Abstract
The pseudo tyrosine kinase receptor 7 (PTK7) is an orphan tyrosine kinase receptor assigned to the planar cell polarity pathway. It plays a major role during embryogenesis and epithelial tissue organization. Here we found that PTK7 is also expressed in normal myeloid progenitors and CD34+ CD38− bone marrow cells in humans. We performed an immunophenotyping screen on more than 300 patients treated for hematologic malignancies. We demonstrated that PTK7 is expressed in acute myeloid leukemia (AML) and is mostly assigned to granulocytic lineage differentiation. Patients with PTK7-positive AML are more resistant to anthracycline-based frontline therapy with a significantly reduced leukemia-free survival in a multivariate analysis model. In vitro, expression of PTK7 in cultured leukemia cells promotes cell migration, cell survival, and resistance to anthracycline-induced apoptosis. The intracellular region of PTK7 is required for these effects. Furthermore, we efficiently sensitized primary AML blasts to anthracycline-mediated cell death using a recombinant soluble PTK7-Fc protein. We conclude that PTK7 is a planar cell polarity component expressed in the myeloid progenitor compartment that conveys promigratory and antiapoptotic signals into the cell and that represents an independent prognosis factor of survival in patients treated with induction chemotherapy.
Introduction
Epithelial tissues are organized by apicobasal polarity and planar cell polarity (PCP). Apicobasal polarity takes place after attachment of epithelial cells to the substratum and seals adjacent cells to each other. PCP organizes subsequently orientation of apical structures and cohorts of cells within the plane of the epithelial monolayer.1 Molecular mechanisms supporting epithelial cell polarity are organized by evolutionarily conserved polarity proteins that also act in nonepithelial polarized cells, such as neurons, endothelial cells, and lymphocytes.2-4 First described in Drosophila melanogaster, a set of PCP genes has been now characterized in vertebrates5 and includes scribble, vangl2, and ptk7. Vangl2 and Scribble are required for cell migration of neuroepithelial cells6 and epithelial cells.7,8 They also have been implicated in dissemination of cancer cells.9-12
The protein PTK7, also known as CCK4,13-15 is an evolutionarily conserved receptor tyrosine kinase (RTK)–like molecule encoded by a gene located on human chromosome 6 (6p21.1).16 PTK7 includes an extracellular region with 7 immunoglobulin domains, a single transmembrane region, and a cytoplasmic region harboring a defective tyrosine kinase domain. No PTK7 ligand has been identified yet. Because of the lack of catalytic activity, PTK7 may act as a coreceptor like other pseudo-kinases.17 Data obtained in Xenopus suggest that PTK7 interacts with the Frizzled-Dishevelled complex at the plasma membrane.18 Recently, PTK7 was shown to modulate vascular endothelial growth factor-induced endothelial cell migration19 and to be a marker of Recent Thymic Emigrant lymphocytes,20 demonstrating that its function is not restricted to epithelial tissues.
As the role of PCP in the hematopoietic system and hematologic malignancies has been poorly investigated, we screened various cell types for PTK7 expression and found that the protein is expressed in human hematopoietic progenitors and is predominantly a myeloid marker. Acute myeloid leukemia (AML) patients have a clonal expansion of myeloid progenitors, and one subgroup expresses PTK7 at the cell surface. PTK7-positive AML patients are more resistant to anthracycline-based frontline therapy with a significantly reduced relapse-free survival (RFS). In vitro experiments done with cultured leukemic cell lines and AML patient cells show that PTK7 plays a role in cell migration and that it is endowed with antiapoptotic functions. These findings demonstrated, for the first time, the implication of PTK7 in AML and stressed the interest of studying this pathway in hematologic malignancies.
Methods
Cell lines and cultures
The following cell lines were used in this study: Baf3, HL60, KG-1A, U937, Kasumi, K562, TF1, Jurkat, NALM6, and RAJI. All cell lines were purchased at the ATCC. Cells were cultured at 37°C in a humidified atmosphere and supplemented with 5% CO2. Culture medium used was RPMI 1640 supplemented with 10% fetal calf serum (FCS, Eurobio), L-glutamine 2mM (Invitrogen), and 20 U/mL penicillin-streptomycin (Invitrogen). Medium was supplemented with interleukin-3 (IL-3, 10 ng/mL) for the Baf3 cell line and granulocyte-macrophage colony-stimulating factor (10 ng/mL) for TF1 and Kasumi cell lines. Geneticin 1 mg/mL (Invitrogen) was added in transfected Baf3 cell cultures. Puromycin 1.3 μg/mL (Sigma-Aldrich) was added in the TF1 cell line transfected with pSuper vector.
Stable Baf3-PTK7 and Baf3 control cells were generated using electroporation (Genepulser system, Bio-Rad) with a mock pcDNA3 vector or with the pcDNA3-human PTK7-Flag construct already described by Shin et al.19 We used directed mutagenesis to create a pcDNA3 vector expressing PTK7 deleted of its intracellular kinase domain (PTK7.ΔKD). This construct was also transfected in the Baf3 cell line. Down-regulation of PTK7 expression was performed using siRNA specific for PTK7 (Dharmacon RNA Technologies) and pSuper shRNAs directed against PTK7. The recombinant PTK7-Fc protein that composes the extracellular region of PTK7 (soluble PTK7) fused to the Fc region of IgG1 was produced in COS cells using a protocol already used for recombinant Nectin4-Fc.21 Of note, both Baf3 and TF1 cell lines used for the study do not express multidrug resistance phenotype (data not shown).
Patient samples and clinical data
Bone marrow and peripheral blood samples were collected from normal donors and patients. A written informed consent was obtained for the use of biologic materials and relevant clinical data in accordance with the Declaration of Helsinki. The study was approved by Institut Paoli-Calmettes Internal Review Board. The cohort of patients presented is mostly constituted of consecutive patients and reflects the accrual of our center over the past 4 years. To evaluate the risk of potential patient selection bias, we compared the clinical/biologic characteristics and outcome between the study cohort and the patients treated in our institution during the previous 3 years (supplemental data, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). No significant differences were found.
Mononuclear cells were isolated from fresh samples using a Ficoll-Hypaque gradient method. Cells were immediately used or frozen until use in FCS, 10% dimethyl sulfoxide.
