Abstract
Inhibition of cyclooxygenase (COX)–derived prostaglandins (PGs) by nonsteroidal anti-inflammatory drugs (NSAIDs) mediates leukocyte killing of bacteria. However, the relative contribution of COX1 versus COX2 to this process, as well as the mechanisms controlling it in mouse and humans, are unknown. Indeed, the potential of NSAIDs to facilitate leukocyte killing of drug-resistant bacteria warrants investigation. Therefore, we carried out a series of experiments in mice and humans, finding that COX1 is the predominant isoform active in PG synthesis during infection and that its prophylactic or therapeutic inhibition primes leukocytes to kill bacteria by increasing phagocytic uptake and reactive oxygen intermediate-mediated killing in a cyclic adenosine monophosphate (cAMP)-dependent manner. Moreover, NSAIDs enhance bacterial killing in humans, exerting an additive effect when used in combination with antibiotics. Finally, NSAIDs, through the inhibition of COX prime the innate immune system to mediate bacterial clearance of penicillin-resistant Streptococcus pneumoniae serotype 19A, a well-recognized vaccine escape serotype of particular concern given its increasing prevalence and multi-antibiotic resistance. Therefore, these data underline the importance of lipid mediators in host responses to in-fection and the potential of inhibitors of PG signaling pathways as adjunc-tive therapies, particularly in the con-text of antibiotic resistance.
Introduction
Antibiotic resistance arising from the selective pressure generated by excessive/inappropriate antibiotic use in human and veterinary practices poses major challenges to the management of infection, particularly with the scarcity of new antibacterial drugs.1 For this reason, there is considerable interest in developing strategies to counteract multidrug microbial resistance either as an inde-pendent pharmaceutical entity or as an adjunct to existing treatment regimes.
Cyclooxygenase (COX) metabolizes phospholipase A2-liberated arachidonic acid to PGH2, which serves as a substrate for downstream synthases to generate prostaglandins (PGs) and thromboxane A2.2 Two isoforms of COX exist, with constitutively expressed COX1 suggested to make PGs to aid physiologic processes while COX2 is inducible at sites of inflammation believed to generate pathophysiologic PGs.3 During inflammation in response to infection, PGs of the E/D series elevate cyclic adenosine monophosphate (cAMP) by activating EP2/EP4 or DP1 receptors,4 respectively. Elevating cAMP inhibits 2 pivotal steps in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-mediated bacterial killing, namely the phosphorylation as well as the translocation of the cytosolic p47phox subunit to cell membrane.5-8 Moreover, by signaling through EP2, PGE2 inhibits FcγR-mediated phagocytosis.9 Therefore, as nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit PG synthesis,10 it is not surprising that targeting COX pathways of arachidonic acid metabolism is attracting current attention as a means of facilitating leukocyte killing of bacteria.11 That notwithstanding, the antibacterial properties of COX1 versus COX2 inhibitors is unknown as is their respective roles in PG synthesis, cAMP expression and therefore cytokine balance during infection in both mouse and in humans. Moreover, it is not known whether NSAIDs interfere with antibiotic-mediated bacterial killing. Finally, it is unknown whether priming the innate response by PG inhibition would enhance leukocyte killing of antibiotic-resistant bacteria by overcoming strategies drug-resistant bacteria have developed to parry antibiotic efficacy.
To investigate this, we carried a series of experiments in mouse and in humans finding that COX1 is the predominant isoform active in synthesizing PGs during infection and that prophylactic as well as therapeutic inhibition of both COX isoforms kills bacteria by increasing phagocytic uptake and reactive oxygen intermediate-mediated killing. Moreover, we report that inhibiting PGs synthesis and/or signaling enhances bacterial killing in humans and that NSAIDs do not interfere with the mode of action of antibiotics but exert an additive effect when used in combination. Finally, we show that priming the innate immune system by PG inhibition enables the effective killing of antibiotic-resistant bacteria. These data highlight the therapeutic potential of inhibitors of PG biosynthetic and/or signaling pathways in combination with existing antibacterial regimes in combating recalcitrant bacterial infections, particularly resistant strains.
Methods
Preparation of bacteria for in vivo experiments
A clinical isolate of Group B Streptococcus (GBS) from adult humans, NCTC10/84 (serotype V) was a gift from Dr. T Lawrence (Center d'Immunologie de Marseille, France). Bacteria were grown in 3% Todd-Hewitt Broth (TH Broth; Difco) without agitation at 37°C in 5% CO2 to an OD600 of 0.3-0.4. A single loop of culture was transferred to an agar plate made up of 3% TH broth and 2% bactericidal agar (TH agar; Oxoid) and incubated overnight at 37°C. Agar plates containing GBS colonies were transferred to 4°C and stored for future use. Twenty-four hours before experimentation one colony of GBS was removed from agar and grown in 3 mL of TH broth at 37°C for 8 hours without agitation. After this time, 50 μL inoculate was added to 5 mL of fresh TH broth and incubated overnight at 37°C. Two milliliters of this solution was added to 18 mL of TH broth and divided into 4 × 50-mL falcon tubes (5 mL in each), to optimize growth and incubated at 37°C until the OD600 reached 0.4, equivalent to 108 colony-forming units per milliliter (cfu/mL). GBS was collected by centrifugation at 800g for 5 minutes, washed with sterile phosphate-buffered saline (PBS), and kept on ice to limit further growth. To elicit peritonitis, GBS (3 × 107 cfu) was inoculated intraperitoneally in 300 μL of sterile PBS.12
Preparation of bacteria for human in vitro experiments
Two clinical isolates of S pneumoniae known to confer different susceptibilities to the beta lactam (β-lactam) antibiotic penicillin, ND6022 (strain 199, serotype 19A, intermediate resistance) and 10839 (strain 193, serotype 19A, susceptible), denoted ST199 and ST193, respectively, were donated by Dr W. Hanage (Imperial College London). S pneumoniae was cultured at 37°C in 5% CO2 on 5% blood Columbia agar (Oxoid) plates made using defibrinated horse blood (TCS Biosciences). Working stocks were made by transferring one colony of S pneumonia or GBS to TH broth (containing 0.5% yeast extract for S pneumonia) and grown to an OD600 of between 0.3 and 0.4. Ten percent glycerol was added and bacteria were stored in single-use aliquots at −80°C. The concentration of each stock aliquot was determined by plating 10-fold serial dilutions onto Colombia blood agar (S pneumoniae) or TH agar (GBS), culturing overnight and counting the resulting number of colonies expressed as cfu/mL.
