Abstract
Steady-state hematopoiesis is sustained through differentiation balanced with proliferation and self-renewal of hematopoietic stem cells (HSCs). Disruption of this balance can lead to hematopoietic failure, as hematopoietic differentiation without self-renewal leads to loss of the HSC pool. We find that conditional knockout mice that delete the transcriptional repressor NKAP in HSCs and all hematopoietic lineages during embryonic development exhibit perinatal lethality and abrogation of hematopoiesis as demonstrated by multilineage defects in lymphocyte, granulocyte, erythrocyte and megakaryocyte development. Inducible deletion of NKAP in adult mice leads to lethality within 2 weeks, at which point hematopoiesis in the bone marrow has halted and HSCs have disappeared. This hematopoietic failure and lethality is cell intrinsic, as radiation chimeras reconstituted with inducible Mx1-cre NKAP conditional knockout bone marrow also succumb with a similar time course. Even in the context of a completely normal bone marrow environment using mixed radiation chimeras, NKAP deletion results in HSC failure. NKAP deletion leads to decreased proliferation and increased apoptosis of HSCs, which is likely due to increased expression of the cyclin-dependent kinase inhibitors p21Cip1/Waf1 and p19Ink4d. These data establish NKAP as one of a very small number of transcriptional regulators that is absolutely required for adult HSC maintenance and survival.
Introduction
Hematopoietic cells must tightly balance proliferation with differentiation and self-renewal. Disruption of this balance can lead to loss of hematopoietic stem cells (HSCs) and hematopoietic failure. To date, most well-characterized genes that modulate the key events in HSC maintenance, proliferation and differentiation are regulators of transcription, and their functions in hematopoiesis have been determined through the generation and examination of knockout animals (reviewed in Teitell and Mikkola1 ). HSC maintenance requires the transcriptional repressors Gfi-12 and Bmi-1.3,4 These factors, along with others (such as members of the GATA family of transcription factors5 ), give rise to a transcriptional network that, through direct regulation, feedback loops, and combinatorial associations, regulates hematopoiesis.6 In addition to regulators of transcription, mutations in cell-cycle regulators, which either suppress or promote proliferation, also disturb this balance leading to hematopoietic failure due to stem cell depletion or exhaustion, respectively (reviewed in Myatt and Lam7 and Ezoe et al8 ). The transcriptional activators or repressors that are required for HSC survival and self-renewal, in part, exert their effect through regulating expression of cell-cycle regulators. Loss of APC9 or Gfi-12 leads to decreased expression of the cyclin-dependent kinase inhibitor p21 Cip1/Waf1. p21 Cip1/Waf1-deficient mice exhibit increased proliferation leading to HSC exhaustion upon serial transplantation.10 Bmi1-deficient HSCs have increased expression of the cyclin-dependent kinase inhibitor p16 Ink4a that may mediate the decreased proliferation that is observed.3,4 This was proven upon generation of Bmi1/p16 Ink4a double-deficient mice, in which most of the hematopoietic defects observed in the Bmi1-deficient mice were alleviated.11 Thus, transcriptional control of proliferation and self-renewal is critical to maintenance and survival of HSCs. Previously, we isolated NKAP on a genetic complementation screen and determined that it functions as a transcriptional repressor.12 In T cells, loss of NKAP expression leads to a severe block in early thymocyte development.12 Here, we demonstrate the obligate requirement for NKAP in adult HSC maintenance and survival.
Methods
Mice
Floxed NKAP mice were described previously.12 Vav-cre transgenic mice were generously provided by Thomas Graf (Centre for Genomic Regulation, Barcelona, Spain).13 Mx1-cre mice were purchased from The Jackson Laboratory.14 Vav-cre or Mx1-cre male transgenics were bred to female mice heterozygous for the floxed NKAP allele. Because NKAP is expressed on the X chromosome, male progeny that contained a single copy of NKAP and expressed a cre-transgene were examined, with male littermate controls. All animal work was performed with the approval of the Institutional Animal Care and Use Committee at the University of Pennsylvania and the Mayo Clinic, in accordance with their guidelines.
Generation of radiation chimeras
Male 129 × B6.SJL F1 recipients were bred in-house, and irradiated with 900 rads prior to transfer of 2 × 106 total bone marrow cells from either mixed B6 × 129 Mx1-cre NKAP cKO mice or wild-type (WT; littermate) mice for straight radiation chimeras, or a 50:50 ratio of mixed B6 × 129 Mx1-cre NKAP cKO:WT 129xB6.SJL F1 bone marrow cells or a 50:50 ratio of mixed B6 × 129 WT littermate:WT 129 × B6.SJL F1 bone marrow cells for mixed radiation chimeras. Mice were analyzed at least 8 weeks after transplantation.
Inducible deletion with poly I-C
FACS analysis
Single-cell suspensions were generated from thymus, spleen, bone marrow, or neonatal liver. Splenic suspensions were treated with ammonium-chloride potassium (ACK) lysis buffer to remove red blood cells. Neonatal liver suspensions were treated with Lympholyte-M (Cedarlane) to enrich for hematopoietic cells. Analysis of T-cell development was performed as previously described.11 Other antibodies used in cell-surface staining include Gr1–fluorescein isothiocyanate (FITC), Gr1-phycoerythrin (PE), CD19-PE, allophycocyanin (APC)-Mac1, Flt3-PE, Flt3-PE-Cy7, Sca1-Pe-Cy5.5, CD150-PE-Cy7, cKit-APC, cKit-APC-Cy7, CD48-Pacific Blue, CD45.1(SJL)-FITC, CD45-PE, CD45-APC, CD71-FITC, Ter119-PE, CD41-bio/streptaviding-PE-Cy5, and CD61-APC. The lineage cocktail was composed of FITC-conjugated antibodies against CD8α, TCRβ, TCRγδ, CD3ϵ, B220, CD19, CD11c, DX5, NK1.1, and Ter119. All antibodies were from eBioscience, Biolegend, or BD Pharmingen. Intracellular flow cytometry to measure protein expression of p53 (Alexa Fluor 647; Cell Signaling Technology), phospho-p53 Ser15 (Alexa Fluor 488; Cell Signaling Technology15 ), Bcl-2 (FITC; eBioscience), Bcl-xL (Alexa Fluor 488; Cell Signaling Technology), and BrdU (FITC; BD Pharmingen) was performed using fix and perm solutions from eBioscience. All analysis was performed using FlowJo 8.8.6 (TreeStar).
Preparation of blood leukocytes for flow cytometric analysis
Peripheral blood was obtained via submandibular puncture in adult mice and was added to an equal volume of heparin solution (20 units/mL in phosphate-buffered saline [PBS]; Sigma-Aldrich). Blood was lysed in ACK lysis buffer on ice for 10 minutes and then quenched with media. Cells were spun at 290g 4°C, for 8 minutes and resuspended in phosphate-buffered saline and filtered through nylon mesh. Cells were resuspended in fluorescence-activated cell sorting (FACS) buffer for surface staining.
CBC/differential of peripheral blood
Peripheral blood was obtained via submandibular puncture in adult mice, and neonates were decapitated for blood collection from the thoracic cavity using Sarstedt microvette tubes. Analysis of complete blood count (CBC)/differential on peripheral blood was performed using a Hemavet HV950FS.