Clinical data and common biologic criteria (blood cell counts, bone marrow morphology, cytogenetics, immunophenotype, and molecular markers) were collected from the Biopathology Department of Institut Paoli-Calmettes, following World Health Organization and European LeukemiaNet recommendations22,23 (supplemental Table 1). Patients were treated with induction chemotherapy (supplemental Table 2, details of this subpopulation) using a standard 3 + 7 regimen (daunorubicin 60 mg/m2 days 1-3 and cytarabine 200 mg/m2 days 1-7, 100 mg/m2 for patients older than 65 years). Patients younger than 65 years with persistent disease at day 15 were further treated using cytarabine 1000 mg/m2 days 15 to 18 and daunorubicin 35 mg/m2 days 15 and 16. Response was evaluated according to International Working Group recommendations.24 Consolidation chemotherapy included intermediate- to high-dose cytarabine cycles. All patients with intermediate- or high-risk disease were eligible for genoidentical or unrelated allogeneic stem cell transplantation up to 65 years. In patients older than 65 years, maintenance therapy using low-dose cytarabine, 6-mercaptopurine, and methotrexate was used. Follow-up was updated in October 2009.
Flow cytometric analysis
For experiments using primary AML blasts, red cells were lysed before analyses (Versalyse, Beckman-Coulter). We used a FACSCANTO II flow cytometer (BD Biosciences) and FACSDIVA software (BD Biosciences) for acquisition and analysis. A 5-color multiparametric protocol was used for all experiments. Gating was systematically performed on blast population using CD45dim, SSClow gate. Mean fluorescence intensity (MFI) of gated cells was acquired for each fluorescence and compared with isotypic control. MFI ratio was defined as the fold increase between MFI (isotypic control) and MFI (antibody of interest). Positivity was defined by an MFI ratio of 5-fold in at least 20% of the gated cells. For the screening, we used an anti–PTK7-PE antibody (Miltenyi Biotec). The complete panel of antibodies used for this study is described in supplemental data.
Biochemistry
The following antibodies were used in this study: rabbit polyclonal anti-PTK7 antibody,19 anti-cKit antibody (R&D Systems), anti-XIAP antibody (Cell Signaling Technology), anti-MCL1 antibody (CST), antitubulin antibody (CST), antiphosphotyrosine antibody (4G10, Millipore), and anti-FLAG antibody (Sigma-Aldrich).
For immunoprecipitation assays, 500 μg of protein was used for each experiment. Lysates were incubated with the appropriate antibody for 2 hours at 4°C; then protein G-Sepharose 4 Fast Flow beads (GE Healthcare) were added and incubated 1 hour at 4°C. Beads were washed in lysis buffer, and then sample buffer was added. Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis or stored at −20°C until further use.
Molecular biology
Total RNA was isolated using RNeasy microkit (QIAGEN) and then retro-transcribed and amplified using SuperSCRIPT-II RT-PCR system (Invitrogen). Polymerase chain reactions (PCRs) were performed in a DYAD thermo cycler (MJ Research) for 35 cycles using the following primers: PTK7 set 1 (forward: 5′-atgctggtgtctacacctg-3′; reverse: 5′-ctacaagatgatccagacca-3′), PTK7 set2 (forward: 5′-cctagcagagattgaagaca-3′; reverse: 5′-gagatccaagaagaagtggc-3′). After separation of PCR products by agarose gel migration, each band was sequenced (GATC Biotechnology).
Migration assays
A Transwell system (Corning Life Sciences) was used to investigate migration potential of PTK7. After 1 hour in serum-free media (SFM), 5 × 104 (96-well plate) to 2 × 105 (24-well plate) cells were suspended in 50 to 100 μL of SFM and were loaded in 5-μm pore, fibronectin-coated transwell insert (fibronectin 40 μg/mL, Sigma-Aldrich, 50 μL/well, incubated overnight). Wells were incubated 1 hour with phosphate-buffered saline + 2% bovine serum albumin before the experiment. The same number of cells was plated in a distinct well to be used as control for migration. The lower chamber was filled with SFM supplemented with different chemoattracting reagents: control, stromal-derived factor-1 (SDF-1α, 100 ng/mL, R&D Systems), 250 ng/mL stem cell factor (SCF, PeproTech), and 20% FCS (Eurobio). Plates were then incubated 4 hours at 37°C, 5% CO2. All experiments were performed in duplicates. The migration ratio was estimated using a Celltiter-Glo luciferase assay (Promega) and was defined as the relative light unit ratio between migration well and control well. The detailed methodology was previously reported by Restouin et al.25
Cell survival and proliferation assays
To investigate the potential role of PTK7 in cell survival, a total of 1 × 104 cells in 100 μL were plated in a 96-well plate and incubated 48 hours at 37°C, 5% CO2. The number of living cells was evaluated using Celltiter-Glo luciferase assay. Experiments were realized in triplicate, and results were expressed as a ratio of relative light unit between experimental wells and control wells. Experimental conditions were defined as follows: addition of growth factors versus SFM, fibronectin-coated wells versus uncoated wells.
Tritiated thymidine proliferation assay was used to determine cell proliferation. Briefly, cells were starved overnight and then put in complete media in triplicate wells. After 48 hours, 100 μCi of tritiated thymidine (GE Healthcare) was added in each well, and cells were incubated 24 hours. Incorporation of tritiated thymidine was evaluated using a Matrix 9600 counter (Packard).
Apoptosis assays
Spontaneous and doxorubicin-induced apoptosis was evaluated using 2 methods: (1) a flow cytometric quantification of annexin V externalization (annexin V FITC staining); and (2) activity of effector caspases (caspases 3 and 7) was measured using a luciferase reporter assay (CaspaseGlow assay, Promega) following the manufacturer's recommendations.
Statistical analyses
Data were summarized by frequency and percentage for categorical variables. For continuous variables, the median and range were computed. All results are presented with their 95% confidence intervals (CIs). Statistical tests were 2-sided (Student t test, χ2 method). To investigate the association between continuous variables and categorical variables, univariate statistical analyses were performed using a nonparametric Wilcoxon rank-sum test or Kruskal-Wallis rank-sum test when appropriate. Survival rates were estimated by the Kaplan-Meier method.26 Overall survival (OS) was defined by the time interval from the date of the AML diagnosis until death from any cause with observation ending at the date of last contact for patients known to be alive. The RFS was defined as the time from the date of complete remission to relapse. Death from other causes was considered as a competitive risk. Multivariate models were constructed using a stepwise selection and included all variables with P value less than .15 in univariate analysis. Patients without event were censored at the date of the last follow-up. Follow-up of the patients treated with allogeneic hematopoietic stem cell transplantation was censored at the time of transplantation. Forest plot analysis was used for subgroup analysis of OS and leukemia-free survival (LFS). The significant results that are presented here were checked using the interaction test method.
Statistical analyses were performed using R.2.10.0 (R Development Core Team) and SAS Version 9.1 (SAS Institute Inc) software.