Animal maintenance, peritonitis, and drug dosing
Wild-type (WT) and COX1-deficient mice (COX1−/−), all C57bl6/J were bred under standard conditions and maintained in a 12-hour light/12-hour dark cycle at 22 ± 1°C and given food and tap water ad libitum in accordance with United Kingdom Home Office regulations. Peritonitis was induced by intraperitoneal injection of either a resolving inoculums of GBS (3 × 107) in 300 μL sterile PBS or 1 mg zymosan A (Sigma-Aldrich). Aspirin (200 mg/kg; Sigma-Aldrich), indomethacin (3 mg/kg; Sigma-Aldrich), NS398 (10 mg/kg; Cayman), SC-560 (5 mg/kg, Cayman), or vehicle alone (1% wt/vol gum tragacanth; Sigma-Aldrich) were dosed orally in 100 μL of gum traganth either 1 hour before or 1 hour after induction of peritonitis. At selected time points after triggering peritonitis, the peritoneum was lavaged (sterile PBS containing 3.0% sodium citrate) and cells counted by hemocytometer after separation from edema by centrifugation with both cells and edema stored at −80°C for cytokine, lipid, and cAMP quantification. To determine effects of NSAIDs on bacterial counts, either peritoneal washouts or peripheral blood was taken by cardiac puncture 3 hours after GBS injection and cfu counted after 24 hours incubation on agar. To rescue effects of NSAIDs on bacterial killing and cytokine synthesis, 100 mg/kg of the PGE2 analog, 9-deoxy-9-methylene-16,16-dimethyl PGE2 (meteneprost; Cayman) or 0.1 mg/kg of di-butyryl-cAMP (db-cAMP; Sigma-Aldrich) were injected intraperitoneally 55 minutes after indomethacin and therefore 5 minutes before GBS injection. For survival assays, mice were injected intraperitoneally with GBS (6 × 107) and monitored every 4 hours. In these experiments, NSAIDs were given orally 1 hour before GBS infection and every 24 hours thereafter while infliximab (Remicade, University College London Hospital Pharmacy) was given as a single-bolus dose of 20 mg/kg intraperitoneally 1 hour before GBS.
S pneumoniae and GBS in human whole blood assays
Healthy male volunteers over the age of 18 who had not taken NSAIDs for 2 weeks were recruited according to the University College London Research Ethics Committee, which approved the protocol (reference number 1309/003), and all subjects gave written informed consent in accordance with the Declaration of Helsinki. Ten milliliters of peripheral blood were collected in EDTA from volunteers who had and had not taken oral 500 mg naproxen. Blood from volunteers who consumed naproxen was taken 1 hour after drug ingestion, seeded onto 24-well plates and made up to a final volume of 500 μL with RPMI media containing 10% fetal bovine serum (FBS) and 1% L-glutamine without antibiotics. Separately, leukocytes isolated from whole human blood without naproxen were pretreated for 10 minutes with a pan EP/DP PG receptor antagonist targeted against EP1, EP2, EP3-III, and DP1 receptors (AH6809 at 50, 100, and 300μM) as well as against individual PG receptors including EP4 (L-161982 at 10μM; Cayman), DP1 (MK-0524 at 0.5μM; Cayman) and IP (CAY10441 at 1μM; Cayman), and/or penicillin at a concentration that is ineffective at killing S pneumoniae ST199 (0.075 μg/mL; Sigma-Aldrich). Due to the insolubility of these compounds in aqueous solution, dimethyl sulfoxide (DMSO) was used at a final concentration of 0.1% or lower. Cells were stimulated with opsonized-GBS or opsonized-S pneumoniae (ST193 or ST199) at a ratio of 10:1 (10 bacteria:1 leukocyte) at 37°C in 5% CO2. After 1 hour of incubation, surviving bacteria were enumerated by overnight incubation on agar plates.
FACS analysis
Peritoneal cells (0.5 × 106) isolated at specified time points were preincubated at room temperature (10 minutes) with 1 μL of mouse SeroBlock FcR (AbD serotec) diluted in fluorescence-activated cell sorting (FACS) buffer (incomplete Dulbecco modified Eagle medium [DMEM], 20mM glucose, 1% wt/vol bovine serum albumin [BSA]). Cells were aliquoted and coincubated with either F4/80 (eBiosciences), GR1, Ly6G (BD Pharmingen), or CD3/CD19 (Serotec) antibodies for 30 minutes at room temperature in the dark before FACS analysis, using isotype antibodies as controls.
Western blotting, cytokines, cAMP, and prostanoids
Western blotting was carried as described previously.13 Briefly, total leukocytes, isolated from the peritoneum, were lysed in lysis buffer (50mM Tris HCl, 250mM NaCl, 3mM EDTA pH8, 1% Triton-X-100, 0.5% NP-40, 10% glycerol, 2mM 1,4-dithithreitol [DTT], 0.1mM phenylmethylsufonyl [PMSF], 0.1mM Na3VO4, 1mM NaF, 1 μg/mL aprotinin, 1 μg/mL pepstatin A, 1 μg/mL bestatin, and 1 μg/mL leupeptin) and protein concentration determined by bicinchoninic acid (BCA) assay (Pierce). Five micrograms of COX1 and 20 μg of COX2 protein were separated by SDS-PAGE using control standards for COX1 (4 μg rat gut mesentery) or COX2 (5 μg of RAW.264.7 stimulated with 1 μg/mL LPS for 24 hours). Separated proteins were transferred onto a polyvinylidene fluoride transfer membrane (Immobilon, Millipore) and incubated with COX1 (1:1000 dilution), COX2 (1:3000) rabbit polyclonal IgG antibody (Santa Cruz Biotechnology) or β-actin mouse monoclonal IgG1 antibody (1;100 000; Sigma-Aldrich) at 4°C overnight under agitation in Tris-HCl containing 1% Tween-20 (Sigma-Aldrich) supplemented with 5% nonfat milk powder (Marvel) and 1% BSA (Sigma-Aldrich). After washing, proteins were incubated with horseradish peroxidase (HRP)–conjugated antibodies for COX1 and COX2 (goat anti–rabbit IgG; Santa Cruz Biotechnology) and β-actin (goat anti–mouse IgG; Santa Cruz Biotech-nology) for 1 hour at room temperature under agitation. Membranes were washed and specified proteins visualized by enhanced chemiluminescence (ECL) hyperfilm. Levels of mediators in the peritoneal cell–free inflammatory exudate were measured by either enzyme-linked immunosorbent assay (ELISA) for tumor necrosis factor (TNF)–α or interleukin (IL)–10 (eBiosciences and BD Biosciences, respectively) or enzyme immunoassay (EIA) for PGE2, 6-keto PGF1α, 2,3-dinor 6-keto PGF1α, PGD2 and cAMP (Cayman) according to the manufacturer's instructions.
