Abstract
Monosomal karyotype (MK), defined as 2 or more monosomies, or a single monosomy in the presence of structural abnormalities, has recently been reported as identifying a distinct subset of acute myeloid leukemia (AML) patients with an extremely poor prognosis. In an effort to confirm this observation, we analyzed the prognostic impact of MK in 1344 AML patients between the ages of 16 and 88 years treated on Southwest Oncology Group protocols. MK was found in 176 (13%) patients. The proportion of patients with MK increased with age, being present in 4% of patients age 30 or younger, but in 20% of those over age 60. Ninety-eight percent of MK cases were within the unfavorable cytogenetic risk category and comprised 40% of this group. The complete remission rate in patients with unfavorable cytogenetics without MK was 34% versus 18% with MK (P < .01). The 4-year overall survival of patients with unfavorable cytogenetics but without MK was 13% in contrast to a 4-year survival of only 3% with MK (P < .01). Thus, MK defines a sizeable subset of patients with unfavorable cytogenetics who have a particularly poor prognosis.
Introduction
Acute myeloid leukemia (AML) is a remarkably heterogeneous group of hematopoietic neoplasms, with distinct clinical, genetic and molecular features. Age at presentation and diagnostic karyotype are among the most important independent prognostic factors in adult patients with AML.1-3 There is general consensus that 3 distinct prognostic cytogenetic risk groups can be defined, where the presence of t(15;17)(q22;q12), t(8;21)(q22;q22) or inv (16)(p13.1q22)/t(16,16)(p13.1;q22) predicts a relatively favorable outcome and, conversely, that cases of AML where cytogenetic analysis shows inv(3)(q21q26.2)/t(3,3)(q21;q26.2), del(5q), -5, -7 or a complex karyotype with 3 or more abnormalities generally have a very poor prognosis.
A recent published report4 suggests that a distinct group of cytogenetic abnormalities, referred to as monosomal karyotype (MK), is able to further refine the cytogenetic classification in adult patients with AML under the age of 60 years and recent abstracts have confirmed this finding in younger and older patients.5-7 In the published report by Breems et al, which was limited to patients younger than age 60, AML patients with MK, defined as 2 or more distinct monosomies or a single monosomy in the presence of other structural abnormalities, had a 4-year overall survival (OS) of 4%, compared with a 21% 4-year OS in patients with other unfavorable karyotypes but without MK.4 Given that the outcomes of these patients depend not only on the clinical, genetic, and molecular characteristics of AML, but also on the chemotherapeutic regimens used for treatment, we investigated the prognostic impact of MK in young and elderly AML patients treated in North America under Southwest Oncology Group (SWOG) clinical trials.
Methods
Patients
A total of 1344 (48% of 2816 patients registered) previously untreated AML patients with available cytogenetic data at diagnosis were enrolled between 1986 and 2009 in 10 successive SWOG phase 2 or phase 3 clinical trials (S8600, S9031, S9034, S9126, S9333, S9500, S9617, S9918, S0106, S0112).8-15 Almost all were initially treated with standard induction therapy consisting of cytarabine and anthracycline. For patients achieving a complete remission, consolidation therapy varied based on protocol design. Only patients with complete and centrally reviewed cytogenetic studies were included in this analysis. Patients with the t(15;17)(q22;q12) were excluded. All participating patients gave informed consent prior to enrollment into the studies. The studies were approved by ethics committees of all participating institutions and conducted in accordance with the Declaration of Helsinki.
