, and perforin. Moreover, whereas DCIR-targeted IFN-α DCs, which were activated with CD40L, primed CD8+ T cells to produce high amounts of type 2 (IL-4, IL-5, and IL-13) cytokines, TLR7/8 activation shifted the balance toward a type 1 response, which is associated with elevated production of proinflammatory cytokines IFN-γ and TNF-α and markedly reduced levels of IL-4, IL-5, and IL-13. Our findings are in accordance with previous observations attributing enhanced protein-based vaccine-induced T-cell responses to TLR7/8 triggering.46,47 In a nonhuman primate model of SIV, a protein antigen delivered along with a TLR7/8 ligand promoted the induction of a Th1 response, as well as the enhanced and durable expansion of multifunctional CD8+ T cells. These cells, which simultaneously produce IFN-γ, TNF-α, and IL-2, are abundant in HIV nonprogressor relative to progressors and associated with long-term protection. Therefore, combining TLR7/8 agonist with a targeted protein-based vaccine should be beneficial to treat chronic diseases in which CD8+ T cells are mediating effector functions.
In our current experimental setting, we cannot exclude the possibility that the TLR agonists we used had also a direct effect on the CD8+ T cells. As some studies previously demonstrated, direct TLR triggering on CD4+ T cells can induce up-regulation of costimulatory molecules and modulate their proliferation.48,49 Nevertheless, it has been demonstrated that the most effective multifunctional CD8+ T-cell response is induced when the antigen is fused to the adjuvant, rather than delivered separately,46 a finding that might explain the lack of CD8+ T-cell responses in melanoma patients vaccinated with NY-ESO and topical TLR7 agonist.50 Thus, our own data support the approach of conjugating TLR agonists to a targeting antigen vaccine, such as DCIR, as the most efficient method to deliver an antigen and adjuvant directly to DCs. More studies, however, are required to formally conclude which activator or a combination of activators and which vaccine formulation will yield the most potent, long-lasting CD8+ T-cell responses in vivo.
In conclusion, targeting clinically relevant antigens through DCIR to various DC subsets will permit induction of strong cytotoxic CD8+ T-cell responses, which are essential for the prevention and treatment of chronic diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank E. Kowalski, S. Coquery, L. Walters, J. Shay, J. Plants, Dr C. H. Yu, F. Marches, M. Gallego, and S. S. Clayton for their help; Dr Ralph Steinman for kindly providing the HIV gag p24 component; Dr C. Harrod at the BIIR, Dr J. Stecher, J. Duncan, surgeons, and nurses at the Baylor Medical Center Plastic Surgery Department for providing access to skin samples; and Dr M. Ramsay, Dr W. Duncan, and C. Samuelsen for continuous support.
This work was supported by the Baylor Health Care Systems Foundation, the National Institutes of Health (RO-1 CA78846, RO-1 CA85540, PO-1 CA84512, U-19 AI-57234; J.B.), and a contract with the National Agency for AIDS Research (ANRS). J.B. holds the W.W. Caruth, Jr Chair for Transplantation Immunology Research. A.K.P. holds the Ramsay Chair for Cancer Immunology.
National Institutes of Health
Authorship
Contribution: E.K. designed and performed the research, analyzed results, made the figures, and wrote the manuscript; A.-L.F. contributed vital new reagents and made a figure; Y.C., J.-P.B., M.L., and L.T.-S. contributed vital technical assistance; S.Z. generated vital new reagents and performed experiments; A.O., O.A.-D., and P.K. generated vital new reagents; Y.R. contributed vital new reagents; A.K.P. designed the research; G.Z. designed the research, generated vital new reagents, and wrote the manuscript; and J.B. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jacques Banchereau, Baylor Institute for Immunology Research, 3434 Live Oak, Dallas, TX 75204; e-mail: jacquesb@baylorhealth.edu; and Gerard Zurawski, Baylor Institute for Immunology Research, 3434 Live Oak, Dallas, TX 75204; e-mail: gerardz@baylorhealth.edu.
References
Author notes
G.Z. and J.B. contributed equally to this study and are senior authors.


![Figure 2. Engineering and characterization of targeted proteins into DCIR mAb. (A) Five constructs are shown. (I) Diagram of mouse IgG1 cross-linked to the target antigen FluMP. (II-III) Diagram of chimeric mAbs (IgG4).doc conjugated to coh.antigen (FluMP [II] or MART-1 [III]). (IV-V) Diagram of chimeric fusion mAb IgG4-antigen (MART-1 [IV] or HIV gag p24 [V]). (B) Staining of HLA-A201–FluMP complexes on CD34+-derived DCs unpulsed (control DCs, gray histogram), or pulsed with 50nM DCIR-targeted FluMP. Cells were activated with 5 μg/mL anti-CD40 mAb (12E12, Baylor Research Institute; BIIR) and stained after 24 hours with phycoerythrin-labeled tetramerized anti–HLA-A201–FluMP Fab (M1D12).39 (C) Cross-presentation of FluMP to CD8+ T cells by autologous HLA-A201+CD34+–derived LCs that were cultured with 8nM (top panel) or 0.8nM (bottom panel) of anti–DCIR.doc-coh.FluMP or IgG4.doc-coh.FluMP conjugate mAbs. Dot plots show the proportions of HLA-A201–FluMP(58-66) peptide tetramer-positive CD8+ T cells after 10 days. (D) Proportions of HLA-A201–FluMP(58-66) tetramer-positive CD8+ T cells induced by DCs that were pulsed for 18 hours with 8nM anti–DCIR.doc-coh.FluMP or control IgG2a.doc-coh.FluMP conjugate mAbs, washed and cultured with autologous CD8+ T cells for 10 days. Graphs show the proportions of HLA-A201–FluMP(58-66) tetramer-positive CD8+ T cells, mean ± SD; n = 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/10/10.1182_blood-2010-01-264960/4/m_zh89991056860002.jpeg?Expires=1768385555&Signature=ptxfRjbQhO9QM8xoGpP6-GFV~4bCTP-8O4j8rn6oJo-meOEiilDrfn69bi1IDfius55mck3u2aD-b~Jt4mhPAVuBF0dKJaCbaUVe13ZayWxLjHvSw-WZ9uSsLpe-1rkRnfq2n7rrJcqECKstuIhnvXoGnjWiRvSwMnZH6hx-EYYx7tK~26jvQv4AFnO3H1FRG2Lnhoo~CXnPkh50I4d6Va4jy0yyXv6C5x708CETXXMegEopdxWjwwJ7hCHcmviggqQXenWw5FWaM1dX0eErORToRMrGJgnU7SAOkj3gETjxIYwS0ijtL26B9ETIA9~tXG~Z9aoeYFVGwwtIztnH9A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)