Opsonization
Stock bacteria were removed from −80°C on the day of experimentation and allowed to thaw at room temperature (10 minutes), incubated in a 25% human serum solution for 30 minutes at 37°C and centrifuged at 12 000g for 10 minutes, discarding the supernatant. Pooled human serum was obtained from normal human volunteers that had not been vaccinated with the Pneumococcal polysaccharide or conjugate vaccine.
Characterizing S pneumoniae susceptibility to penicillin
To test the susceptibility of S pneumoniae to penicillin, a minimum inhibitory concentration (MIC) test was performed. Bacteria were cultured at 37°C on 5% blood Columbia agar in 5% CO2 in the presence of a benzylpenicillin Etest strips (AB BIODISK) with MIC of 0.016-256 μg/mL and 32-0.002 μg/mL. Twenty-four hours later, MIC values were determined where the edge of the inhibition ellipse intersected the strip (supplemental data and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). It was concluded that the MIC value of ST199 was between 0.094 μg/mL and 0.047 μg/mL (mean: 0.07 μg/mL) and 0.012 μg/mL for ST193. Thus, 0.075 μg/mL penicillin was used, at which concentration ST193 would be fully susceptible and ST199 partially resistant to penicillin.
Blood flash lysis
Three milliliters of blood from each volunteer was transferred into 50-mL Falcon tubes followed by 42 mL of deionized water for 19 seconds, after which time 5 mL of 9% saline (to generate final concentration of 0.9%) was added to maintain osmolarity. After the mixture was allowed to equilibrate for 5 minutes on ice, cells were centrifuged at 400g for 10 minutes at 4°C, the remaining supernatant removed, and white blood cell pellet resuspended in PBS.
C3 binding assay
Complement factor interaction with S pneumoniae was assessed using flow cytometry assays as described.14,15 Briefly, 1 × 106 cfu S pneumoniae or GBS was incubated in 10 μL of human serum (diluted to 20% or 50% in PBS) for 30 minutes at 37°C with/without 400μM naproxen, washed twice with 500 μL of PBS/0/1% Tween-20 and resuspended in 50 μL of PBS/0.1% Tween-20 containing 1:300 dilution of fluorescein isothiocyanate (FITC)–conjugated polyclonal goat anti–human C3 antibody (ICN). FITC labeling of bacteria was determined by FACSCalibur (BD Biosciences) with gating based on the analysis of at least 25 000 bacteria as described previously.14 The very large differences in results for control and sera with naproxen were compared using the geometric mean fluorescence intensities of bacteria positive for C3b.
Phagocytosis assays
FITC-labeled zymosan (Invitrogen; 0.5 mg per mouse) was injected intraperitoneally 1 hour after oral treatment with gum traganth (control) or 200 mg/kg aspirin. Phagocytosis, using naive peritoneal macrophages, was measured at 10, 15, 30, and 60 minutes after FITC-labeled zymosan by performing a peritoneal lavage with ice-cold 2mM EDTA (ethylenediaminetetraacetic acid; Amresco). Cells was quenched in 0.002% trypan blue for 5 minutes and then fixed in ice-cold 4% paraformaldehyde (Acros organics). Cells were washed of unbound FITC-labeled zymosan 3 times before analyzing phagocytosis using FACS. Control mice were used at each time-point (mice with 0.5 mg of zymosan without FITC). For in vitro phagocytosis on human blood leukocytes, S pneumoniae was fluorescently labeled with 6-carboxyfluorescein succinimidyl ester (FAMSE; Molecular Probes) as described previously.16 Human peripheral blood leukocyteswere seeded at 2.5 × 105 per 24-well plate and pretreated with andwithout 400μM naproxen for 30 minutes at 37°C, 5% CO2. Cells were then stimulated with FAMSE labeled, opsonized penicillin-susceptible (ST193) S pneumoniae at a ratio of 10:1. After 1.5, 3, and 5 minutes, 100 μL was removed from triplicate wells and fixed using 1% paraformaldehyde (Sigma-Aldrich) and quenched with 500 μL 0.002% trypan blue and analyzed by FACSCalibur (Becton Dickinson).
NADPH oxidase activity
Leukocytes (1 × 105) were incubated with 50μM Amplex Red Ultra (Invitrogen), 0.1 U/mL horseradish peroxidase (R&D Systems) with/without (phorbol myristate [PMA]; 1 ng/mL). Fluorescence was measured using excitation in the range of 530-560 nm and emission detection at 590 nm every 30 seconds with data analyzed using Omega Data Analysis software Version 1.02 (BMG Labtech) determining the rate of O2 consumption between 0 and 15 minutes. The absolute concentration of hydrogen peroxide (H2O2) was ascertained by adding an excess of Amplex Red Ultra and HRP to varying concentrations of H2O2 (Calbiochem), allowing the reaction to proceed for 15 minutes, which ensured all the H2O2 was converted into O2.
NSAID/live bacterial coincubation assay
To exclude NSAIDs possessing direct antibiotic properties against bacteria, dual COX inhibitors naproxen and indomethacin as well as the COX2-specific inhibitor NS398 were incubated with GBS in a 6% plasma/DMEM solution in the absence of mammalian leukocytes. After 1 hour, numbers of surviving bacteria were incubated on Todd Hewitt agar for enumeration 24 hours later. NS398 and indomethacin were used at concentrations concordant with their IC80 and at levels 10× higher,17 while naproxen was used at a concentration equivalent to serum levels of healthy volunteers after ingestion of 500 mg twice daily18 and a dose 10× lower. Penicillin was used as a reference at 0.075 μg/mL bringing about greater than 99% killing of bacteria.