Cytogenetic studies
At diagnosis, samples from bone marrow aspirates were analyzed in SWOG-approved laboratories for cytogenetic abnormalities using standard culturing and banding techniques. Karyotype designation was in accordance to the most current version of the International System for Human Cytogenetic Nomenclature (ISCN) at the time of the cytogenetic analysis.16 Abnormalities were considered clonal and therefore mentioned in the karyotype when at least 2 metaphases had the same aberration in the case of either a structural abnormality or an additional chromosome. If there was loss of a chromosome, it had to be present in at least 3 metaphase cells to be considered a clonal monosomy. Cytogenetic abnormalities were grouped according to published criteria adopted by SWOG as favorable, intermediate, unfavorable, and unknown.2 The following abnormalities were tallied by chromosome: loss of a chromosome (monosomy), extra copy of a chromosome (trisomy or tetrasomy), structural cytogenetic abnormalities (deletion, inversion translocation, or addition of chromosomal material of unknown origin), marker chromosomes, and ring chromosomes. Also, the following specific cytogenetic abnormalities were tallied separately: inv(3)(q21q26.2)/t(3,3)(q21;q26.2), t(6;9)(p23;q34), t(6;11)(q27;q23), t(11;19)(q23;p13.1), t(11;19)(q23;p13.3), t(9;22)(q34;q11.2), del(5q), del(7q), del(9q), del(12p), abnormalities of 3q, 9q, 11q, 20q, 21q, 17p, and t(11q23)(MLL rearrangements). The karyotype analysis was based on 20 or more metaphase cells for more than 90% of patients included in this analysis.
Statistical analysis
The Fisher exact test was used to test associations between categorical variables (eg, to compare complete response across SWOG cytogenetic risk groups). OS was measured from the date of entry onto the patient's clinical trial until death from any cause, with observation censored at the date of last contact for patients last known to be alive. The Kaplan-Meier method was used to estimate survival curves and 4-year OS.17 Kaplan-Meier estimates were only calculated for subgroups with more than 4 patients. Log-rank tests were used to assess differences between survival curves. A Cox regression analysis was used to assess regression models for OS.18
Results
Incidence of cytogenetic abnormalities in AML patients
Initially, the frequency of distinct cytogenetic abnormalities in all 1344 AML patients (between 16 and 88 years of age) managed on SWOG protocols was investigated. A normal karyotype was detected in 41% of patients (n = 552 patients), while 792 patients (59%) had an abnormal karyotype. Ninety-five patients presented with inv(16)(p13.1q22) or t(16;16)(p13.1;q22). Seventy patients had a t(8;21); 60 of these patients had the abnormality without del(9q) and without a complex karyoptype. Single and multiple autosomal monosomies were noted in 95 patients (7%) and 129 patients (10%), respectively. A complex karyotype (CK), defined as 3 or more chromosomal abnormalities, was noted in 244 patients (18%). In total, 180 patients (13%) fulfilled criteria for a MK (as defined by Breems et al4 ); 129 patients had 2 or more monosomies (± other structural abnormalities) and the remaining 51 patients had a single autosomal monosomy plus at least 1 other structural abnormality. Of the 180 patients with MK, 2 had favorable cytogenetics, 2 had unknown cytogenetic risk, and the remaining 176 patients had unfavorable cytogenetics. For the purposes of this study, we excluded the 4 patients with MK without unfavorable cytogenetics, and focused our analyses on the remaining 1340 patients.
Baseline characteristics of patients with MK
The proportion of patients with MK+ AML increased with age. Whereas only 4% of patients younger than age 30 had a MK, the fraction increased to 20% for those over age 60 (see Table 1). No sex distribution differences were noted between AML patients in the MK and non-MK groups (p = 0.37). Virtually all patients with MK (98%) fell into the unfavorable risk group by SWOG criteria, and 95% had a complex karyotype. Monosomies of 5 and 7 were the most common, with monosomy 5 present in 53 cases of MK (30%) and monosomy 7 in 68 (39%). However, almost half of the MK cases (43%) had neither monosomy 5 nor 7. Among MK+ patients, single monosomies were detected in all chromosomes with the exception of chromosomes 1, 2, 3, 4, 9, 10, 14, 19, and 21. All chromosomes were affected among patients with multiple monosomies.