FACS sorting
To isolate HSC, MPP (multipotent progenitor), and CMP (common myeloid progenitor), bone marrow cells from C57BL/6 mice were harvested, and positively selected for CD117+ cells using magnetic beads. For isolation of CLP and Pre-Pro B, bone marrow cells were harvested from C57BL/6 mice, and depleted of the following Lin+ cells using magnetic beads on a Vario MACS (Miltenyi Biotec): CLP (common lymphoid precursor) - Gr-1, Mac-1, Ter119, CD3ϵ, CD45R, and CD19; Pre-Pro B – Gr-1, Mac-1, Ter119, and CD3ϵ. The cells were stained with antibodies and then sorted: HSC (Lin−ckithi Sca-1+CD27−), MPPs (Lin−ckithiSca-1+CD27+), CMPs (Lin−ckithiSca-1+), CLP (IL7R+ckitloSca-1+) and Pre-Pro B cells (CD43+B220+CD19−IgM−). In radiation chimeras, bone marrow suspensions were stained with antibodies against lineage markers, cKit, Sca1, CD45.1 (SJL) and CD45.2 (B6) as described “in FACS analysis” and sorted for (F1 129x)SJL LSKs (lin−Sca1+cKit+CD45.1+CD45.2+) or WT or cKO LSKs (lin−Sca1+cKit+CD45.1−CD45.2+). All sorting was performed on a FACSAria (Becton Dickinson) with a purity more than 98%.
Real-time quantitative PCR analysis
mRNA was isolated from sorted populations. cDNA generated with Superscript II was amplified and detected using TaqMan probes (Applied Biosystems) for E2A, GATA2, Bmi1, cMyc, Hes1, cMyb, p19Ink4d, p21Cip1/Waf1, p27Kip1, Mcl1, NKAP and p53 as well as 18S rRNA as an internal control. An ABI RT-PCR System was used, and relative expression was calculated via the 2-ΔΔCT method.16
Immunohistochemistry
Tissues from WT and NKAP cKO mice were fixed in formalin overnight. For bone marrow samples, the bones were decalcified prior to embedding. All mounting, sectioning and hematoxylin/eosin staining were performed by either the Tissue and Molecular Cell Analysis core facility at the Mayo Clinic or the Children's Hospital of Philadelphia Pathology Core Facility. Images were acquired using Axiovision 4.8 software on an Axioplan2 (Carl Zeiss Microimaging), using either a 10× (Figure 3F), 20× (Figures 1C, 2B), or 40× (Figures 1E) objective.
BrdU analysis of HSC proliferation
Deletion in WT/SJL and (mx1-cre NKAP) cKO/SJL radiation chimeras was induced by a single intraperitoneal injection of poly-IC. Fourteen hours prior to killing at day 4 after induction, mice were given a single intraperitoneal injection of BrdU (1 mg). Flow cytometry was performed to analyze proliferation in HSCs as described in “FACS analysis” and “FACS sorting” for surface staining, prior to permeabilization and staining with anti-BrdU per manufacturer's protocol (BD Pharmingen).
Chromatin IPs
Chromatin immunoprecipitations (ChIPs) were performed as in Pajerowski.12 Briefly, Gal4-293T cells17 were transfected with a plasmid encoding full-length NKAP fused to the GAL4 DNA binding domain or a plasmid encoding the isolated GAL4-DBD. ChIPs were performed using the EZ Chip kit (Millipore) according to the manufacturer's protocol, using 2 μg of anti-Gal4 (RK5C1; Santa Cruz Biotechnology). Precipitates were analyzed by quantitative polymerase chain reaction (Q-PCR) using previously published primers in the human p21 promoter.18,19 Q-PCR was also performed using input DNA so that the percent recovery of the input target sequence by ChIP could be calculated.
Results
Multilineage blocks in hematopoiesis in vav-cre NKAP conditional knockout mice
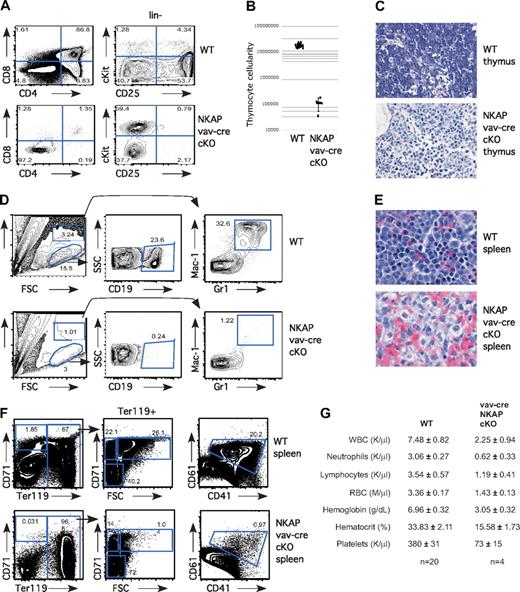
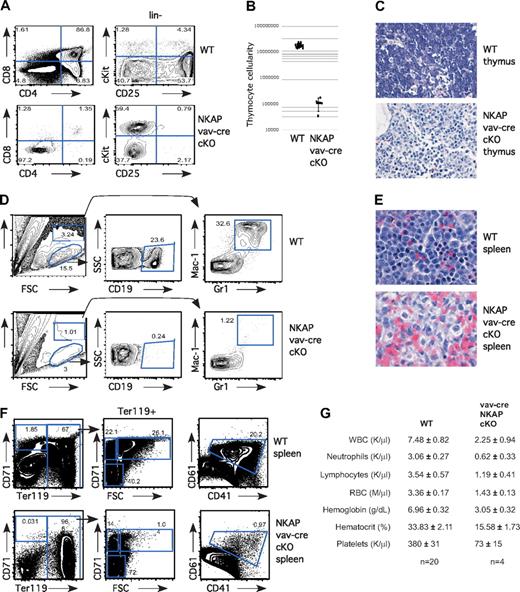
Previously, we demonstrated that NKAP is critical for T-cell development at the DN3 to DP (double positive) transition, using lck-cre NKAP conditional knockout mice (cKO; Pajerowski12 ). To determine whether NKAP is critical earlier in T-cell development, as well as in other hematopoietic lineages, we generated vav-cre NKAP conditional knockout mice. The vav-cre transgene is expressed in HSCs and in all downstream hematopoietic lineages.13 Vav-cre NKAP cKO mice have perinatal lethality, dying 1-3 days after birth, and exhibit a dramatic block in hematopoiesis. No differences were observed between wild-type littermates, or mice with only the vav-cre transgene or the floxed NKAP allele in the absence of a cre transgene (data not shown). In the thymus, T-cell development from vav-cre NKAP conditional knockout mice was blocked early, at the CD4−CD8− DN (double negative) stage (Figure 1A). Within the DN population, cells accumulated at the earliest stage in T-cell development, c-Kit+CD25− (Figure 1A). Consistent with an early block in T-cell development, total thymic cellularity was decreased in the vav-cre NKAP cKO animals by approximately 2 orders of magnitude (Figure 1B). Hematoxylin and eosin (H&E) staining of thymic sections confirmed the paucity of hematopoietic cells, and contained primarily stroma (Figure 1C). Therefore, there is an early and severe block in T-cell development in the absence of NKAP. Similarly, examination of the neonatal liver (Figure 1D) or spleen (data not shown) demonstrated that there were almost no B cells (defined by CD19+) or granulocytes (defined by Mac-1+Gr1+) in vav-cre NKAP conditional knockout mice. Examination of splenic H&E sections confirmed the absence of cells with either a lymphoid or granulocyte morphology (Figure 1E). We failed to detect any CD41+/CD61+ developing megakaryocytes in the neonatal liver of vav-cre NKAP cKO mice (Figure 1F). In addition, when erythropoiesis was examined,20 we failed to detect any pro-erythroblasts (CD71+Ter119−) or erythroblast-A (CD71+ Ter119+FSChi) in vav-cre NKAP cKO mice, although later stages of erythropoeisis including erythroblast-B (CD71+ Ter119+FSClo) and erthryoblast-C (CD71−Ter119+) were present, indicating that differentiation into the erythroid lineage had also been halted (Figure 1F). Therefore, at birth, NKAP is required for the generation of lymphocytes, granulocytes, megakaryocytes and erythrocytes, demonstrating that vav-cre NKAP cKO mice have multilineage blocks in hematopoiesis. To understand the basis for the perinatal lethality, CBCs with differential analysis of peripheral blood of 1-day-old pups was performed. As shown in Figure 1G, there was a significant (P < .001) defect in hemoglobin levels (3.05 ± .32 in vav-cre NKAP cKO compared with 6.96 ± .32 in WT littermates) as well as in hematocrit (15.58 ± 1.73 in vav-cre NKAP cKO compared with 33.83 ± 2.11 in WT littermates), resulting in severe anemia that was likely the cause of death in the vav-cre NKAP cKO mice.