Results
COX, PG, and cytokine profiles throughout infectious inflammation
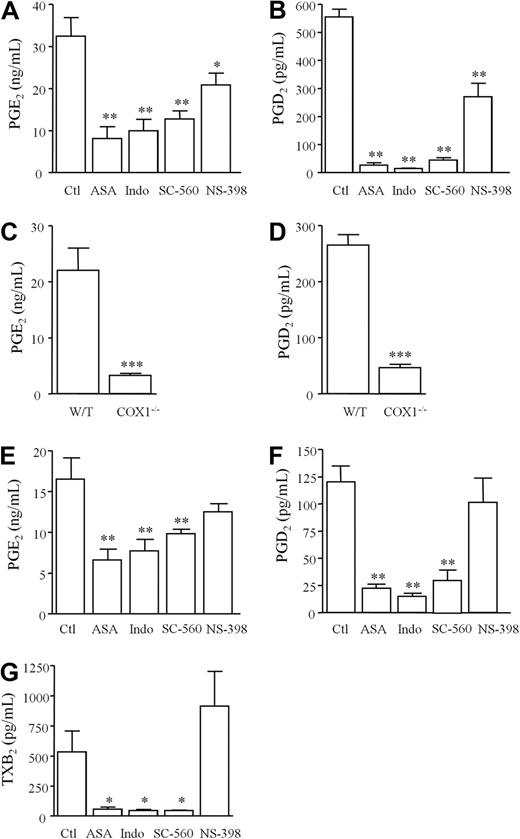
After intraperitoneal injection of GBS to WT mice, inflammation (total leukocyte infiltrate) peaked between 6 and 24 hours and waned slowly thereafter (Figure 1A). Profiles of individual leukocytes are illustrated in Figure 1B-C. Unlike COX2, total leukocyte COX1 was constitutively expressed in the naive peritoneum and throughout infection (Figure 1D) with cell-free peritoneal exudate levels of PGE2 (Figure 1E) and 6-keto PGF1α (PGI2 metabolite, Figure 1F) peaking at 3 hours. 2,3-dinor 6-keto PGF1α, the β oxidation metabolite of 6-keto PGF1α and principle urinary metabolite of PGI2 in humans, peaked as inflammation resolved (Figure 1F). As with lipids, inflammatory cytokines TNFα (Figure 1G) and IL-10 (Figure 1H) were also maximal early in inflammatory exudates in response to GBS (3 hours). To discern the relative contribution of COX1 and COX2 to PG synthesis during GBS-elicited peritonitis, selective COX inhibitors (SC-560 for COX1 and NS-398 for COX2) and nonselective COX inhibitors (aspirin and indomethacin) were used. Results of these experiments revealed that inhibition of COX1 brought about a greater reduction in PGE2 (Figure 2A) and PGD2 (Figure 2B) at 3 hours than did inhibition of COX2; findings supported using COX1−/− mice (Figure 2C-D) bearing GBS-induced peritonitis. An equally predominant role for COX1 in PG generation was also found in a noninfectious peritonitis triggered by zymosan (Figure 2E-F). As NS-398 was used at a concentration that did not inhibit plasma TxB2 (Figure 2D), a marker of COX1 activity, these data reveal a predominant role for COX1 in the generation of PGs during infectious and noninfectious inflammation, as shown previously.19,20
Characterization of infectious peritonitis. Injecting GBS intraperitoneally to WT mice triggers an immediate (A) leukocytes infiltrate comprising (B) GR1-positive polymorphonuclear leukocytes (PMNs) and F4/80-positive macrophages as well as (C) CD3-positive T and CD19-positive B cells. Total peritoneal leukocytes were prepared for (D) COX1 and COX2 protein expression while (E) PGE2, (F) prostacyclin, measured as its stable metabolites 6-keto PGF1α and 2,3-dinor 6-keto PGF1α, (G) TNFα, and (H) IL-10 were determined in the cell-free inflammatory exudates. Values are expressed as the mean ± SEM of 5-12 mice/group.
Characterization of infectious peritonitis. Injecting GBS intraperitoneally to WT mice triggers an immediate (A) leukocytes infiltrate comprising (B) GR1-positive polymorphonuclear leukocytes (PMNs) and F4/80-positive macrophages as well as (C) CD3-positive T and CD19-positive B cells. Total peritoneal leukocytes were prepared for (D) COX1 and COX2 protein expression while (E) PGE2, (F) prostacyclin, measured as its stable metabolites 6-keto PGF1α and 2,3-dinor 6-keto PGF1α, (G) TNFα, and (H) IL-10 were determined in the cell-free inflammatory exudates. Values are expressed as the mean ± SEM of 5-12 mice/group.
COX1 is the predominant isoforms functional during infectious peritonitis. Selective COX inhibitors (SC-560 for COX1 and NS-398 for COX2) and nonselective COX inhibitors (aspirin and indomethacin) were dosed orally to WT animals 1 hour before GBS injection. Cell-free peritoneal exudate levels of (A) PGE2 and (B) PGD2 were measured 3 hours after GBS with similar results obtained for (C) PGE2 and (D) PGD2 using COX1 knockout mice. In addition, COX inhibitors were dosed to mice 1 hour before intraperitoneal zymosan (noninfectious stimulus) with cell-free peritoneal exudate levels of (E) PGE2 and (F) PGD2 measured 3 hours later. NS-398 was used at dosing levels that do not inhibit (G) COX1-derived plasma TxB2 measured 3 hours after intraperitoneal GBS injection. Data were analyzed by one-way ANOVA and Dunnett multiple comparison test or by unpaired Student t test. Values are expressed as the mean ± SEM of between 5-12 mice/group. * P < .05; ** P < .01 and *** P < .001.
COX1 is the predominant isoforms functional during infectious peritonitis. Selective COX inhibitors (SC-560 for COX1 and NS-398 for COX2) and nonselective COX inhibitors (aspirin and indomethacin) were dosed orally to WT animals 1 hour before GBS injection. Cell-free peritoneal exudate levels of (A) PGE2 and (B) PGD2 were measured 3 hours after GBS with similar results obtained for (C) PGE2 and (D) PGD2 using COX1 knockout mice. In addition, COX inhibitors were dosed to mice 1 hour before intraperitoneal zymosan (noninfectious stimulus) with cell-free peritoneal exudate levels of (E) PGE2 and (F) PGD2 measured 3 hours later. NS-398 was used at dosing levels that do not inhibit (G) COX1-derived plasma TxB2 measured 3 hours after intraperitoneal GBS injection. Data were analyzed by one-way ANOVA and Dunnett multiple comparison test or by unpaired Student t test. Values are expressed as the mean ± SEM of between 5-12 mice/group. * P < .05; ** P < .01 and *** P < .001.