Results
PTK7 is a hematopoietic receptor expressed in normal CD34+ cells and primary AML blasts
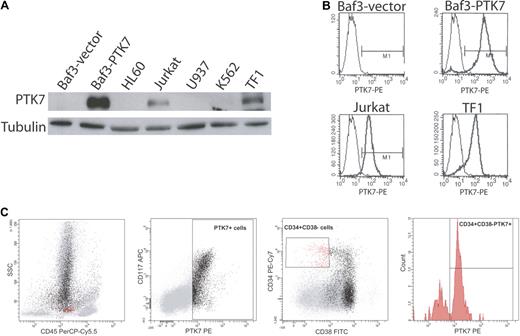
We screened cell lines of various origins for expression of PTK7 by Western blot and flow cytometry. As control, we used the murine PTK7-negative Baf3 cell line in which we expressed a full-length version of human PTK7 (Figure 1A-B). As expected, PTK7 is expressed in human colon cancer cells, such as Caco2 cells,14 and in breast cancer cell lines (data not shown). Unexpectedly, PTK7 is also expressed in myeloid (TF1) and lymphoid (Jurkat) cell lines, as evidenced by Western blot (Figure 1A), flow cytometry (Figure 1B), and reverse-transcribed PCR (supplemental Figure 1). We next evaluated the expression of PTK7 by flow cytometry on normal donor peripheral blood (n = 4) and bone marrow samples (n = 5; Figure 1C). There was no significant expression of PTK7 on mature PBMCs (data not shown), whereas PTK7-positive cells were detected in the bone marrow (4%–9% of total cells). Five-color flow cytometric analysis showed that PTK7 was expressed from CD34+ CD38− progenitors (mean frequency of CD45dim CD34+ CD38− PTK7+ cells = 64%) to committed myeloid progenitors. The expression of PTK7 is lost during monocytic and erythroid differentiation, and there was no significant expression on lymphoid committed progenitors or on the common subsets of mature lymphoid cells (data not shown). Thus, PTK7 appears to be a rather specific marker of myeloid progenitors within the hematopoietic compartment.
Expression of PTK7 in leukemia cell lines and in normal donor bone marrow. (A) Western blot analysis of leukemic cell lysates using a polyclonal anti-PTK7 antibody. These data were confirmed by flow cytometry using a monoclonal anti-PTK7 PE antibody (B). (C) Flow cytometric data on a normal donor marrow. PTK7+ cells are in black, and CD45dim CD34+ CD38− cells are in red. CD45dim CD34+ CD38− PTK7+ cells represent 65% of this population.
Expression of PTK7 in leukemia cell lines and in normal donor bone marrow. (A) Western blot analysis of leukemic cell lysates using a polyclonal anti-PTK7 antibody. These data were confirmed by flow cytometry using a monoclonal anti-PTK7 PE antibody (B). (C) Flow cytometric data on a normal donor marrow. PTK7+ cells are in black, and CD45dim CD34+ CD38− cells are in red. CD45dim CD34+ CD38− PTK7+ cells represent 65% of this population.
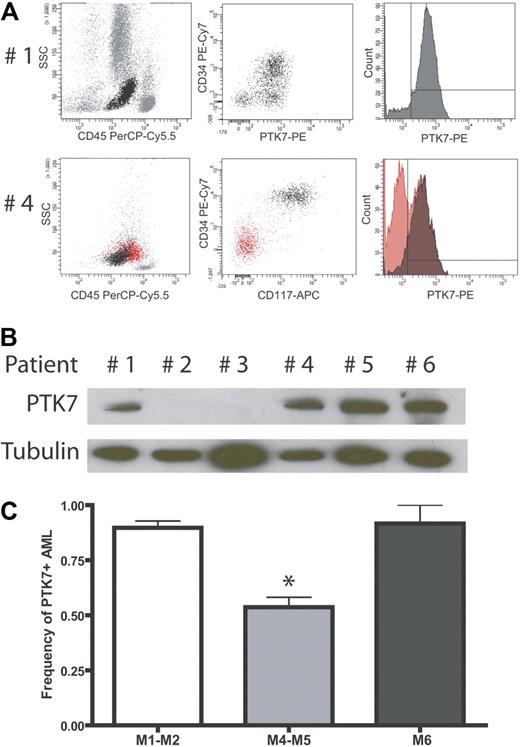
We next evaluated the expression of PTK7 in AML where this immature cell compartment is expanded. A total of 323 AMLs or other hematologic malignancies were analyzed, and the detailed results of the screening are shown in Table 1. Figure 2A shows typical results obtained from 2 AML patients. The patient 1 sample contains almost only myeloblastic cells (CD45+CD34+CD117+) that were 95% PTK7+. Patient 4 had a mixed blast population of granulocytic (CD33+CD117+) and monocytic (CD14+CD117−) origins (AML French-American-British [FAB] M4). In that case, only myeloblastic cells were positive for PTK7 (Figure 2A). Expression of PTK7 was confirmed by Western blot using a polyclonal anti-PTK7 antibody to probe protein extracts from blast cells of patients 1 and 4, as well as from patients 5 and 6 that also have PTK7-positive AML. As negative controls, we probed protein extracts from AML samples of patient 2 and patient 3 that do not express PTK7 in flow cytometry experiments (data not shown; Figure 2B). As seen in Table 1, PTK7 is expressed in 184 of the 257 AML patients (72%) with a median MFI of 13-fold (range, 0-192 MFI in PTK7+ patients: 20-fold range, 5-192). To a lesser extent, PTK7 was also expressed in acute lymphoblastic leukemia: 11 of 39 patients (27%) are PTK7+ (MFI in PTK7+ patients: 9-fold range, 5-58). Interestingly, the 5 biphenotypic acute leukemia tested expressed PTK7 with a median MFI of 43-fold (range, 15-110). There was no detected expression of PTK7 in chronic lymphoid diseases (n = 13, mainly chronic lymphocytic leukemia and low-grade non-Hodgkin lymphoma). In chronic myeloid disorders (n = 9, myeloproliferative syndromes and chronic myelomonocytic leukemia), we did not find significant differences compared with normal donors (ie, expression of PTK7 in myeloid progenitors).
AML: PTK7 is expressed in a subgroup of AMLs. (A) Flow cytometric data using the monoclonal anti-PTK7 antibody. (Top panel) An AML patient with 95% of PTK7+ cells. (Bottom panel) An AML patient with a mixed blast population. In this sample, myeloid cells (CD34+ CD117+) are PTK7+, whereas monocytic blasts are PTK7−. Of note, these monocytic blasts expressed significant levels of CD11b and CD14 (data not shown). (B) Western blot of fresh AML protein extracts using a polyclonal antibody directed against PTK7. (C) Correlation of PTK7 expression with cytologic findings using the FAB classification: FAB M1, M2, and M6 AMLs frequently express PTK7 compared with FAB M4 or M5 AMLs (Fisher exact test, P < .001).