Severe multilineage blocks in hematopoiesis in vav-cre NKAP cKO neonates. (A) Total thymocytes from wild-type (WT) and vav-cre NKAP cKO neonates were examined for surface expression of CD4 and CD8. Lineage-negative thymocytes from WT and vav-cre NKAP cKO neonates were examined for surface expression of c-Kit and CD25. (B) Total thymocyte number in wild-type (n = 13) or vav-cre NKAP cKO (n = 4). Results are shown on a logarithmic plot. A line indicated the average cellularity, and error bars reflect SD. (C) H&E staining of fixed sections of thymus from wild-type or vav-cre NKAP cKO neonates. (D) Neonatal liver was homogenized, and enriched for hematopoietic cells. Hematopoietic cells from WT and vav-cre NKAP cKO neonatal liver were analyzed for presence of B cells (CD19) and granulocytes (Gr-1/Mac1). (E) H&E sections of fixed sections of spleen from wild-type or vav-cre NKAP cKO neonates. (F) Total splenocytes from WT and vav-cre NKAP cKO neonates were analyzed for erythopoiesis by staining with CD71 and Ter119, and for megakaryocyte precursors by staining with CD41 and CD61. (G) CBCs with differential were analyzed on a Hemavet from 4 one-day-old vav-cre NKAP cKO and 20 wild-type littermates. In all of the parameters shown, the difference between WT and vav-cre NKAP cKO pups using the Student t test is significantly significant (P < .001). Data are shown as the average ± SEM.
Severe multilineage blocks in hematopoiesis in vav-cre NKAP cKO neonates. (A) Total thymocytes from wild-type (WT) and vav-cre NKAP cKO neonates were examined for surface expression of CD4 and CD8. Lineage-negative thymocytes from WT and vav-cre NKAP cKO neonates were examined for surface expression of c-Kit and CD25. (B) Total thymocyte number in wild-type (n = 13) or vav-cre NKAP cKO (n = 4). Results are shown on a logarithmic plot. A line indicated the average cellularity, and error bars reflect SD. (C) H&E staining of fixed sections of thymus from wild-type or vav-cre NKAP cKO neonates. (D) Neonatal liver was homogenized, and enriched for hematopoietic cells. Hematopoietic cells from WT and vav-cre NKAP cKO neonatal liver were analyzed for presence of B cells (CD19) and granulocytes (Gr-1/Mac1). (E) H&E sections of fixed sections of spleen from wild-type or vav-cre NKAP cKO neonates. (F) Total splenocytes from WT and vav-cre NKAP cKO neonates were analyzed for erythopoiesis by staining with CD71 and Ter119, and for megakaryocyte precursors by staining with CD41 and CD61. (G) CBCs with differential were analyzed on a Hemavet from 4 one-day-old vav-cre NKAP cKO and 20 wild-type littermates. In all of the parameters shown, the difference between WT and vav-cre NKAP cKO pups using the Student t test is significantly significant (P < .001). Data are shown as the average ± SEM.
Abrogation of hematopoiesis in the bone marrow
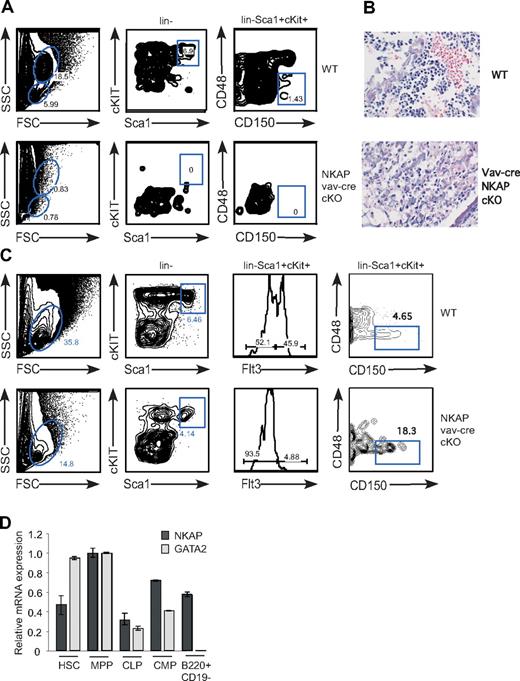
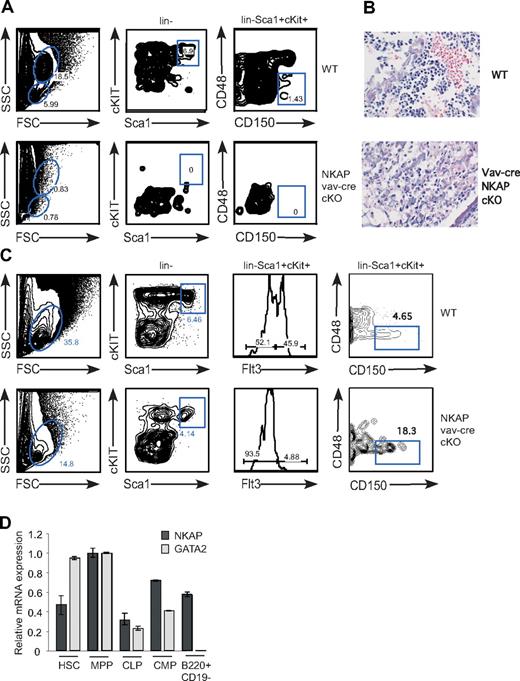
During late embryogenesis, HSCs populate the bone marrow, which becomes the primary site of hematopoiesis after birth (reviewed in Cumano and Godin21 ). HSCs are found within the “LSK population” (defined by lin−Sca1+cKit+), and long-term HSCs have been best characterized within this pool using the expression of the SLAM family receptors CD48 and CD150 (“SLAM-HSCs”22 ). While LSKs and SLAM-HSCs were found in the bone marrow of wild-type neonates, neither were found in bone marrow of vav-cre NKAP conditional knockout mice, indicating that NKAP is critical to bone marrow hematopoiesis (Figure 2A). H&E stains of bone marrow sections confirmed the lack of hematopoiesis in vav-cre NKAP cKO bone marrow (Figure 2B). Examination of the neonatal liver, the primary site of hematopoiesis during embryogenesis, demonstrated that LSK cells and SLAM-HSCs are present in vav-cre NKAP conditional knockout mice, but are reduced compared with wild-type (Figure 2C and data not shown). Within the LSK pool, one of the first differentiative events is the up-regulation of Flt3+, which defines a pool of nonself-renewing MPPs. These MPPs are almost absent in vav-cre NKAP cKO mice, indicating a block in the earliest events in hematopoiesis in the absence of NKAP (Figure 2C). Q-PCR from FACS-sorted wild-type LSKs and MPPs confirmed that NKAP is expressed in these cells (Figure 2D), indicating that the loss of NKAP may be directly altering the HSC pool. However, while embryonic SLAM-HSCs are present in the liver of vav-cre NKAP conditional knockout mice, the complete absence of HSCs or evidence of hematopoiesis in the bone marrow suggests that NKAP may be critical for the maintenance and survival of adult bone marrow HSCs.