NSAIDS enhance bacterial killing in rodents
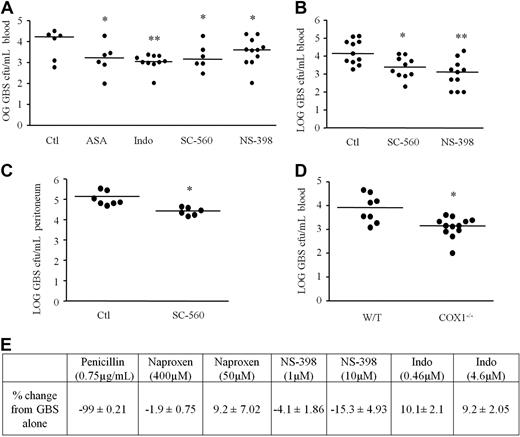
Selective and nonselective COX inhibitors were given either as a single dose 1 hour before intraperitoneal GBS injection (Figure 3A) or therapeutically 1 hour after GBS, (Figure 3B). Three hours after inoculation, peripheral blood (Figure 3A-B) or peritoneal lavage fluid (Figure 3C) were taken and cultured on agar for 24 hours to determine GBS cfu numbers. Regardless of dosing regimes, there was consistent and significant enhancement of bacterial killing with all categories of NSAIDs by up to 90%. Enhanced bacterial killing was also observed in COX1 deficient mice (Figure 3D) thereby excluding an off-target effect of COX inhibitors. COX2−/− mice not used in these studies as they develop spontaneous sterile peritonitis.21 Therefore, we have extended the previous findings of others5,9,11 by showing that NSAIDs prime the innate immune response to kill bacteria efficiently when given either prophylactically or therapeutically. We confirmed that these effects were not due to NSAIDs acting directly on bacteria (Figure 3E) finding that naproxen, indomethacin, and NS-398 incubated with GBS in the absence of leukocytes did not affect bacteria viability, whereas penicillin was 99% efficacious in this in vitro assay.
COX inhibition enhances bacteria killing in rodents. Mice were given dual (indomethacin/aspirin), COX1 (SC-560) or COX2 (NS-398) selective inhibitors orally either (A) 1 hour before or (B) therapeutically 1 hour after intraperitoneal inoculation of GBS. To exclude dampened bacterial translocation as a potential explanation for reduced plasma GBS titres after COX inhibition, peritoneal exudate levels of (C) GBS were measured 3 hours after inoculation. GBS was also injected intraperitoneally to (D) COX1 knockout mice with plasma taken 3 hours later for overnight cfu culture number determination as well as (E) incubated in culture media only with NSAIDs in the absence of leukocytes. Data were analyzed by one-way ANOVA and Dunnett multiple comparison test or by unpaired Student t test. Values are expressed as the mean ± SEM of 5-12 mice/group. * P < .05 and ** P < .01.
COX inhibition enhances bacteria killing in rodents. Mice were given dual (indomethacin/aspirin), COX1 (SC-560) or COX2 (NS-398) selective inhibitors orally either (A) 1 hour before or (B) therapeutically 1 hour after intraperitoneal inoculation of GBS. To exclude dampened bacterial translocation as a potential explanation for reduced plasma GBS titres after COX inhibition, peritoneal exudate levels of (C) GBS were measured 3 hours after inoculation. GBS was also injected intraperitoneally to (D) COX1 knockout mice with plasma taken 3 hours later for overnight cfu culture number determination as well as (E) incubated in culture media only with NSAIDs in the absence of leukocytes. Data were analyzed by one-way ANOVA and Dunnett multiple comparison test or by unpaired Student t test. Values are expressed as the mean ± SEM of 5-12 mice/group. * P < .05 and ** P < .01.
cAMP reverses NSAID-induced bacterial killing and cytokine synthesis
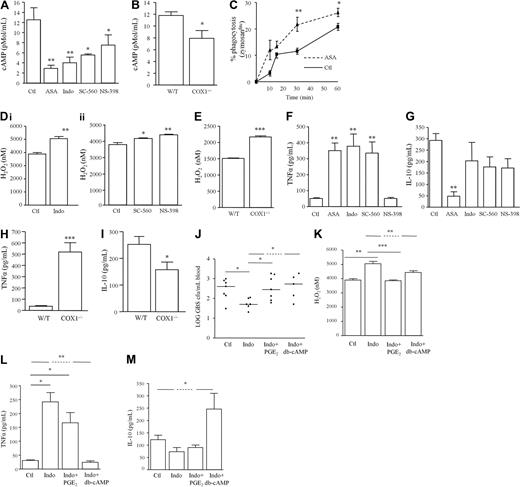
In the above experiments where bacterial killing was enhanced after COX inhibition (Figure 3A-C), cAMP levels in the cell-free peritoneal lavage fluid were dampened (Figure 4A) with similar effects obtained with COX1−/− mice (Figure 4B). This reduction in cAMP was associated with increased peritoneal leukocyte phagocytic capacity (Figure 4C) and NADPH oxidase activity as measured by H2O2 production in both NSAID-treated WTs (Figure 4Di-ii) and COX1 knockouts (Figure 4E). Given that cAMP inhibits proinflammatory signaling pathways, inhibition of COX1 triggered TNFα synthesis in inflammatory exudates (Figure 4F) with a modest reduction in IL-10 (Figure 4G). A similar cytokine profile was obtained using COX1−/− mice (Figure 4H-I). To determine that changes in cAMP consequent to COX inhibition are instrumental in modulating bacterial killing as well as NADPH oxidase activity and cytokine synthesis, mice were given the PGE2 analog 15(S)-15 methyl PGE2 (Metenprost) or dibutyrl-cAMP 55 minutes after indomethacin. One hour after indomethacin and therefore 5 minutes after PGE2/cAMP treatment, mice were injected intraperitoneally with GBS. Overnight culture on agar plates of peripheral blood taken 3 hours after GBS confirmed that enhanced bacterial killing with indomethacin was reversed by 15(S)-15 methyl PGE2 and dibutyrl-cAMP (Figure 4J). Indomethacin-induced NADPH oxidase activity in peritoneal leukocytes (Figure 4K) as well as changes in exudate levels of TNFα (Figure 4L) and IL-10 (Figure 4M) were also reversed by 15(S)-15 methyl PGE2 or dibutyrl-cAMP. These data implicate cAMP in PG-mediated suppression of bacterial killing and proinflammatory cytokine synthesis.
COX inhibition alters phagocytosis, cytokine synthesis, and bacterial killing mechanisms in a cAMP-dependent manner. GBS was injected intraperitoneally to (A) WT mice dosed orally 1 hour earlier with indomethacin (dual COX inhibitor), SC-560 (COX1 inhibitor) or NS-398 (COX2 inhibitor) as well as to (B) COX1 knockout mice. Three hours after GBS injection (A-B) cAMP was measured in cell-free exudates while (C) phagocytosis and (D-E) NADPH oxidase activity was determined in total leukocytes. Cell-free exudate levels of (F-G) TNFα and IL-10 were also determined in NSAID-treated WTs and (H-I) COX1 knockouts. This COX-inhibited differential change in WT mice (J) bacterial killing, (K) NADPH oxidase activity and (L-M) cytokine synthesis was reversed by 15(S)-15 methyl PGE2 (EP agonist) or db-cAMP given 5 minutes before GBS and therefore 55 minutes after NSAIDs. Data are represented and analyzed by an unpaired Student t test or ANOVA followed by either Dunnett or Bonferroni multiple comparison tests. Values are expressed as the mean ± SEM of 5-12 mice/group. * P <.05, ** P < .01, *** P < .001.