AML: PTK7 is expressed in a subgroup of AMLs. (A) Flow cytometric data using the monoclonal anti-PTK7 antibody. (Top panel) An AML patient with 95% of PTK7+ cells. (Bottom panel) An AML patient with a mixed blast population. In this sample, myeloid cells (CD34+ CD117+) are PTK7+, whereas monocytic blasts are PTK7−. Of note, these monocytic blasts expressed significant levels of CD11b and CD14 (data not shown). (B) Western blot of fresh AML protein extracts using a polyclonal antibody directed against PTK7. (C) Correlation of PTK7 expression with cytologic findings using the FAB classification: FAB M1, M2, and M6 AMLs frequently express PTK7 compared with FAB M4 or M5 AMLs (Fisher exact test, P < .001).
Expression of PTK7 in AML is associated with distinct cytologic, immunophenotyping, and cytogenetic profiles
We analyzed the potential association between PTK7 expression and the biologic characteristics of 257 AML patients at diagnosis (supplemental Table 1). We found that PTK7 expression was correlated with FAB classification (Figure 2C). Granulocytic lineage leukemia (ie, FAB-AML M1, M2) expressed significantly more PTK7 compared with monocytic lineage leukemia (FAB AML M4 and M5) with a median MFI of 16- and 6-fold, respectively (P = .005). PTK7 was also highly expressed in FAB AML M6 leukemia (n = 12) with a median MFI of 22-fold. Interestingly, in a majority of the myelomonocytic leukemia (FAB AML M4) n = 47), expression of PTK7 was restricted to the granulocytic committed blasts and poorly expressed in their monocytic counterparts (eg, patient 4 in Figure 2A).
Those cytologic findings were confirmed by an immunophenotyping approach (Table 2). In all FAB subgroups, we found a significant association of PTK7 expression with the expression of myeloid and stem cell lineage markers (CD34 and CD117, both with P < .001) and a limited coexpression with monocytic lineage and differentiation markers (CD11b and CD15, both with P < .001).
Finally, we looked at the potential correlation between expression of PTK7 and specific cytogenetic or molecular subgroups. We found that PTK7 is differentially expressed among those clusters, independently of the FAB classification (Table 3). There is a high level of PTK7 expression in promyelocytic leukemia (acute promyelocytic leukemia [APL], t(15;17), core binding factor AML, ie, t(8;21), and inversion(16)), in contrast to subgroups with 3q or 11q23 (ie, MLL) abnormalities.
Expression of PTK7 in AML is associated with distinct clinical features and with a poor clinical outcome
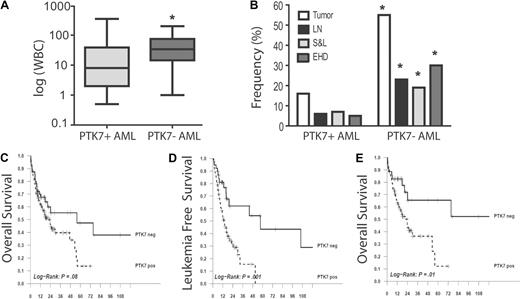
Univariate analysis showed that PTK7+ AMLs were associated with a significantly lower white blood cell (WBC) count at diagnosis compared with PTK7− AMLs (8 g/L vs 33 g/L, P = .004, Figure 3A) and with a lower frequency of extramedullary disease (16% vs 52%, P < .001, Figure 3B). This lower frequency was found in all extramedullary locations (lymph nodes, spleen, and liver, extrahematopoietic locations) and was not related to FAB (data not shown). There was no difference in age, sex, or bone marrow blast count between the PTK7− and PTK7+ populations.
PTK7 expression is associated with poor clinical outcome. (A) Correlation between PTK7 expression and WBC at diagnosis. The PTK7+ AMLs are significantly associated with lower WBC count in the whole population (Wilcoxon test, P = .001) but also when myeloid and monocytic subgroups were analyzed separately. (B) Correlation of PTK7 expression and extramedullary disease. LN indicates lymph node involvement; S&L, spleen and/or liver enlargement; and EHD, extrahematologic disease. Frequency was lower in PTK7+ AML (χ2 test, P < .001, .01, < .001, and < .001 for the respective columns), and this was also confirmed in analysis of subgroup patients. (C-E) Survival curves (OS and LFS, time scale is in months) using the Kaplan-Meier method. Only patients with homogeneous treatment were analyzed (n = 182). Differences shown here were confirmed in the Cox multivariate analysis model. (E) OS in intermediate cytogenetic risk group patients.
PTK7 expression is associated with poor clinical outcome. (A) Correlation between PTK7 expression and WBC at diagnosis. The PTK7+ AMLs are significantly associated with lower WBC count in the whole population (Wilcoxon test, P = .001) but also when myeloid and monocytic subgroups were analyzed separately. (B) Correlation of PTK7 expression and extramedullary disease. LN indicates lymph node involvement; S&L, spleen and/or liver enlargement; and EHD, extrahematologic disease. Frequency was lower in PTK7+ AML (χ2 test, P < .001, .01, < .001, and < .001 for the respective columns), and this was also confirmed in analysis of subgroup patients. (C-E) Survival curves (OS and LFS, time scale is in months) using the Kaplan-Meier method. Only patients with homogeneous treatment were analyzed (n = 182). Differences shown here were confirmed in the Cox multivariate analysis model. (E) OS in intermediate cytogenetic risk group patients.
We next analyzed the impact of PTK7 expression on complete remission rate, OS, and LFS in the patients treated with standard induction chemotherapy (n = 182, supplemental Table 2). Of note, patients with APL (n = 18) were excluded from this analysis because of their specific treatment.