Absence of HSCs from bone marrow of vav-cre NKAP cKO neonates. (A) Early hematopoiesis in total bone marrow single-cell suspensions from WT and vav-cre NKAP cKO neonates was analyzed for LSK (lin−Sca1+cKit+) and SLAM-HSC (lin−Sca1+cKit+CD48−CD150+). (B) Representative H&E staining of fixed bone marrow sections from WT and vav-cre NKAP cKO neonates is shown. Please note that at birth there is little accumulation of fat deposits, which develop with age. (C) Early hematopoietic progenitors were examined in liver from wild-type or NKAP cKO neonates. The resulting suspensions were stained with a lineage cocktail, c-Kit, Sca-1, Flt3, CD48, and CD150 to analyze LSK, as well as MPP (lin−Sca1+cKit+Flt3+), and SLAM-HSC (lin−Sca1+cKit+CD48−CD150+). (D) HSC (Lin−ckithiSca-1+CD27−), MPP (Lin−ckithiSca-1+CD27+), CMP (Lin−ckithiSca-1−), CLP (Lin−IL7R+ckitloSca-1+), and Pre-Pro B cells (CD43+B220+CD19−) were isolated by FACS, and expression of NKAP, GATA2 and 18S was analyzed by Q-PCR. Results shown are the average from triplicate amplifications of a single population sort, normalized to 18S, and then standardized between samples to the expression of either NKAP or GATA2 in the MPP population (= 1.0). Error bars represent SD.
Absence of HSCs from bone marrow of vav-cre NKAP cKO neonates. (A) Early hematopoiesis in total bone marrow single-cell suspensions from WT and vav-cre NKAP cKO neonates was analyzed for LSK (lin−Sca1+cKit+) and SLAM-HSC (lin−Sca1+cKit+CD48−CD150+). (B) Representative H&E staining of fixed bone marrow sections from WT and vav-cre NKAP cKO neonates is shown. Please note that at birth there is little accumulation of fat deposits, which develop with age. (C) Early hematopoietic progenitors were examined in liver from wild-type or NKAP cKO neonates. The resulting suspensions were stained with a lineage cocktail, c-Kit, Sca-1, Flt3, CD48, and CD150 to analyze LSK, as well as MPP (lin−Sca1+cKit+Flt3+), and SLAM-HSC (lin−Sca1+cKit+CD48−CD150+). (D) HSC (Lin−ckithiSca-1+CD27−), MPP (Lin−ckithiSca-1+CD27+), CMP (Lin−ckithiSca-1−), CLP (Lin−IL7R+ckitloSca-1+), and Pre-Pro B cells (CD43+B220+CD19−) were isolated by FACS, and expression of NKAP, GATA2 and 18S was analyzed by Q-PCR. Results shown are the average from triplicate amplifications of a single population sort, normalized to 18S, and then standardized between samples to the expression of either NKAP or GATA2 in the MPP population (= 1.0). Error bars represent SD.
Mx1-cre NKAP cKO mice demonstrate a cell-intrinsic, critical function for NKAP in HSC maintenance and survival
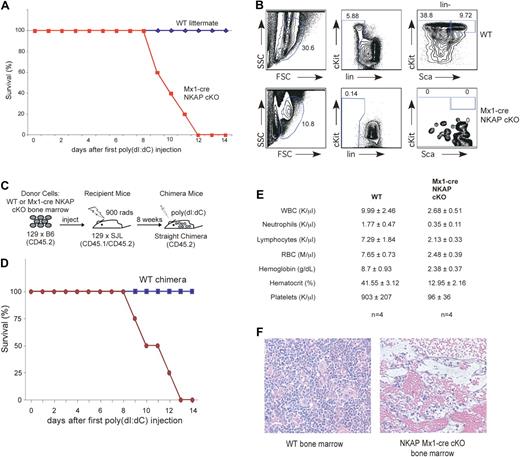
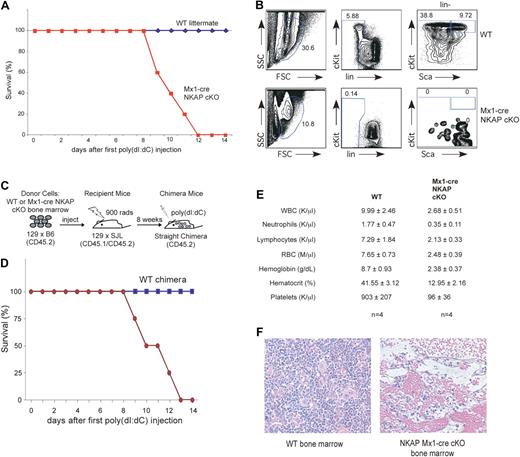
To explicitly examine the role of NKAP in adult HSC maintenance and survival, floxed NKAP mice were crossed to interferon-inducible Mx1-cre transgenic mice.14 To induce cre and delete NKAP, Mx1-cre NKAP floxed mice and wild-type control littermates (containing either the floxed allele or the cre transgene) were injected intraperitoneally with 200 μg of poly-IC at days 0, 2, and 4. Prior to induction of cre expression, Mx1-cre NKAP floxed mice were indistinguishable from their wild-type littermates (data not shown). Unexpectedly, the Mx1-cre NKAP cKO mice died between 8 to 12 days after the poly-IC treatment was completed, while no lethality was observed in wild-type littermates (Figure 3A). Analysis of 3 Mx1-cre NKAP cKO mice at day 8 demonstrated a complete loss of HSCs (as defined by LSKs) in the bone marrow implicating NKAP as being critical for HSC maintenance and survival, while poly-IC treatment of control littermates did not alter this pool (Figure 3B; for all subsequent analysis HSCs will be defined using LSK markers). However, in the Mx1-cre model, NKAP has the potential to be deleted in all cells, and therefore it cannot define whether the effects observed are intrinsic to the HSCs or whether the effects on HSCs are indirect, such as, for example, a result of abrogation of the hematopoietic niche. To distinguish between these possibilities, radiation chimeras were generated, in which Mx1-cre NKAP cKO bone marrow or wild-type littermate bone marrow was used to reconstitute hematopoiesis in lethally irradiated recipients (shown schematically in Figure 3C). Upon poly-IC injection to induce deletion, 4/4 mice reconstituted with Mx-1 cre NKAP cKO bone marrow died between 8 to 13 days after the first injection, as was observed in Mx1-cre NKAP cKO mice and indicating that the HSC failure is cell autonomous (Figure 3D). No lethality was observed in the 4 mice reconstituted with WT bone marrow. In addition, when wild-type bone marrow was used to reconstitute irradiated Mx-1 cre NKAP mice, no lethality was observed upon poly-IC induction (n = 2, data not shown), demonstrating that the lethality observed in the Mx1-cre NKAP conditional knockout mice was due to hematopoietic failure. Examination of the CBC demonstrates that the Mx1-cre NKAP cKO radiation chimera had severe anemia (hemoglobin levels of 2.38 ± .37), which was likely the cause of death (Figure 3E). The abrogation of normal hematopoiesis was confirmed in H&E sections of Mx1-cre NKAP conditional knockout bone marrow 12 days after induction (Figure 3G). Thus, deletion of NKAP from HSCs leads to their disappearance and complete hematopoietic failure, leading to lethality.