COX inhibition alters phagocytosis, cytokine synthesis, and bacterial killing mechanisms in a cAMP-dependent manner. GBS was injected intraperitoneally to (A) WT mice dosed orally 1 hour earlier with indomethacin (dual COX inhibitor), SC-560 (COX1 inhibitor) or NS-398 (COX2 inhibitor) as well as to (B) COX1 knockout mice. Three hours after GBS injection (A-B) cAMP was measured in cell-free exudates while (C) phagocytosis and (D-E) NADPH oxidase activity was determined in total leukocytes. Cell-free exudate levels of (F-G) TNFα and IL-10 were also determined in NSAID-treated WTs and (H-I) COX1 knockouts. This COX-inhibited differential change in WT mice (J) bacterial killing, (K) NADPH oxidase activity and (L-M) cytokine synthesis was reversed by 15(S)-15 methyl PGE2 (EP agonist) or db-cAMP given 5 minutes before GBS and therefore 55 minutes after NSAIDs. Data are represented and analyzed by an unpaired Student t test or ANOVA followed by either Dunnett or Bonferroni multiple comparison tests. Values are expressed as the mean ± SEM of 5-12 mice/group. * P <.05, ** P < .01, *** P < .001.
PG control of cytokine synthesis
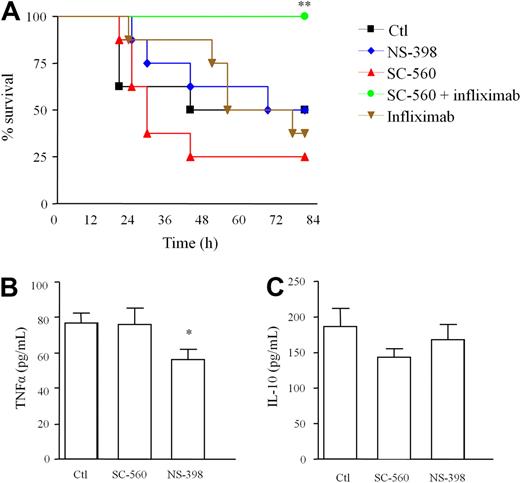
TNFα was substantially elevated when COX1 inhibitors were given before infection (Figure 4E). This was associated with enhanced mortality, which was completely reversed by blocking TNFα with infliximab (Figure 5A). However, COX1 inhibitors did not alter cytokines when given therapeutically, 1 hour after GBS injection (Figure 5B). NS398 was also without effect on TNFα levels when given before GBS (Figure 4E) and even modestly reduced TNFα (Figure 5B) when administered 1 hour after infection. These data suggest that PG/cAMP control of TNFα synthesis is COX1 mediated and occurs within the first 60 minutes of infection. Therefore, prophylactic inhibition of PG synthesis triggers a cytokine storm leading to death, effects not seen when COX is inhibited in a clinically relevant, therapeutic setting after infection is established.
Prophylactic but not therapeutic COX inhibition triggers pro-inflammatory cytokine synthesis. WT mice were injected intraperitoneally with GBS 1 hour after oral administration of NS-398 (COX2 inhibitor), SC-560 (COX1 inhibitor) or SC-560 plus infliximab with (A) complete reversal of COX1-mediated cytokine storm (TNFα synthesis, Figure 4F) afforded by infliximab. SC-560 and NS-398 were dosed orally 1 hour after GBS injection causing little effects on (B-C) inflammatory cytokine levels in cell-free inflammatory exudates 3 hours later. Survival analysis was completed on 8 mice/group and the log-rank test was used to compare each group against one another. ** values represent P < .01 for controls versus Sc-560/infliximab. For cytokines, values are expressed as the mean ± SEM of 5-7 mice/group and analyzed by one-way ANOVA and Dunnett multiple comparison test. *values represent P < .05. Survival experiments had 8 mice/group.
Prophylactic but not therapeutic COX inhibition triggers pro-inflammatory cytokine synthesis. WT mice were injected intraperitoneally with GBS 1 hour after oral administration of NS-398 (COX2 inhibitor), SC-560 (COX1 inhibitor) or SC-560 plus infliximab with (A) complete reversal of COX1-mediated cytokine storm (TNFα synthesis, Figure 4F) afforded by infliximab. SC-560 and NS-398 were dosed orally 1 hour after GBS injection causing little effects on (B-C) inflammatory cytokine levels in cell-free inflammatory exudates 3 hours later. Survival analysis was completed on 8 mice/group and the log-rank test was used to compare each group against one another. ** values represent P < .01 for controls versus Sc-560/infliximab. For cytokines, values are expressed as the mean ± SEM of 5-7 mice/group and analyzed by one-way ANOVA and Dunnett multiple comparison test. *values represent P < .05. Survival experiments had 8 mice/group.
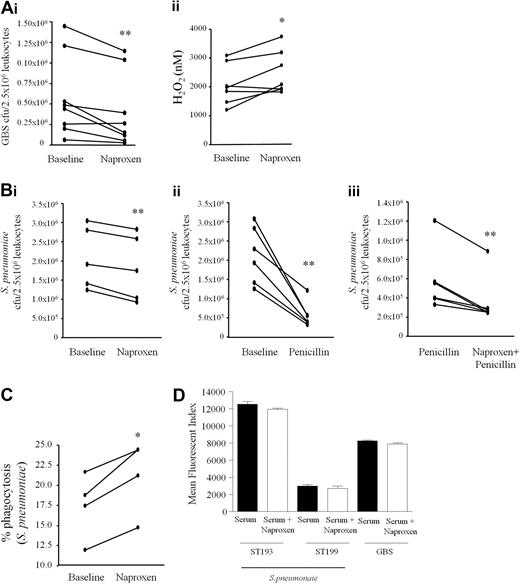
COX inhibition enhances bacterial killing in human whole blood assays
To determine whether COX inhibition is antibacterial in human, peripheral blood of 8 healthy males between 25 and 50 years old was taken before and 1 hour after oral naproxen (500 mg) and incubated ex vivo with opsonized GBS. Naproxen significantly enhanced whole blood leukocyte bacterial killing (Figure 6Ai) concomitant with enhanced blood leukocyte NADPH oxidase activity (Figure 6Aii). At these anti-inflammatory doses naproxen did not impair antibiotic activity but worked additively with penicillin as penicillin plus naproxen exerted significantly greater bacterial killing compared with penicillin alone (Figure 6Bi-iii). In these experiments, as with mice (Figure 4C), COX inhibition also enhanced blood leukocyte uptake of FAMSE-labeled S pneumoniae (ST193, Figure 6C) consistent with PGE2 being and inhibitor of phagocytosis.9 Importantly, NSAIDs did not alter opsonization (C3 deposition) of target bacteria (Figure 6D), providing further evidence of a direct immune-priming effect of NSAIDS on leukocyte antimicrobial activity. Thus, from studies on both humans and rodents we found that inhibiting COX enhances bacterial uptake as well as killing in a cAMP-dependent manner and does not interfere with the mode of action of antibiotics or opsonization of target bacteria.