Complete remission rate was 73% with no difference between PTK7+ and PTK7− patients (74% vs 72% respectively, P = not significant). With a 21-month follow-up, the 2-year probabilities of OS and of LFS in the PTK7+ versus PTK7− subgroups were 46% versus 56% (P = .08) and 30% versus 63% (P = .001), respectively (Figure 3C-3D; supplemental Figure 2, the cumulative incidence of relapse). The multivariate analysis (supplemental Tables 2-3) showed that PTK7 is an independent prognosis factor of OS (hazard ratio [HR] = 2.5; 95% CI, 1.4-4.6, P = .001) and LFS (HR = 3.4; 95% CI, 1.8-6.7, P = .002). Interestingly, analysis of subgroups using a Forest plot method showed that the effect of PTK7 expression on OS and LFS was the most significant in the intermediate cytogenetic risk group population (HR = 5.3; 95% CI, 1.8-15.7, P = .01, Figure 3E). Among the different cytogenetic clusters, we demonstrate that PTK7 expression was associated with a significantly worse outcome in FLT3-ITD mutated patients (median OS: 11 months for FLT3-ITD+ PTK7+ patients vs 23 months for FLT3-ITD+ PTK7− patients, data not shown). Taken together, our data suggest that PTK7-positive AMLs were more resistant to chemotherapy therapy, which translated into a significantly reduced survival.
PTK7 promotes cell migration of leukemia cells
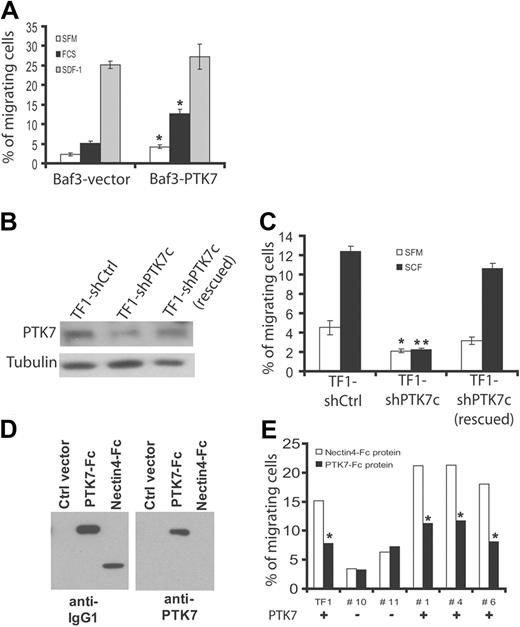
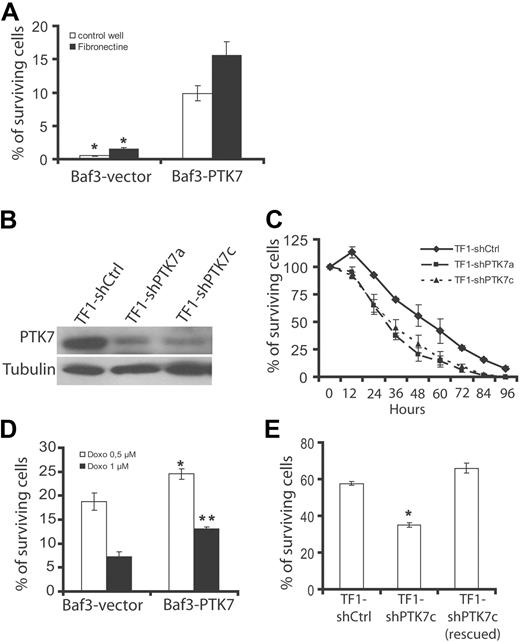
The clinical results may suggest that expression of PTK7 confers particular properties to leukemia cells. A recent report has revealed a promigratory role of PTK7 in presomitic cells during gastrulation.27 To test whether PTK7 plays a role in leukemia cell migration, we used Baf3 cells expressing PTK7 (Figure 1A) and evaluated cell migration in a Transwell system assay (Figure 4A). Ectopic expression of PTK7 (Baf3-PTK7) increases spontaneous cell migration in SFM and FCS-induced migration compared with Baf3 cells transfected with a control vector (Baf3-vector). Stimulation of cells with SDF-1α, a potent chemotactic agent, induced equivalent chemotaxis in the 2 cell populations. TF1 cells are erythroleukemia cells that coexpress endogenous PTK7 and c-Kit (CD117; Figure 1A). Stimulation of TF1 cells with SCF, a ligand for c-Kit, promotes transmigration in a Transwell system assay. We down-regulated PTK7 expression using a specific shRNA (shPTK7c; Figure 4B) and found that both spontaneous cell migration and SCF-induced cell migration were decreased in these cells compared with cells transfected with a control shRNA (shCtrl; Figure 4C). Importantly, expression and activation of c-Kit were not affected by down-regulation of PTK7 (supplemental Figure 3). We designed an shRNA-resistant PTK7 plasmid and reexpressed it in TF1 shPTK7c cells obtaining TF1 shPTK7c (rescued) cells (Figure 4B). Using this strategy, we could efficiently restore cell migration demonstrating the specificity of the effect observed with shPTK7c (Figure 4B-C).
Role of PTK7 in cell migration. (A) Measurement of chemotaxis (Transwell system) of Baf3 cells expressing (Baf3-PTK7) or not (Baf3-vector) PTK7 in the presence of SFM, 20% FCS, or SDF-1. After 4 hours, the cell migration ratio was evaluated using a luminometric assay. Expression of PTK7 increases cell migration in both SFM and FCS conditions. Cell migration induced by SDF-1 was not statistically different between the 2 cell lines. (B) Down-regulation of PTK7 by 2 different shRNAs (ie, shPTK7a and shPTK7c) in TF1 cells stimulated with SCF (250 ng/mL). Protein extracts were revealed by anti-PTK7 or antitubulin antibodies. (C) Decreased expression of PTK7 impairs cell migration. Reexpression of a PTK7 mutant resistant to shPTK7c restores cell migration (B-C). (D) Production of a soluble recombinant PTK7-Fc protein that is recognized by anti-IgG1 and anti-PTK7 antibodies by Western blot. As a control, recombinant Nectin4-Fc is only recognized by anti-IgG1 antibody. (E) Measurement of cell migration of a PTK7+ cell line (TF1) and PTK7+ (+) or PTK7− (−) primary AML blasts isolated from patients. Cells were incubated in the presence of 5 μg/mL of soluble protein and stimulated by SCF as in panel B. Cell migration was measured in a Transwell system. Student t test was used for statistics; all P values < .05.