NKAP deletion in Mx1-cre NKAP cKO mice results in lethality and hematopoietic failure. (A) Kaplan-Meier survival curve data of WT (n = 5) and Mx1-cre NKAP cKO (n = 5) treated with poly-IC. (B) Mx-1 cre NKAP cKO and a wild-type littermate were injected intraperitoneally with 200 μg of poly-IC at day 0, 2 and 4. At day 8, HSCs in the bone marrow were analyzed by flow cytometry. Shown is representative data from 3 separate Mx1-cre and wild-type littermate pairs. (C) Schematic representation of the experimental design to generate WT and Mx1-cre NKAP cKO radiation chimeras. (D) Kaplan-Meier survival curve data of WT (n = 4) and Mx1-cre NKAP cKO (n = 4) radiation chimeras treated with poly-IC. (E) CBCs with differential at day 14 (WT chimeras) or at killing when moribund (Mx1-cre NKAP cKO chimeras). Results are from 4 animals in each group. In all of the parameters shown, the difference between WT and vav-cre NKAP cKO pups using the Student t test is significantly significant (P < .002). Data are shown as the average ± SEM. (F) H&E staining of fixed femur sections from a WT and a moribund Mx1-cre NKAP cKO at day 12 after poly-IC induction.
NKAP deletion in Mx1-cre NKAP cKO mice results in lethality and hematopoietic failure. (A) Kaplan-Meier survival curve data of WT (n = 5) and Mx1-cre NKAP cKO (n = 5) treated with poly-IC. (B) Mx-1 cre NKAP cKO and a wild-type littermate were injected intraperitoneally with 200 μg of poly-IC at day 0, 2 and 4. At day 8, HSCs in the bone marrow were analyzed by flow cytometry. Shown is representative data from 3 separate Mx1-cre and wild-type littermate pairs. (C) Schematic representation of the experimental design to generate WT and Mx1-cre NKAP cKO radiation chimeras. (D) Kaplan-Meier survival curve data of WT (n = 4) and Mx1-cre NKAP cKO (n = 4) radiation chimeras treated with poly-IC. (E) CBCs with differential at day 14 (WT chimeras) or at killing when moribund (Mx1-cre NKAP cKO chimeras). Results are from 4 animals in each group. In all of the parameters shown, the difference between WT and vav-cre NKAP cKO pups using the Student t test is significantly significant (P < .002). Data are shown as the average ± SEM. (F) H&E staining of fixed femur sections from a WT and a moribund Mx1-cre NKAP cKO at day 12 after poly-IC induction.
NKAP is required for HSC maintenance and survival in a replete environment
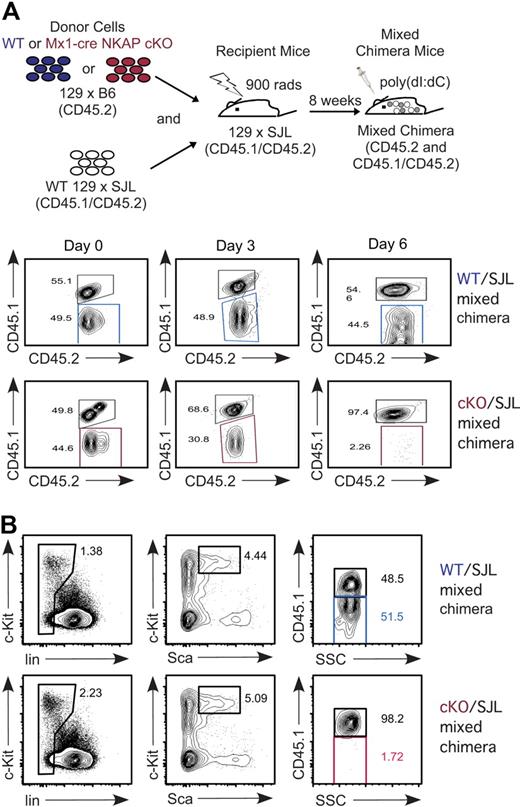
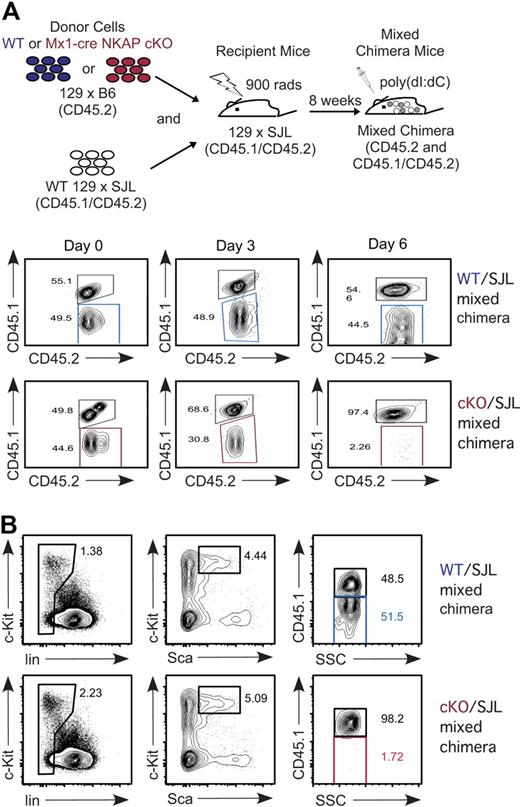
Maintenance and survival of HSCs require an interplay with their niche in the bone marrow.23 It was possible that NKAP-deficient HSCs act to disrupt the normal niche, leading to a feedback loop that disrupts hematopoiesis. To determine whether the effects observed were independent of any secondary effects on the niche, mixed bone marrow chimeras were generated. Mx1-cre NKAP cKO or WT littermate bone marrow was mixed equally with WT 129 × B6.SJL F1 bone marrow prior to reconstitution of irradiated 129 × B6.SJL F1 recipients (as outlined in Figure 4A, this produces either “cKO/SJL” or “WT/SJL” mixed chimeras). The CD45.1 allelic marker was used to differentiate Mx1-cre NKAP cKO/WT littermate hematopoietic cells from 129 × B6.SJL F1. As granulocytes are short-lived and must be continually produced, examination of peripheral granulocytes was used as a surrogate for HSCs in a longitudinal study in a cohort of mice. As shown in Figure 4A, prior to poly-IC induction, there was approximately equal distribution in Gr1+ granulocytes between the different donor cells in the peripheral blood. However, by day 3, loss of Mx1-cre NKAP cKO granulocytes was observed, resulting in a skewing of the granulocyte population toward the WT 129 × B6.SJL F1 donor cells that was not observed in the control WT/SJL chimera. By day 6, there was almost a complete loss of Mx1-cre NKAP cKO derived granulocytes, indicating a complete abrogation of granulopoiesis. At day 12, no Mx1-cre NKAP cKO HSCs were detected in the mixed chimeras; all HSCs were derived from WT 129 × B6.SJL (Figure 4B). The control WT/SJL retained the 50:50 mix in the HSC pool (Figure 4B). Therefore, even in a replete environment with normal hematopoiesis and a normal niche, NKAP deficiency results in rapid loss of HSCs.
