To the editor:
Alpha-thalassemia commonly results from deletions or point mutations in one or both alpha-globin genes, located on chromosome 16p13.3. Rarely, alpha-thalassemia is caused by deletions in a region, located 30 to 70 kb upstream of the alpha-globin genes, containing 4 remote, multispecies conserved sequences (MCS-R 1-4), required to regulate alpha-globin expression.1 Natural deletions in humans, analysis of interspecific hybrids, stable transfectants, and studies of transgenic mice indicate that deletion of MCS-R 2 leads to almost complete down-regulation of alpha-gene expression.2,3
Hemoglobin H (HbH) disease, the clinically significant intermediate form of alpha-thalassemia, is characterized by mild to moderate (sometime severe) microcytic, hypochromic hemolytic anemia, jaundice, hepatosplenomegaly, and occasionally mild thalassemia-like bone modifications. Most commonly HbH disease results from deletion or dysfunction of 3 of 4 alpha-globin genes, and rarely from deletions in the upstream regulatory region.2,4-6
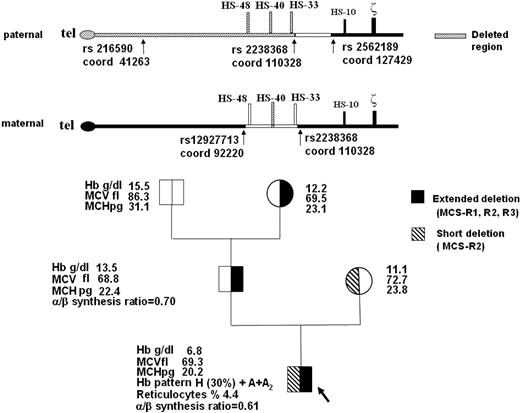
Here we describe a severe case of HbH disease in a 11-year-old Italian boy due to deletions of variable extent of both upstream regulatory regions, with all 4 downstream alpha-globin genes intact. The hematologic characteristics of the proband and his family are reported in Figure 1. The patient had moderate jaundice, marked hepatosplenomegaly, and mild but typical facial thalassemia-like modifications; he maintained the hemoglobin level between 6.0 and 8.0 g/dL and needed several red blood cell transfusions for worsening of anemia. The patient lacks MCS-R2 in both chromosomes, and MCS-R1 and MCS-R3 in one chromosome (Figure 1).
Family pedigree, hematologic data, and schematic representation of the telomeric region of paternal and maternal chromosome 16 (p16.3). The common deletion or nondeletion defects in the proband and in his parents, and the presence of hyper-unstable alpha-globin variants were excluded with conventional DNA analysis technique (GAP-PCR and alpha-gene sequencing). Multiplex ligation-dependent probe amplification (MLPA) of the extended alpha-globin gene cluster using a set of 25 probes covering a region of 170 kb in the alpha-cluster (MRC-Holland)4 detected the deletion of the 2 MCS-R2 region probes (coordinates ligation site 103553-54 and 103712-13, respectively) in homozygosity in the proband, suggesting the complete lack of MCS-R2 region, and in heterozygosity in his parents. In the proband and in his father, MLPA analysis revealed a deletion extending up to the first telomeric probe (coordinates ligation site 43321-22), located on POL3K gene, suggesting the removal of the telomere.10 Family segregation studies of SNPs along the telomeric region of chromosome 16 (coordinates from 41263 to 127429, according GenBank accession number AE006462.1) showed in the father an apparent homozygosity and absence of Mendelian segregation for rs216590 (coord 41263) and for rs2238368 (coord 110328) polymorphisms, whereas the rs2562189 (coord 127429) was heterozygous. These data are indicative of a deletion extending at least from the telomere to rs2238368 (coord 110328), which caused the loss of MCS-R1, MCS-R2, and MCS-R3 regions. We could not define the centromeric breakpoint of the deletion. The same deletion was identified in the paternal grandmother. In the mother, heterozygosity for rs12927713 (coord 92220) and rs22388368 (coord 110328) suggested a smaller deletion. The direct sequencing of the rearranged fragment, obtained with a GAP-PCR using 2 primers flanking the deletion, allowed us to exactly define the extension (3361 bp), the breakpoints (5′ at 103192/3 and 3′ at 106553/4 position), and the presence of 39 orphan nucleotides.