Role of PTK7 in cell migration. (A) Measurement of chemotaxis (Transwell system) of Baf3 cells expressing (Baf3-PTK7) or not (Baf3-vector) PTK7 in the presence of SFM, 20% FCS, or SDF-1. After 4 hours, the cell migration ratio was evaluated using a luminometric assay. Expression of PTK7 increases cell migration in both SFM and FCS conditions. Cell migration induced by SDF-1 was not statistically different between the 2 cell lines. (B) Down-regulation of PTK7 by 2 different shRNAs (ie, shPTK7a and shPTK7c) in TF1 cells stimulated with SCF (250 ng/mL). Protein extracts were revealed by anti-PTK7 or antitubulin antibodies. (C) Decreased expression of PTK7 impairs cell migration. Reexpression of a PTK7 mutant resistant to shPTK7c restores cell migration (B-C). (D) Production of a soluble recombinant PTK7-Fc protein that is recognized by anti-IgG1 and anti-PTK7 antibodies by Western blot. As a control, recombinant Nectin4-Fc is only recognized by anti-IgG1 antibody. (E) Measurement of cell migration of a PTK7+ cell line (TF1) and PTK7+ (+) or PTK7− (−) primary AML blasts isolated from patients. Cells were incubated in the presence of 5 μg/mL of soluble protein and stimulated by SCF as in panel B. Cell migration was measured in a Transwell system. Student t test was used for statistics; all P values < .05.
A classic approach to block RTK functions consists of the use of a soluble receptor able to compete for ligand binding. PTK7 exhibits the conventional features of RTK, in particular, an extracellular region with a not yet identified ligand. We produced the extracellular region of PTK7 fused to the Fc region of IgG1 (soluble PTK7-Fc) and purified it from COS cell supernatants (Figure 4D). As a control, we used Nectin4-Fc.21 When TF1 cells are stimulated with SCF and incubated with 5 μg/mL of soluble PTK7-Fc, the response to SCF in Transwell assays was impaired. Such an effect was not evidenced with the same amount of Nectin4-Fc protein used as a control (Figure 4E). The soluble PTK7-Fc protein had no significant effect on the residual migratory properties of TF1 shPTK7c cells. The blocking effect of PTK7-Fc protein was next evaluated on primary AML samples (n = 5) in the presence of SCF. We found that soluble PTK7-Fc decreased cell migration in all cases of PTK7+ AML (fold decrease, 2.0; range, 1.8-2.2), whereas migration of PTK7− AML was not modified (fold decrease, 1.1; range, 0.8-1.2; Figure 4E). We concluded that expression of PTK7 promotes cell migration of leukemia cells and that a recombinant soluble PTK7-Fc protein can efficiently repress this function.
PTK7 expression increases resistance to apoptosis
Resistance to chemotherapy in PTK7+ AML patients may suggest an increased ability of leukemic cells to proliferate or to survive when PTK7 is present. Proliferation assay using a tritiated thymidine incorporation method did not show any difference between Baf3-vector and Baf3-PTK7 cells or between TF1 shCtrl and TF1 shPTK7c cells (supplemental Figure 4). Baf3 is an IL-3-dependent cell line. To test a potential survival effect of PTK7, we cultured Baf3 cells expressing or not PTK7 in SFM and in the absence of IL-3 (Figure 5A). PTK7 expression does not allow IL-3-independent cell growth (data not shown). Nevertheless, Baf3-PTK7 cells present higher survival than Baf3-vector cells when cultured on uncoated or fibronectin-coated wells (Figure 5A). Conversely, knockdown of PTK7 in TF1 cells by 2 different shRNAs (shPTK7a and shPTK7c; Figure 5B) is associated with lower survival in serum-starved conditions compared with control (shCtrl-transfected cells; Figure 5C).
Role of PTK7 in cell survival. (A) Effect of growth factor deprivation evaluated in the Baf3 model. After 24 hours of incubation, most of the Baf3 cells transfected with the empty vector (Baf3-vector) are dead, whereas a percentage of Baf3-PTK7 remains alive (Trypan blue dye exclusion assay and luminometric cell viability assay). (B) Western blot using anti-PTK7 antibody. Down-regulation of PTK7 expression in TF1 cells by 2 different shRNAs (shPTK7a and shPTK7c). (C) Reduced cell survival of TF1 cells expressing low amounts of PTK7 in a growth factor deprivation assay. (D-E) Induction of apoptosis by anthracyclines. After 24 hours of incubation, the number of surviving cells was evaluated by a cell viability luminometric assay in the Baf3 (D) and TF1 (E) cell systems. Student t test was used for statistics; all P values < .05.
Role of PTK7 in cell survival. (A) Effect of growth factor deprivation evaluated in the Baf3 model. After 24 hours of incubation, most of the Baf3 cells transfected with the empty vector (Baf3-vector) are dead, whereas a percentage of Baf3-PTK7 remains alive (Trypan blue dye exclusion assay and luminometric cell viability assay). (B) Western blot using anti-PTK7 antibody. Down-regulation of PTK7 expression in TF1 cells by 2 different shRNAs (shPTK7a and shPTK7c). (C) Reduced cell survival of TF1 cells expressing low amounts of PTK7 in a growth factor deprivation assay. (D-E) Induction of apoptosis by anthracyclines. After 24 hours of incubation, the number of surviving cells was evaluated by a cell viability luminometric assay in the Baf3 (D) and TF1 (E) cell systems. Student t test was used for statistics; all P values < .05.
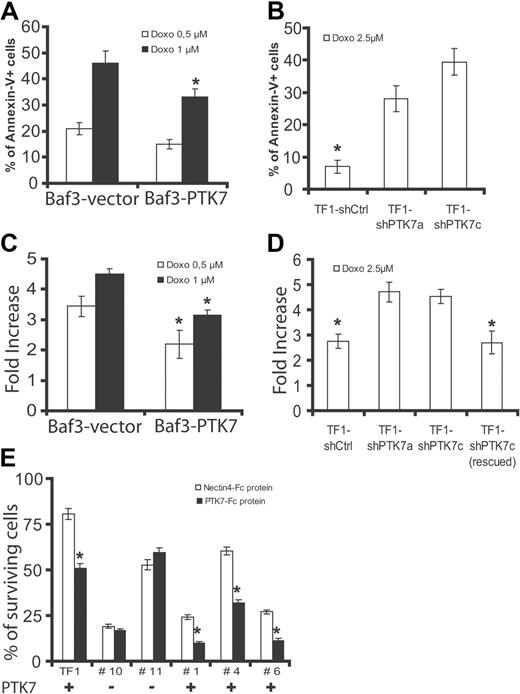
Chemotherapeutic activity of anthracyclines, such as doxorubicin, is the result of a proapoptotic effect on leukemia cells. We next evaluated the response of cells expressing or not PTK7 to doxorubicin in survival assays. Expression of PTK7 increases survival of Baf3 at 2 concentrations of doxorubicin (0.5 and 1μM; Figure 5D). We evaluated apoptosis in these cells by measuring annexin V staining and activity of executive caspases (ie, caspases 3 and 7). Expression of PTK7 provokes a significant decrease of annexin V staining (Figure 6A) and caspase 3/7 activity (Figure 6C). Similar experiments were performed in TF1 cells expressing (TF1 shCtrl and TF1 shPTK7c, rescued) or not (TF1 shPTK7c) the receptor. Decreased expression level of PTK7 reduces cell survival at a 2.5μM concentration of doxorubicin, this effect being abolished by expression of an shRNA-resistant PTK7 form (Figure 5E). This is correlated with a decrease in apoptosis as evidenced by annexin V staining and measurement of caspase 3/7 activity (Figure 6B,D). Having shown that a recombinant soluble PTK7-Fc protein can partially block PTK7 cell migration property (Figure 4E), we incubated TF1 cells and fresh AML samples expressing or not PTK7 with 5 μg/mL of recombinant soluble PTK7-Fc or Nectin4-Fc proteins. Cell survival was measured after exposure to doxorubicin (2.5μM). We observed a decreased cell survival of TF1 cells and PTK7+ AML samples after anthracycline exposure in the presence of soluble PTK7-Fc protein compared with PTK7− samples (Figure 6E). Altogether, these experiments, carried on cell lines and AML samples, demonstrate that expression of PTK7 allows higher cell survival and resistance of leukemia cells to apoptosis induced by anthracyclines. Similar results were obtained in the TF1 cell model using the nucleosidic analog cytarabine (supplemental Figure 5).