Cell-autonomous requirement for NKAP in HSC maintenance and survival. (A) Schematic representation of the experimental design to generate WT and Mx1-cre NKAP cKO mixed radiation chimeras. In the study of mixed chimeras here and in subsequent figures, WT littermate cells are denoted by blue, Mx1-cre NKAP cKO cells are denoted by red, and competing SJL+ cells are in gray. Longitudinal study evaluating Gr1+ peripheral blood for the relative contribution of WT littermate and Mx1-cre NKAP cKO (CD45.2+CD45.1−), compared with competing WT SJL (CD45.2+CD45.1+) after poly-IC induction. Shown is representative data from 4 WT/SJL and 4 cKO/SJL mixed chimeras. (B) Analysis of contribution of each donor to the HSC pool in the mixed chimeras 12 days after poly-IC induction. Shown is representative data from 2 WT/SJL and 2 cKO/SJL mixed chimeras.
Cell-autonomous requirement for NKAP in HSC maintenance and survival. (A) Schematic representation of the experimental design to generate WT and Mx1-cre NKAP cKO mixed radiation chimeras. In the study of mixed chimeras here and in subsequent figures, WT littermate cells are denoted by blue, Mx1-cre NKAP cKO cells are denoted by red, and competing SJL+ cells are in gray. Longitudinal study evaluating Gr1+ peripheral blood for the relative contribution of WT littermate and Mx1-cre NKAP cKO (CD45.2+CD45.1−), compared with competing WT SJL (CD45.2+CD45.1+) after poly-IC induction. Shown is representative data from 4 WT/SJL and 4 cKO/SJL mixed chimeras. (B) Analysis of contribution of each donor to the HSC pool in the mixed chimeras 12 days after poly-IC induction. Shown is representative data from 2 WT/SJL and 2 cKO/SJL mixed chimeras.
NKAP is required for survival and proliferation of HSCs
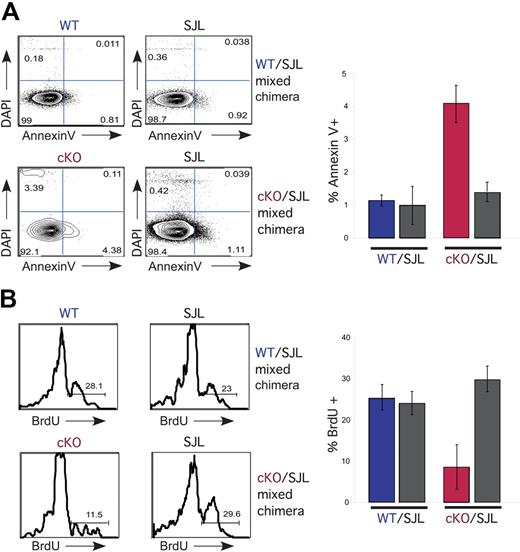
At the time point examined above (12 days, Figure 4B), Mx1-cre NKAP cKO HSCs cannot be isolated. To examine the effects of NKAP deficiency in HSCs in mixed chimeras, a single injection of poly-IC was performed and bone marrow harvested 4 days later. Fourteen hours prior to examination, mice were injected with BrdU to measure proliferation. At this time point, Mx1-cre NKAP cKO HSCs were present, however, there were fewer than control WT 129 × B6.SJL HSCs from the same mouse (data not shown). There was an approximately 4-fold increase in annexin V+ HSCs compared with WT 129 × B6.SJL from the same mouse, or compared with HSCs from the control WT/SJL chimeras (Figure 5A). A representative FACS plot is shown, along with average data from 4 cKO/SJL and 4 control WT/SJL chimeras. Similarly, Mx1-cre NKAP cKO HSCs were found to proliferate less than controls, as shown by BrdU incorporation (Figure 5B). Therefore, NKAP is required for HSC survival and proliferation.
NKAP-deficient HSCs exhibit decreased survival and proliferation. (A) At day 4 after a single injection of poly-IC, bone marrow LSK cells were analyzed for viability using DAPI and annexin V, as well as CD45.1 to separate SJL from WT littermate or Mx1-cre NKAP cKO hematopoietic progenitors. A representative FACS analysis is shown at right, and the average from 3 WT/SJL and 3 cKO/SJL mixed chimeras is shown at left. The Mx1-cre NKAP cKO LSKs are signicantly different from the other 3 samples, P < .01 by Student t test. Error bars represent SD. (B) At day 4 after a single injection of poly-IC, bone marrow LSK cells were analyzed for proliferation by BrdU incorporation, as well as CD45.1 to separate SJL from WT littermate or Mx1-cre NKAP cKO hematopoietic progenitors. BrdU was injected intraperitoneally 14 hours prior to killing. A representative FACS analysis is shown at right, and the average from 3 WT/SJL and 3 cKO/SJL mixed chimeras is shown at left. The Mx1-cre NKAP cKO LSKs are signicantly different from the other 3 samples, P < .04 by Student t test. Error bars represent SD from the mean.
NKAP-deficient HSCs exhibit decreased survival and proliferation. (A) At day 4 after a single injection of poly-IC, bone marrow LSK cells were analyzed for viability using DAPI and annexin V, as well as CD45.1 to separate SJL from WT littermate or Mx1-cre NKAP cKO hematopoietic progenitors. A representative FACS analysis is shown at right, and the average from 3 WT/SJL and 3 cKO/SJL mixed chimeras is shown at left. The Mx1-cre NKAP cKO LSKs are signicantly different from the other 3 samples, P < .01 by Student t test. Error bars represent SD. (B) At day 4 after a single injection of poly-IC, bone marrow LSK cells were analyzed for proliferation by BrdU incorporation, as well as CD45.1 to separate SJL from WT littermate or Mx1-cre NKAP cKO hematopoietic progenitors. BrdU was injected intraperitoneally 14 hours prior to killing. A representative FACS analysis is shown at right, and the average from 3 WT/SJL and 3 cKO/SJL mixed chimeras is shown at left. The Mx1-cre NKAP cKO LSKs are signicantly different from the other 3 samples, P < .04 by Student t test. Error bars represent SD from the mean.