Prognostic impact of MK in AML
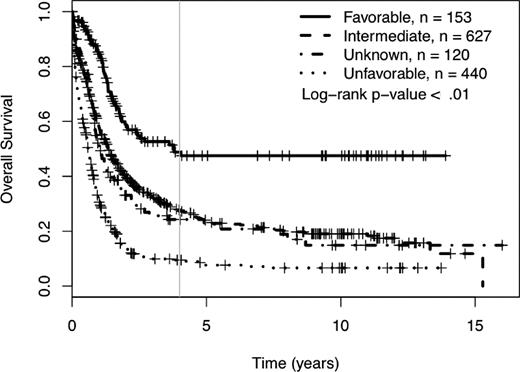
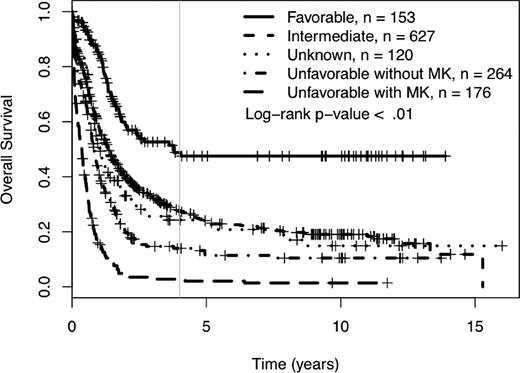
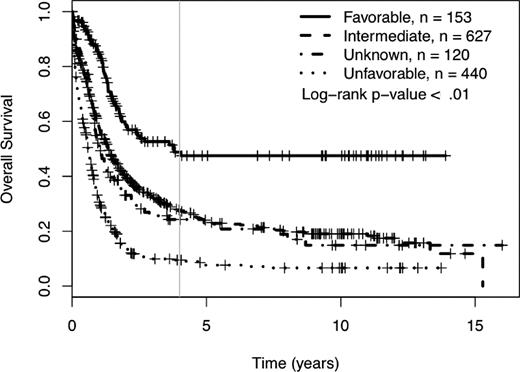
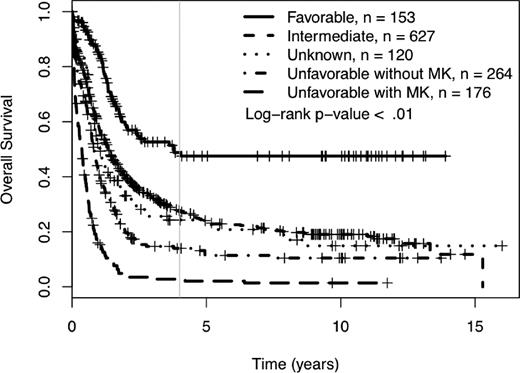
We determined the impact of MK on complete remission (CR) rates and OS of patients treated in SWOG protocols. Using SWOG's conventional cytogenetic risk categorization of AML, the complete remission rates were 71% for favorable risk patients, 46% for intermediate risk patients, 42% for those in the unknown category, 34% for those with unfavorable cytogenetics without MK, and 18% for those with unfavorable cytogenetics with MK (P < .01; supplemental Table 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The 4-year OS was 49% for patients with favorable cytogenetics, 27% for those with intermediate cytogenetics, 24% for those with unknown cytogenetic risk, and 9% for those with unfavorable risk (see Table 1 and Figure 1). As shown in Table 1 and Figure 2, among patients with unfavorable cytogenetics, those who were MK− had a 4-year OS of 13% (95% CI = 10, 20), while those who were MK+ had a 4-year OS of 3% (95% CI = 1, 8; log rank P < .01).
Four-year overall survival Kaplan-Meier plots according to SWOG conventional cytogenetic risk category definitions. Crosses mark censored observations. A gray vertical line marks 4 years.
Four-year overall survival Kaplan-Meier plots according to SWOG conventional cytogenetic risk category definitions. Crosses mark censored observations. A gray vertical line marks 4 years.