Family pedigree, hematologic data, and schematic representation of the telomeric region of paternal and maternal chromosome 16 (p16.3). The common deletion or nondeletion defects in the proband and in his parents, and the presence of hyper-unstable alpha-globin variants were excluded with conventional DNA analysis technique (GAP-PCR and alpha-gene sequencing). Multiplex ligation-dependent probe amplification (MLPA) of the extended alpha-globin gene cluster using a set of 25 probes covering a region of 170 kb in the alpha-cluster (MRC-Holland)4 detected the deletion of the 2 MCS-R2 region probes (coordinates ligation site 103553-54 and 103712-13, respectively) in homozygosity in the proband, suggesting the complete lack of MCS-R2 region, and in heterozygosity in his parents. In the proband and in his father, MLPA analysis revealed a deletion extending up to the first telomeric probe (coordinates ligation site 43321-22), located on POL3K gene, suggesting the removal of the telomere.10 Family segregation studies of SNPs along the telomeric region of chromosome 16 (coordinates from 41263 to 127429, according GenBank accession number AE006462.1) showed in the father an apparent homozygosity and absence of Mendelian segregation for rs216590 (coord 41263) and for rs2238368 (coord 110328) polymorphisms, whereas the rs2562189 (coord 127429) was heterozygous. These data are indicative of a deletion extending at least from the telomere to rs2238368 (coord 110328), which caused the loss of MCS-R1, MCS-R2, and MCS-R3 regions. We could not define the centromeric breakpoint of the deletion. The same deletion was identified in the paternal grandmother. In the mother, heterozygosity for rs12927713 (coord 92220) and rs22388368 (coord 110328) suggested a smaller deletion. The direct sequencing of the rearranged fragment, obtained with a GAP-PCR using 2 primers flanking the deletion, allowed us to exactly define the extension (3361 bp), the breakpoints (5′ at 103192/3 and 3′ at 106553/4 position), and the presence of 39 orphan nucleotides.
A series of naturally occurring human deletions that remove MCS-R elements, reducing the expression of the remote alpha-globin genes, have been identified.2-7 It has been shown experimentally that deletion of MCS-R2 alone is sufficient to down-regulate alpha-globin expression to less than 3% of normal, consistent with the notion that MCS-R2 is the most important regulatory element.8,9 In our patient, the homozygous deletion of MCS-R2 is associated with HbH disease, a phenotype less severe than expected from the predicted reduction of alpha-globin chain expression. Moreover, it should be pointed out that in the paternal chromosome, MCS-R1 and MCS-R3 are also deleted. Therefore, in this patient the residual production of alpha-globin chains is due only to the presence of paternal MCS-R4 and maternal MCS-R1, -R3, -R4, which seem therefore to play a significant role in the control of alpha-globin gene expression. The clinical findings of our patient (in particular the grade of anemia and percentage of HbH) are more severe compared with those in similar patients with deletions removing the upstream regulatory region.2 All of these patients with HbH disease have a combination of deletions of the upstream regulatory region with the common 3.7- or 4.2-kb alpha-globin gene deletion, whereas in our patient all 4 alpha-globin genes are intact.
In conclusion, the patient here described represents the first true human “knockout” MCS-R2 mutation, with a clinically relevant phenotype despite the presence of all 4 alpha-genes, but less severe than expected. Despite their rarity, these mutations should be investigated in the gene-mapping screening programs for alpha-thalassemia. This report adds significant information on the control of the alpha-gene cluster, proving that the complete loss of the major regulatory MCS-R2 element severely down-regulates the expression of alpha-globin genes but is not associated with a complete absence of alpha-chain production.
Authorship
Acknowledgments: The authors thank Laura Placido for editorial assistance. This study was supported by grants from L.R.11 1990 Regione Autonoma Sardegna.
Contribution: R.G. designed the research and drafted the manuscript; M.C.S. and M.E.P. performed the research, analyzed the data and drafted the manuscript; D.L. and R.C. performed the research and analyzed the data; and R.P. did the clinical study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Renzo Galanello, Ospedale Regionale Microcitemie, Via Jenner s/n, 09121 Cagliari, Italy; e-mail: renzo.galanello@mcweb.unica.it.