Increased mRNA expression of p21 Cip1/Waf1 in NKAP-deficient HSCs
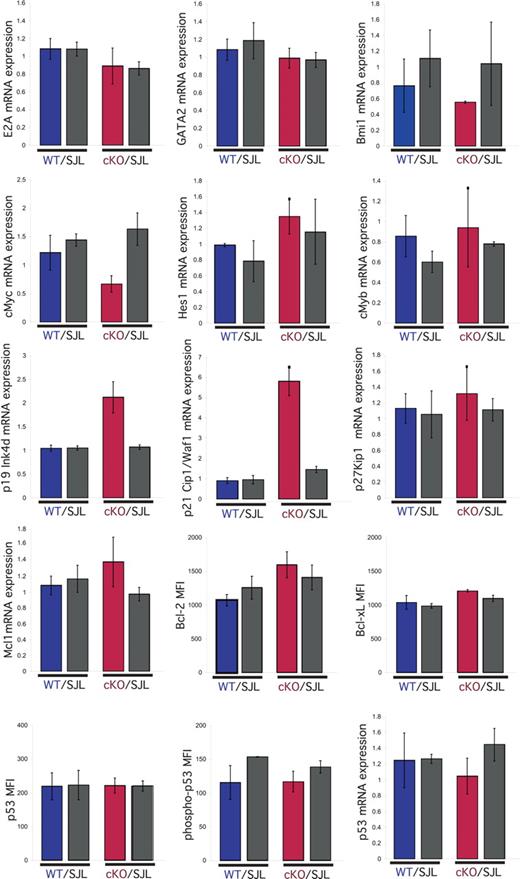
To gain insight into the molecular mechanism by which deletion of NKAP results in decreased proliferation and survival, LSKs from cKO/SJL and control WT/SJL mixed chimeras were purified by FACS sorting. mRNA was generated and examined by Q-PCR for the expression of genes previously implicated in regulating HSC maintenance, survival and/or proliferation: E2A,24 GATA2,25 Bmi1,3,4 cMyc,19 cMyb,26 p19 Ink4d,27 p21 Cip1/Waf1,10 p27 Kip1,28 Mcl1,29 and p53.30 In addition, the expression of Bcl-2, Bcl-xL, p53 and phospho-p53 Ser15 were examined by intracellular flow cytometry. Of these genes, only the p21 Cip1/Waf1 and p19Ink4d genes displayed altered mRNA expression in Mx1-cre NKAP cKO LSKs with a statistically significant difference of greater than 2-fold from the controls (SJL LSKs from the same mouse, and WT littermate and SJL from the control WT/SJL chimera, P < .05). The difference in expression in cMyc in the Mx1-cre NKAP cKO HSCs was less than 2 fold and not statistically significant (P > .1). Also, because there is no HSC defect in Mx1-cre cMyc heterozygous mice,31 this alteration is presumably not responsible for the phenotype observed. Interestingly, both p21 Cip1/Waf1 and p19 Ink4d are cyclin-dependent kinase inhibitors (CKIs), and both were increased in Mx1-cre NKAP cKO HSCs. p21 Cip1/Waf1 expression was increased approximately 5-fold and p19 Ink4d approximately 2-fold in the NKAP-deleted HSCs relative to each of the 3 controls. p19 Ink4d is a member of the INK family of CKIs that inhibit cyclin D/cdk4 and cyclin D/cdk6 complexes in early G0/G1 (reviewed in Sherr and Roberts32 ). p21 Cip1/Waf1, a member of the Cip/Kip family of CKIs, inhibits the function of cyclin E/cdk2 and cyclin A/cdk2 complexes at late G0/G1.32 While p21 Cip1/Waf1 is normally a positive regulator of cyclin D/cdk complexes, overexpression of p21 Cip1/Waf1 can also inhibit cyclin D/cdk4 or cyclin D/cdk6 complexes.32 Therefore, the combined overexpression of p19 Ink4d and p21 Cip1/Waf1 CKIs may lead to inhibition of cyclin/cdk complexes at the G0/G1 to S transition, and thus be responsible for the decreased proliferation observed in NKAP-deficient LSKs.
Direct regulation of the p21 promoter by NKAP
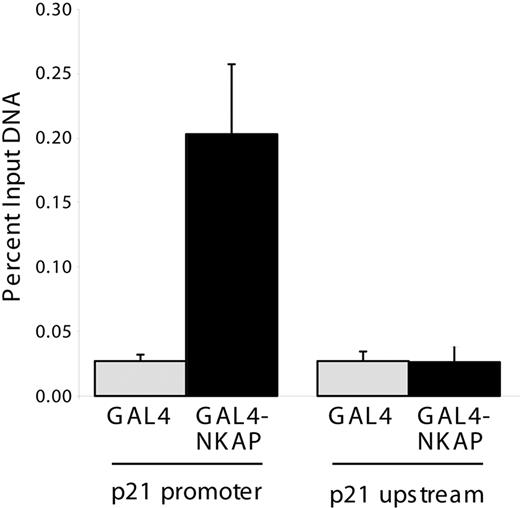
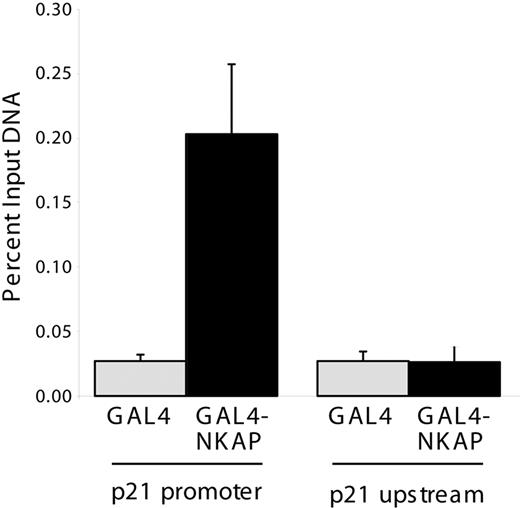
p21 Cip1/Waf1 mRNA expression is regulated by p53, however, no alterations in p53 mRNA, protein or phospho-protein levels were observed in Mx1-cre NKAP cKO LSKs (Figure 6), demonstrating that the increased expression of p21 Cip1/Waf1 in NKAP-deficient LSKs occurs in a p53-independent manner. p21 Cip1/Waf1 expression is also regulated by Notch signaling dependent upon a CSL site in its promoter.33 To examine whether NKAP directly associates with the p21 Cip1/Waf1 promoter, ChIP of NKAP were performed as previously described.12 NKAP fused to the Gal4 DNA-binding domain (Gal4DBD-NKAP) or the isolated Gal4DBD were transfected into a 293 cell line that contains a stably integrated Gal4-TK-reporter,17 and proteins were precipitated with anti-Gal4 antibodies. Gal4DBD and Gal4DBD-NKAP associated with the integrated Gal4 reporter to similar extents (data not shown), demonstrating equivalent precipitation. To examine the association of NKAP with the p21 Cip1/Waf1 promoter, previously characterized ChIP primers for the Notch binding site within this promoter were used.18 Gal4DBD-NKAP associated with the p21 Cip1/Waf1 promoter (Figure 7) to approximately 5-fold higher extent than Gal4DBD (P < .01, Student t test), but did not associate to a greater extent with a sequence approximately 4 kb upstream of the p21 transcriptional start site.19 Thus, NKAP, or a complex of proteins containing NKAP, can directly associate with the p21 Cip1/Waf1 promoter. As NKAP has been shown to be a transcriptional repressor,12 loss of this association likely results in the increased p21 Cip1/Waf1 mRNA expression in NKAP-deficient LSKs.
Analysis of genes involved in proliferation and survival in NKAP-deficient HSCs. At day 4 after a single injection of poly-IC, bone marrow LSK cells from WT/SJL and (Mx1-cre NKAP) cKO/SJL mixed radiation chimeras were purified by FACS sorting. mRNA was generated, and examined by Q-PCR for expression of E2A, GATA2, Bmi1, cMyc, Hes1, cMyb, p19, p21 Cip1/Waf1, p27, Mcl1, and p53. Results shown are the average of triplicate amplifications from independent sorts from 2 mice in each group, normalized to 18S RNA. For each gene analyzed, expression was normalized to the expression in one of the replicates of WT littermate LSK. Other graphs (p53, phospho-p53 Ser15, Bcl-2, and Bcl-xL) show mean fluorescence intensity (MFI) from intracellular flow cytometry in LSK cells within each population, and the graphs are the average MFI in each population from 3 mice in each group. Error bars represent SD.