PTK7 expression increases resistance to apoptosis in an anthracycline-induced cytotoxicity assay. (A-B) Annexin V staining was measured by flow cytometry after exposure of Baf3 (A) and TF1 (B) cells to anthracyclines (concentrations of doxorubicin are indicated in panels A and B). Expression of PTK7 was associated with a decreased frequency of annexin V-positive cells in both models. (C-D) Apoptosis was also evaluated by measuring executive caspase activity (caspases 3 and 7, luminometric assay). PTK7 expression was associated with a decreased induction of caspase activity in both models. (E) Same as in Figure 4B, except that TF1 cells and primary AML samples were sensitized to apoptosis (24-hour incubation, doxorubicin 2.5μM) and that recombinant proteins (PTK7-Fc and Nectin4-Fc) were added to compete with this effect. Percentage of surviving cells was measured as in Figure 5A. Student t test was used for statistics; all P values < .05.
PTK7 expression increases resistance to apoptosis in an anthracycline-induced cytotoxicity assay. (A-B) Annexin V staining was measured by flow cytometry after exposure of Baf3 (A) and TF1 (B) cells to anthracyclines (concentrations of doxorubicin are indicated in panels A and B). Expression of PTK7 was associated with a decreased frequency of annexin V-positive cells in both models. (C-D) Apoptosis was also evaluated by measuring executive caspase activity (caspases 3 and 7, luminometric assay). PTK7 expression was associated with a decreased induction of caspase activity in both models. (E) Same as in Figure 4B, except that TF1 cells and primary AML samples were sensitized to apoptosis (24-hour incubation, doxorubicin 2.5μM) and that recombinant proteins (PTK7-Fc and Nectin4-Fc) were added to compete with this effect. Percentage of surviving cells was measured as in Figure 5A. Student t test was used for statistics; all P values < .05.
The intracellular region that includes the tyrosine kinase domain is required for PTK7 functions
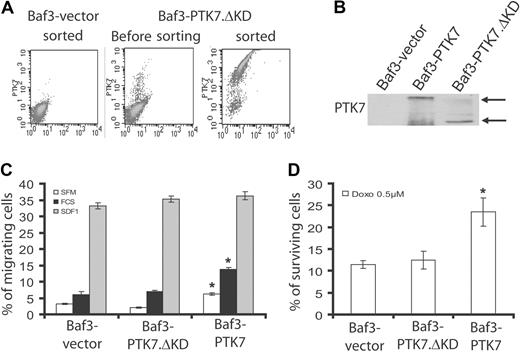
Using a recombinant soluble PTK7-Fc molecule, we showed that the extracellular region of PTK7 is required for PTK7 activity. PTK7 also possesses a juxtamembrane region and a catalytically inactive tyrosine kinase domain within the intracellular region. We constructed a mutant of PTK7 with a deletion of the kinase domain (from residue 789 to residue 1070; PTK7.ΔKD). We next expressed PTK7.ΔKD in Baf3 cells and selected cell populations expressing identical amounts of PTK7 and PTK7.ΔKD at the cell surface (Figure 7A). Expression of the proteins was verified with an anti-PTK7 antibody specific for the PTK7 extracellular domain (Figure 7B). Although expression of PTK7 promotes cell migration and cell survival as previously shown, deletion of the kinase domain completely abolishes these effects (Figure 7C-D). These data demonstrate that, despite the absence of enzymatic activity, the kinase domain of PTK7 conveys signals necessary for its cell migration and cell survival properties.
The intracellular region is required for PTK7 functions. We constructed a PTK7 protein deleted of the intracellular domain (PTK7.ΔKD) and transfected the construct in the Baf3 cell line. (A) Cells were sorted by flow cytometry according to PTK7 expression at the cell surface. (B) In a Western blot with anti-PTK7 antibody, amounts of PTK7 and PTK7.ΔKD proteins were comparable in Baf3-PTK7 and Baf3-PTK7.ΔKD cells. Effect of this truncated form of PTK7 was evaluated in a cell migration assay (C) and in an anthracycline-induced apoptosis assay (D). In both settings, the results obtained for the Baf3-PTK7.ΔKD cell line were comparable with Baf3-vector. Student t test was used for statistics; all P values < .05.
The intracellular region is required for PTK7 functions. We constructed a PTK7 protein deleted of the intracellular domain (PTK7.ΔKD) and transfected the construct in the Baf3 cell line. (A) Cells were sorted by flow cytometry according to PTK7 expression at the cell surface. (B) In a Western blot with anti-PTK7 antibody, amounts of PTK7 and PTK7.ΔKD proteins were comparable in Baf3-PTK7 and Baf3-PTK7.ΔKD cells. Effect of this truncated form of PTK7 was evaluated in a cell migration assay (C) and in an anthracycline-induced apoptosis assay (D). In both settings, the results obtained for the Baf3-PTK7.ΔKD cell line were comparable with Baf3-vector. Student t test was used for statistics; all P values < .05.
Discussion
The present study describes, for the first time, an implication of the planar cell polarity receptor PTK7 in hematologic diseases. In a large series of samples, we demonstrate that PTK7 is expressed in more than two-thirds of AMLs and that its expression is correlated with myeloid lineage differentiation. Accordingly, PTK7 is expressed in 4% to 9% of normal donor bone marrow cells together with CD34 and CD117 markers. These findings are consistent with high levels of ptk7 mRNA in myeloblastic-derived AMLs found in a high-throughput tyrosine kinase expression screening.28 Based on these results, we decided to functionally characterize PTK7 using cultured leukemia cells and AML blasts and to analyze PTK7 expression in a large cohort of AML patients to study potential clinical and biologic correlations.