Four-year overall survival Kaplan-Meier plots according to revised cytogenetic risk category definition. Monosomal karyotype (MK) refers to ≥ 2 autosomal monosomies or 1 autosomal monosomy with at least 1 structural abnormality. Crosses mark censored observations. A gray vertical line marks 4 years.
Four-year overall survival Kaplan-Meier plots according to revised cytogenetic risk category definition. Monosomal karyotype (MK) refers to ≥ 2 autosomal monosomies or 1 autosomal monosomy with at least 1 structural abnormality. Crosses mark censored observations. A gray vertical line marks 4 years.
Next, we determined the prognostic impact of MK in different cohorts of patients. CR rates for patients age 30 or younger, 31 to 40, 41 to 50, 51 to 60 and older than 60 without MK were 54%, 60%, 67%, 52%, and 27%, respectively, and with MK were 50%, 27%, 14%, 24%, and 13%, respectively. Thus, for every subset except those under age 30, MK was associated with a lower CR rate (although only 4 patients were in the MK+, age 30 or younger cohort). The association of MK and survival is shown in Table 1. MK+ AML was particularly unfavorable in older patients. The 4-year OS among the 156 patients age 41 or older with MK+ AML was less than 1%, compared with 21% for MK− AML in this age group (log rank P < .01). Among patients younger than age 41, the small group with MK+ AML likewise did more poorly than those MK− disease, but the impact wasn't quite as striking; 4-year OS for patients under age 41 with MK− AML was 40% (95% CI 35, 47) and for MK+ AML was 22% (95% CI 8, 57; log-rank P < .01). For several of the cytogenetic subgroups included in the unfavorable risk category, including monosomy 7 (p = 0.06), inv(3)/t, (3,3) del(5q) (p = 0.50) and t(9;22) (p = 0.67), presence or absence of MK did not appear to significantly influence prognosis. The subgroup of unfavorable cytogenetics where presence or absence of MK appeared to have the biggest impact was in those patients with del(7q) who had a 4-year OS of 29% if MK−, but 0% if MK+ (log rank P < .01). Among those who were classified as unfavorable based on having complex cytogenetics, the 4-year OS was 13% if MK−, and 3% if MK+ (log rank P < .01). There was no apparent difference between those defined as MK+ based on having 2 or more monosomies compared with those with a single monosomy plus additional structural change(s).
A Cox multivariate analysis was performed taking into account age, presence of complex karyotype, and presence of a MK. All 3 variables were significantly associated with OS: MK hazard ratio (HR) 2.1 (P < .01), complex HR 1.5 (P < .01), and age HR 2.4 (P < .01; supplemental Table 3).
Prognostic impact of single monosomies, other abnormalities, and age on OS
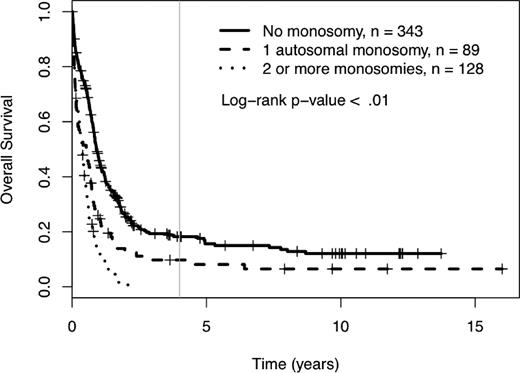
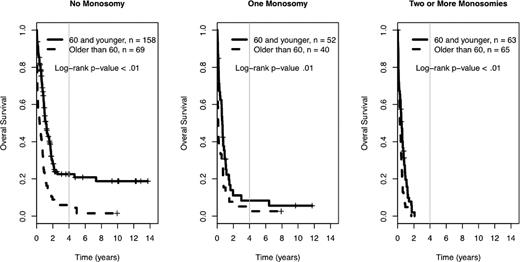