Analysis of genes involved in proliferation and survival in NKAP-deficient HSCs. At day 4 after a single injection of poly-IC, bone marrow LSK cells from WT/SJL and (Mx1-cre NKAP) cKO/SJL mixed radiation chimeras were purified by FACS sorting. mRNA was generated, and examined by Q-PCR for expression of E2A, GATA2, Bmi1, cMyc, Hes1, cMyb, p19, p21 Cip1/Waf1, p27, Mcl1, and p53. Results shown are the average of triplicate amplifications from independent sorts from 2 mice in each group, normalized to 18S RNA. For each gene analyzed, expression was normalized to the expression in one of the replicates of WT littermate LSK. Other graphs (p53, phospho-p53 Ser15, Bcl-2, and Bcl-xL) show mean fluorescence intensity (MFI) from intracellular flow cytometry in LSK cells within each population, and the graphs are the average MFI in each population from 3 mice in each group. Error bars represent SD.
Chromatin immunoprecipitation of NKAP at the p21 promoter. 293:Gal4-TK-luc cells were transfected with Gal4DBD or Gal4DBD-NKAP and ChIP was performed using anti-Gal4. DNA recovered by ChIP was compared with input DNA from each transfection by Q-PCR to measure the quantity of p21 promoter or upstream genomic sequence. Data shown are from 3 independent immunoprecipitations. Error bars show SD.
Chromatin immunoprecipitation of NKAP at the p21 promoter. 293:Gal4-TK-luc cells were transfected with Gal4DBD or Gal4DBD-NKAP and ChIP was performed using anti-Gal4. DNA recovered by ChIP was compared with input DNA from each transfection by Q-PCR to measure the quantity of p21 promoter or upstream genomic sequence. Data shown are from 3 independent immunoprecipitations. Error bars show SD.
Discussion
Hematopoietic cells must tightly balance proliferation with differentiation and self-renewal. Mutations in cell-cycle regulators, which either suppress or promote proliferation, disturb this balance leading to hematopoietic failure due to stem cell depletion or exhaustion, respectively (reviewed in Myatt and Lam7 and Ezoe et al8 ). Transcriptional activators or repressors that are required for HSC survival and self-renewal, in part, exert their effect through regulating expression of cell-cycle regulators. The loss of NKAP expression in HSCs leads to increased expression of the cyclin-dependent kinase inhibitors p21 Cip1/Waf1 and p19 Ink4d. Overexpression of both p21 Cip1/Waf1 and p19 Ink4d inhibits the function of cyclin D/cdk complexes that regulate entry into cell cycle from G0/G1. Interestingly, mice deficient for all mammalian D-type cyclins (D1/D2/D3 triple knockout) have a similar HSC defect34 compared with NKAP deficiency. Mice deficient in all 3 D-type cyclins die late in embryogenesis and have severe blocks in all hematopoietic lineages.34 At E14.5, the absolute number of LSK cells is decreased almost 6-fold compared with WT littermates, indicating a requirement for D-cyclins in HSCs and hematopoiesis. This requirement was shown to be cell intrinsic using radiation chimeras.34 Dramatic effects on hematopoiesis were also observed in cdk4/cdk6 double-knockout mice.35 Therefore, the similarities between vav-cre NKAP cKO and cyclin-D1/D2/D3 triple knockout, and cdk4/cdk6 double knockout, may be due to the inhibition of D-type cyclins by increased expression of the CKIs p21 Cip1/Waf1 and p19 Ink4d. The exact role of p21 Cip1/Waf1 and/or p19 Ink4d overexpression to HSC survival and maintenance in NKAP-deficient HSCs remains to be determined. Initial examination of p21 KO/Mx1-cre NKAP cKO mice indicate that loss of p21 alone is insufficient to rescue the HSC defect (V.S.S., unpublished results, April 2010), indicating that additional genes are also important. In particular, because loss of NKAP expression in HSCs also leads to decreased survival, as measured by annexin V staining, it may be that additional genes are dysregulated in NKAP-deficient HSCs and therefore deficiency in both p21 Cip1/Waf1 and p19 Ink4d may not be sufficient to rescue the HSC defect upon NKAP deletion.
We have shown that the transcriptional repressor NKAP is absolutely required, in a cell-intrinsic manner, for the maintenance and survival of adult HSCs. The loss of NKAP during embryonic development leads to multilineage blocks in hematopoiesis, although HSCs as defined by the LSK/SLAM family markers are present. This difference between the vav-cre and Mx1-cre data may simply reflect an absolute requirement for NKAP within adult HSCs, compared with fetal HSCs. Adult and fetal HSCs are not equivalent, as demonstrated by the requirement for Sox17 in fetal but not adult HSCs.36 Alternatively, it may be that NKAP deletion is incomplete in vav-cre mice, which accounts for the presence of HSCs, albeit at decreased frequency. However, our data clearly demonstrates the obligate role for NKAP in adult HSC maintenance and survival.
Previously, we found that NKAP is a novel transcriptional repressor that functions, in part, as a component of the Notch corepressor complex and thus, is a negative regulator of Notch signaling. However, the dramatic phenotype observed in vav-cre NKAP cKO mice cannot be explained by altered Notch signaling alone. Notch1 signaling has been shown to be important in the generation, but not maintenance, of definitive hematopoiesis.37,38 Overexpression of truncated, constitutively active Notch1 (ICN1) in HSCs during in vitro differentiation resulted in impaired development (in both percentage and absolute numbers) of granulocyte lineages.39 However, retroviral transduction of bone marrow progenitors with ICN1 altered lymphopoiesis in favor of T-cell development at the expense of B cell development, while myelopoiesis remained intact.40 In addition, poly-IC induction in Mx1-cre RBP-J cKO mice results in almost complete deletion leading to abrogation of T-cell development, but not to lethality or an HSC defect.38,41 Therefore, constitutive activation of Notch (via ICN1), or loss of RBP-J, does not produce a dramatic block in hematopoiesis like that observed in vav-cre or Mx1-cre NKAP cKO mice. These data indicate that NKAP must also regulate pathways independent of Notch that lead to the loss of HSCs when dysregulated. p19 Ink4d has not been demonstrated to be positively regulated by Notch, but is up-regulated upon treatment with HDAC inhibitors.42,43 Previously, we demonstrated that NKAP is a transcriptional repressor that associates with HDAC3, and that the HDAC3-binding domain is required for transcriptional repression by NKAP.12 Similarly, p21 Cip1/Waf1 expression is also increased upon treatment with HDAC inhibitors.44 Investigation into the molecular mechanisms responsible for the up-regulation of p19 Ink4d and p21 Cip1/Waf1 will give further insight into regulation of gene expression by NKAP.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Thomas Graf for sharing his vav-cre transgenic line with us, and Jay Thompson for his help with microscopy. We thank Avinash Bhandoola, Dave Allman, Warren Pear, and members of their laboratories for thoughtful discussions and assistance with flow cytometry.
This work was supported by National Institutes of Health grants R21AI069031 and R01AI083279 to V.S.S.
National Institutes of Health
Authorship
Contribution: A.G.P., M.J.S., K.G., R.S., and M.N.-H. performed the research and analyzed the data; A.G.P. and V.S.S. wrote the manuscript; M.J.S., K.M., and V.S.S. revised the manuscript; and all authors contributed to the design of the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Virginia Smith Shapiro, PhD, 401C Guggenheim Bldg, Mayo Clinic, Rochester, MN 55905; e-mail: Shapiro.virginia1@mayo.edu.