COX inhibition primes human whole blood to kill bacteria. Peripheral blood of 8 healthy male volunteers between the ages of 25 and 50 was taken before and 1 hour after ingestion of 500 mg naproxen and incubated ex vivo with opsonized GBS. After 60 minutes aliquots were taken to determine (A i) numbers of viable bacteria determined by overnight incubation on agar plates or (A ii) leukocyte NADPH oxidase activity. Blood from volunteers taken naproxen was further incubated with (B i-iii) penicillin (0.075 μg/mL) to determine NSAID inhibitory/additive effects on bacterial killing with antibiotics. (C) Blood was also treated 400 μM naproxen for 30 minutes and stimulated with FAMSE labeled S pneumoniae (ST193) 5 minutes to determine bacterial phagocytosis and to show that (D) NSAIDs do not interfere with opsinization of bacteria. Data were analyzed by paired Student t test. * values represent P < .05 and ** values represent P < .01.
COX inhibition primes human whole blood to kill bacteria. Peripheral blood of 8 healthy male volunteers between the ages of 25 and 50 was taken before and 1 hour after ingestion of 500 mg naproxen and incubated ex vivo with opsonized GBS. After 60 minutes aliquots were taken to determine (A i) numbers of viable bacteria determined by overnight incubation on agar plates or (A ii) leukocyte NADPH oxidase activity. Blood from volunteers taken naproxen was further incubated with (B i-iii) penicillin (0.075 μg/mL) to determine NSAID inhibitory/additive effects on bacterial killing with antibiotics. (C) Blood was also treated 400 μM naproxen for 30 minutes and stimulated with FAMSE labeled S pneumoniae (ST193) 5 minutes to determine bacterial phagocytosis and to show that (D) NSAIDs do not interfere with opsinization of bacteria. Data were analyzed by paired Student t test. * values represent P < .05 and ** values represent P < .01.
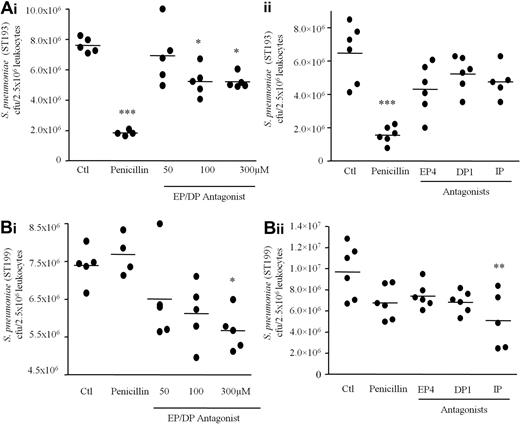
COX inhibition overcomes antibiotic resistance in human whole blood assays
Finally, we questioned whether COX inhibition would prime innate immune-mediated responses to kill antibiotic-resistant bacteria. This hypothesis was tested by incubating human whole blood with a pan-EP/DP PG receptor antagonist targeted against EP1, EP2, EP3-III, and DP1 receptors as well as against individual PG receptors including EP4, DP1, and IP with/without penicillin and subsequently spiking human blood with S pneumoniae serotype 19A which was either penicillin susceptible (ST193) or penicillin-resistant (ST199). After 1 hour, blood samples were taken and incubated overnight on agar. Penicillin and pan-EP/DP1 receptor antagonism significantly reduced ST193 numbers (Figure 7Ai). Although a trend toward a reduction in ST193 was seen when individual EP4, DP1, and IP receptors were antagonized, results were not significant (Figure 7Aii). Importantly, while S pneumoniae ST199 was refractory to penicillin, inhibiting EP/DP signaling with AH6809 (Figure 7Bi) or specific inhibition of IP (Figure 7Bii) brought about a significant killing of antibiotic-resistant S pneumoniae by whole blood with EP4 and DP1 antagonism causing a trend toward reduced ST199 cfu numbers (Figure 7Bii).
Inhibiting PG signaling primes human whole blood to kill antibiotic-resistant bacteria. Healthy human peripheral blood was pre-incubated with/without penicillin and a pan EP/DP PG receptor antagonist targeted against EP1, EP2, EP3-III, and DP1 receptors as well as against individual PG receptors including EP4, DP1, and IP. Whole blood was then stimulated with either (A) serum-opsonized penicillin-susceptible (ST193) or (B) intermediate penicillin-resistant (ST199) S pneumonia for 1 hour. After which time, numbers of surviving S pneumoniae (cfu) were quantified 24 hours later on agar plates. Data were analyzed by one-way ANOVA and Bonferroni multiple comparison test. * values represent P < .05, ** values P < .01, and *** values P < .001.
Inhibiting PG signaling primes human whole blood to kill antibiotic-resistant bacteria. Healthy human peripheral blood was pre-incubated with/without penicillin and a pan EP/DP PG receptor antagonist targeted against EP1, EP2, EP3-III, and DP1 receptors as well as against individual PG receptors including EP4, DP1, and IP. Whole blood was then stimulated with either (A) serum-opsonized penicillin-susceptible (ST193) or (B) intermediate penicillin-resistant (ST199) S pneumonia for 1 hour. After which time, numbers of surviving S pneumoniae (cfu) were quantified 24 hours later on agar plates. Data were analyzed by one-way ANOVA and Bonferroni multiple comparison test. * values represent P < .05, ** values P < .01, and *** values P < .001.