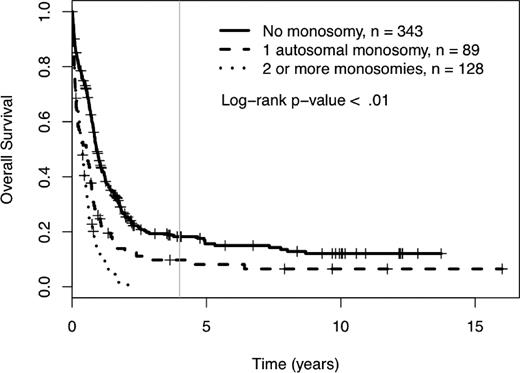
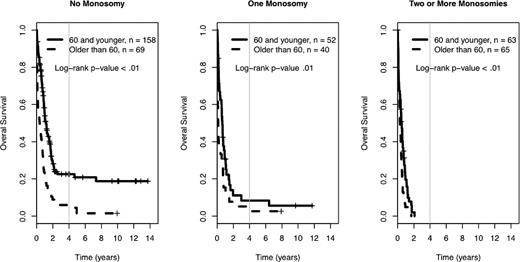
Among the 560 AML patients with unknown or unfavorable cytogenetic risk (in the cohort of 1340 patients), 217 patients had loss of 1 or more autosomal chromosomes (this includes the 176 already defined as MK+). Sixty-five patients presented with monosomies of sex chromosomes (-X or -Y). The most common single autosomal monosomy was loss of chromosome 7 (n = 51); no other single autosomy was noted in more than 6 patients in this cohort (chromosome 17, n = 6). In patients with multiple monosomies, the most commonly affected chromosomes were 17 (n = 60), 7 (n = 51), 5 (n = 50), and 18 (n = 45). The presence of single autosomal monosomies identified a cohort of patients with a poor prognosis, where the 4-year OS of these 89 patients was 10% (95% CI 5%, 19%). Similarly, the presence of multiple monosomies (128 AML patients) also predicted an extremely poor prognosis with a 4-year OS of 0% (see Figure 3, P < .01). The only monosomy (single or multiple) that had non-zero 4-year survival and more than 4 patients in the category was the group of patients with a single monosomy 7. Their 4-year survival rate was 1% (95% CI 0%, 9%). The presence of 1 and 2 or more monosomies also predicted a significantly worse prognosis, compared with patients with no monosomies, when the analysis was restricted to patients older than 60 years of age (log-rank P < .01, supplemental Figure 2). In comparison, the 384 patients with unknown or unfavorable risk and nonmonosomal cytogenetic abnormalities had a 4-year OS of 18% (95% CI 14%, 24%). A higher percentage of patients older than 60 had at least 1 automosal monosomy compared with younger patients (18% vs 30%, P < .01). Also, age greater than 60 was associated with a significantly worse OS, independent of number of monosomies (see Figure 4).
Four-year overall survival Kaplan-Meier plots in relation to number of autosomal chromosomal monosomies among patients with unfavorable karyotypes. Crosses mark censored observations. A gray vertical line marks 4 years.
Four-year overall survival Kaplan-Meier plots in relation to number of autosomal chromosomal monosomies among patients with unfavorable karyotypes. Crosses mark censored observations. A gray vertical line marks 4 years.
Prognostic impact of age in the 4-year overall survival Kaplan-Meier plots in relation to number of autosomal chromosomal monosomies among patients with unfavorable karyotypes. Crosses mark censored observations. A gray vertical line marks 4 years. All log-rank P < .01.
Prognostic impact of age in the 4-year overall survival Kaplan-Meier plots in relation to number of autosomal chromosomal monosomies among patients with unfavorable karyotypes. Crosses mark censored observations. A gray vertical line marks 4 years. All log-rank P < .01.