Our in vitro data show that PTK7 expression promotes increased migratory properties and resistance to apoptosis in leukemic cell lines and primary AML blasts. Recent data obtained in endothelial cells demonstrate a promigratory role of PTK7 and a regulatory function on the vascular endothelial growth factor receptor signaling pathway.19 Similarly, stimulation of TF1 cells with the c-Kit ligand (SCF) provokes cell migration in a PTK7-dependent manner. Even if the molecular basis remains unknown, it thus appears that PTK7 acts as a cosignaling receptor for some RTKs. We were unable to show coimmunoprecipitation between c-Kit and PTK7 in the presence or absence of SCF, or to demonstrate tyrosine phosphorylation of PTK7 on c-Kit activation (data not shown).
We also showed that the frequency of extramedullary disease at diagnosis is lower in PTK7+ AML. At present, it is difficult to speculate on possible causes of discrepancy. One hypothesis could be that movements of PTK7-expressing AML blasts are restricted to the bone marrow microenvironment by in situ chemokines not present, or in too low concentration, in the peripheral blood and extramedullary tissues. As PTK7 is expressed in normal hematopoietic phenotypically defined early progenitor cells, it is also possible that the more immature PTK7-positive AML blasts have higher bone marrow-homing capacities than PTK7-negative blasts.
Recent advances have highlighted the importance of cell adhesion and cell migration pathways in hematopoietic stem cell behavior and differentiation.29 Signaling pathways, such as SDF-1/CXCR4, are implicated in the homing process, as well as in stem cell survival, proliferation, and interaction with the stem cell niche.30 In our hands, expression of PTK7 in Baf3 cells did not modify response to SDF-1 (Figures 4A, 7B). Nevertheless, multiple pathways could play a role, and it will be important to evaluate the bone marrow-homing properties of normal CD34+PTK7+ cells and their leukemic counterparts. More generally, it will be important to address the role of PTK7 in hematopoietic cell differentiation.
Strikingly, expression of PTK7 was associated with an increased relapse rate resulting in shorter OS and LFS. This effect was independent from other known prognostic factors, including cytogenetics. This indicates that PTK7 detection by flow cytometry may add prognostic information. Over the past 10 years, identification of cytogenetic and/or molecular prognostic factors has led to the design of risk-adjusted therapeutic strategies.31 However, the whole variability in patient's outcome is not fully explained, and there is still a need for new prognostic factors. We showed that the impact of PTK7 expression was the highest in the intermediate-risk cytogenetic, the most undefined group, which represents more than 50% of AML cases.22,32 Of course, the prognostic value of PTK7 expression has to be validated in large prospective independent series of patients. We also found a high expression of PTK7 in a majority of low-risk AMLs (such as APL or core binding factor AMLs). In these leukemias, PTK7 expression does not seem to impair prognosis, and its influence seems balanced by the presence of the translocations leading the pathogenesis of these particular diseases.
As for the antiapoptotic role of PTK7, we show that this effect is measurable in serum-starved and doxorubicin-induced conditions. The cytoplasmic domain of PTK7 is important in this mechanism, suggesting that the receptor triggers an intracellular antiapoptotic signaling pathway. No direct interactor of PTK7 has been yet described, although a recent report suggests that PTK7 belongs to a protein complex with Dishevelled, a cytoplasmic PDZ protein.18 Dsh has no obvious antiapoptotic functions, but, interestingly, it was recently demonstrated that Scribble, another PDZ protein and a genetic interactor of PTK7, induces tumor growth by triggering defects of cell polarity and by exerting an antiapoptotic effect in carcinoma cells.9-12
Future work will have to investigate the molecular basis of PTK7 implication in cell migration and resistance to apoptosis. We did not find a significant difference of the antiapoptotic BCL-2 pathway in leukemia cells expressing or not PTK7 by investigating the expression of MCL-1 (data not shown). XIAP (X-linked inhibitor of apoptosis) is a protein that not only acts as an antiapoptotic molecule but also potentially modulates cell migration33 and chemotherapeutic response34 via B-RAF stabilization. We found that XIAP is constitutively expressed in TF1 cells, as it was already shown in other leukemia cells35 and evidenced a decreased induction of XIAP in TF1 cells lacking PTK7 after an anthracycline-induced stress (supplemental Figure 6). Future work will have to define the relationship between XIAP induction and PTK7 function.
Our data suggest that PTK7 may be considered as a therapeutic target in the treatment of AML patients, especially as its expression is mostly restricted to the myeloid lineage but also potentially to the stem cell compartment.36 Interestingly, a soluble PTK7 receptor can sensitize AML blasts to anthracycline-induced apoptosis. Canonical (β-catenin dependent) and noncanonical Wnt pathways have been implicated in leukemogenesis.37-39 The PCP pathway is classically described as a noncanonical Wnt pathway acting independently of β-catenin. After having demonstrated that the kinase domain of PTK7 is implicated in promigratory and antiapoptotic functions, it remains now to discover which signaling molecules are associated and/or activated by PTK7 engagement. These findings, as well as the discovery of PTK7 ligand(s), should help to gain more insights on the role of this PCP protein in normal hematopoiesis and leukemogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the staff from the Hematology Department at the Institut Paoli-Calmettes for their devoted care for the patients. Clinical data and samples are managed at the Clinical Research Office and Center de Ressources Biologiques (Tumorothèque PACA) of Institut Paoli-Calmettes, respectively.
This work was supported by Fondation de France (Comité Leucémie; N.V., T.P.), Association pour la Recherche contre le Cancer, La Ligue Contre le Cancer (Label Ligue; J.-P.B.), and EUCAAD (FP7 program), Basic Science Research Program from KRF of Korea (S.-T.L.).
Authorship
Contribution: T.P. designed and performed research, analyzed the data, and wrote the paper; A.-C.L. and C.A. performed research and analyzed the data; S.M. performed research and contributed to vital reagents; C.C., S.A., F.P., M.J.S., M.S., V.S., Y.H., A.A., S.-T.L., and W.-S.S. contributed to vital reagents; B.E. and D.S. analyzed the data; N.V. analyzed the data and wrote the paper; and J.-P.B. designed research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Paul Borg, Centre de Recherche en Cancérologie de Marseille, CRCM U891 Inserm, 27 bd Leï Roure BP 30059, 13273 Marseille Cedex 09, France; e-mail: jean-paul.borg@inserm.fr.