Discussion
The presence of multiple autosomal monosomies or a single autosomal monosomy in the presence of 1 of more structural abnormalities has been used to define a new subgroup of cytogenetic abnormalities (MK) with very unfavorable prognosis in AML patients younger than 60 years of age.4 This initial description has been confirmed in several other abstract presentations.5-7 The impact of MK on treatment outcome and MK's relationship with age at diagnosis in patients with AML enrolled in North American cooperative group trials has not been defined previously.
Initially, we determined the incidence and baseline characteristics of patients with MK treated on SWOG trials. MK was identified in 13% of patients and occurred more frequently with increased age at diagnosis, being present in only 4% of patients age 30 or younger, but in 20% of those older than age 60. Ninety-eight percent of MK cases were seen in patients with unfavorable cytogenetics, and MK accounted for 40% of patients with unfavorable cytogenetics. Overall, MK was associated with a very poor prognosis and effectively divides patients with unfavorable cytogenetics into 2 groups, those who are MK− with a 4-year survival of 13% and those who are MK+ with a 4-year survival of 3% (P < .01; Figure 2). This difference could not be attributed to the older age distribution of MK+ patients. Age at diagnosis continues to be an important independent prognostic factor in AML, as the negative impact of MK in younger patients (< 40 years) was more modest.
The negative impact of a single autosomal monosomy and the additional detrimental effect of multiple monosomies on OS were confirmed in our cohort of patients. As demonstrated previously,4 the presence of any autosomal monosomy, not only -5, -7 and -17, is associated with a dismal prognosis. Age older than 60 years further worsened the prognostic impact of single or multiple automosal monosomies in our cohort (Figure 4). In our cohort of patients, presence or absence of MK did not affect the already poor outcome of patients within several of the cytogenetic subgroups in the unfavorable risk category, including monosomy 5/7, inv(3)/t(3:3), and del(5q), but did influence the outcome of those with del(7q) or complex karyotypes (Table 1).
Diagnostic karyotype continues to emerge as the most significant prognostic factor in adult patients with AML.19 Several attempts have been made recently to refine the pretreatment cytogenetic classification of adult patients with AML.4-7,19 In agreement, our results demonstrate that presence of MK identifies AML patients with a very unfavorable prognosis. Although this study was not designed to test the utility of any specific therapeutic outcome, the only MK+ patients (n = 2) we were able to identify who were alive and disease-free more than 6 years from diagnosis were treated with allogeneic hematopoietic cell transplantation (data not shown).
The results presented here have several implications. First, future prospective or retrospective studies analyzing results in patients with unfavorable risk should take into account, either by stratification or other means, potential differences in those patients who are MK+ versus those who are MK−. While age and unfavorable or complex cytogenetics are extremely important prognostic indicators, the presence or absence of the MK phenotype adds important information. Second, the extremely poor prognosis in patients with MK+ disease should be kept in mind when considering therapeutic options for such patients. The low likelihood of complete remission (18%) combined with only a 3% probability of survival at 3 years suggests that palliative or experimental therapies should be strongly considered for this group of patients.
In summary, our analysis of AML patients treated in SWOG-sponsored clinical trials confirms that the presence of MK divides patients with unfavorable cytogenetics into 2 distinct groups. We further define the impact of the presence or absence of MK in various subgroups of patients and show that the impact of MK is further influenced by the age of the patient. Finally, we confirm the importance of any monosomy on prognosis, and that multiple monosomies further worsen prognosis.
The online version of this article contain a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors are grateful to the Southwest Oncology Group AML Committee for access to the SWOG AML trials database.
Authorship
Contribution: B.C.M., M.O., and F.R.A. reviewed and analyzed the data and wrote the manuscript; and M.F. and D.R. contributed to reviewing the data and writing and editing the manuscript. All authors had access to primary clinical trial data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruno C. Medeiros, MD, Stanford Cancer Center, 875 Blake Wilbur Dr, Rm 2329, Stanford, CA 94305-5821; e-mail: bruno.medeiros@stanford.edu.