Discussion
Some PGs elevate cAMP, an intracellular second messenger that differentially regulates cytokines (up-regulates IL-10 and inhibits TNFα) but also inhibits phagocytosis as well as reactive oxygen and nitrogen intermediates, phagosomal acidification and lysosomal enzyme release.22 Thus, during infection cAMP dampens host responses to bacteria underscored by studies showing that its pharmacologic manipulation in vitro7-9,23 and in vivo leads to early lethality and enhanced bacterial load.23,24 We found in rodents that prophylactic as well as therapeutic inhibition of both COX isoforms enhanced host ability to kill bacteria by dampening cAMP in a PG-dependent manner. Extending these studies to humans, we showed that NSAIDs in whole blood assays enhanced bacterial phagocytic uptake and killing without interfering with antibiotics, but acting in concert with them to kill bacteria. Finally, we report that by priming the innate immune system through COX inhibition, NSAIDs promote the killing of antibiotic-resistant bacteria by blood leukocytes. Collectively, these data highlight the significance of lipid mediators in host responses to infection and the potential of inhibitors of PG signaling pathways or products of lipoxygenases25-27 as adjunctive therapies in the setting of antibiotic resistance. Of particular relevance to clinical disease, we demonstrate that NSAIDs enhance whole blood killing of penicillin-resistant serotype 19A S pneumoniae. Other investigators have demonstrated that this nonvaccine serotype is currently increasing in prevalence and isolates are frequently resistant to multiple antibiotics. This novel therapeutic use of NSAIDs may potentially enhance antibiotic mediated killing of bacterial pathogens including S pneumoniae and GBS and reduce the therapeutic level of antibiotic required, thereby reducing the likelihood of antibiotic resistance occurring.
COX1−/− mice or COX1 inhibitors given prophylactically (ie, 1 hour before infection) in WTs bearing a GBS-triggered inflammation elevated TNFα. COX2 inhibitors had little effect on TNFα despite dampening PGs and cAMP. Moreover, neither COX1 nor COX2 inhibition modulated TNFα when given therapeutically, 1 hour after GBS injection. As COX2 is expressed from 3 hours and COX1 is constitutively expressed in the naive cavity, these data suggest that PG control of cAMP-modulated TNFα is COX1 mediated and occurs within the first 60 minutes of infection. After this time the regulation of TNFα synthesis becomes COX independent. These results highlight differential roles of COX isoforms in controlling proinflammatory cytokine generation during infection/injury and support the historical concern surrounding the use of conventional (ie, nonselective) NSAIDs in humans for fever and in sepsis due to their propensity to trigger proinflammatory cytokines.28,29 Indeed, inhibiting COX1 triggered a cytokine storm (Figure 4E,G) most likely responsible for hastened death in animals given sublethal GBS as these effects were completely reversed with infliximab (Figure 5A). Therefore, we suspect that elevated TNFα in humans after nonselective COX inhibition with ibuprofen28,29 stems from the predosing regimen that inhibited constitutive COX1 as a result of priming the innate immune system to generate a Th1-type cytokine response. Although it is well appreciated that prophylactic treatment with NSAIDs (particularly ibuprofen and indomethacin) cause significant elevations of proinflammatory cytokines (ie, TNFα, IL-6, and IL-8) in the plasma of humans after inducing endotoxemia,28-30 it is not known what effect therapeutic dosing with either nonselective or COX2 selective inhibitors would have on plasma proinflammatory cytokine levels. But, in the context of NSAIDs being used therapeutically in combination with antibiotics to treat drug-resistant infections, it is likely that cytokine synthesis will not be triggered. In the event a cytokine storm ensues and despite the reservations of TNFα blockade in sepsis,31 concomitant treatment with infliximab may efficiently dampen the associated symptoms.
Reports also suggest that NSAIDs increase the risk of developing Group A Streptococcal (GAS) necrotizing fasciitis, impair its diagnosis/management, and accelerate its course of infection.32-34 However, critical assessment of current data does not support a causal role for NSAIDs in the development of this disease state or a worsening of infection once established.35 Indeed, there are suggestions that NSAIDs may alleviate its symptoms resulting in delayed diagnosis and treatment. In support of this a recent case-control study found that NSAIDs do not increase the risk of developing severe sepsis or septic shock as a consequence of necrotizing fasciitis in adults with community acquired bacterial infections.36 They contend that NSAID exposure during evolving bacterial infection is associated with delayed prescription of effective antibiotic therapy, which is hypothesized to be due to NSAIDs masking the progression of the disease by suppressing the associated inflammation. Interestingly, a recent genetic study using advanced recombinant inbred mice revealed a potentially major causal role for PGE2 in conferring susceptibility to invasive GAS infections.37 They demonstrated that within quantitative trait loci, hypothesized to be involved in modulating severity of GAS infections, genes for both mPGES-1 and mPGES-2, (Ptges and Ptges1, respectively) showed marked up-regulation in mice strains susceptible to GAS, but showed no change or slight decrease in resistant strains after infection. Thus, these results associated high expression of mPGES-1 (and to a lesser extent mPGES-2) with increased susceptibility to death, bacteremia, and bacterial dissemination.
Antibiotics suppress the growth of microorganisms by inhibiting bacteria cell wall synthesis, protein synthesis or nucleic acid/ribonucleic acid metabolism.38 Despite this, bacteria have developed mechanisms of drug resistance including modifications leading to loss or decreased affinity of the drug for its target; reducing the number and/or size of external membrane pore diameter, thereby limiting antibiotic permeability; producing drug detoxifying enzymes; or actively effluxing antibiotics by energy-dependent pumps.38 As a result, there is a pressing need to develop alternatives to overcome antibiotic resistance. Current solutions include efflux pump inhibition,39,40 phage therapy,41 or bacteriocins (bacteria used in probiotic application).42 However, few such strategies have made it to the clinic. Given its constitutively expressed nature and predominant role in PG synthesis during bacterial infection, potential strategies for targeting drug-resistant bacteria based on COX pathways of arachidonic acid metabolism may include dampening COX1 and/or inducible mPGES, antagonizing cAMP-elevating PG signaling receptors (EP2, DP1 or IP) or inhibiting COX2. Selective inhibition of COX1 may not exert the same cardiovascular side effects as COX2 inhibitors; in fact it should be as beneficial as low-dose aspirin and cause little gastric toxicity as both COX1 and COX2 need to be inhibited to cause gastric ulceration.43
If selective inhibition of constitutively expressed COX1 perturbs physiologic pathways required to maintain health, then inhibiting inducible mPGE2S during inflammation may be more tractable. From data presented here, inhibiting COX2 would also be an attractive target as its inhibition effectively kills bacteria without triggering proinflammatory cytokine synthesis. Indeed, its cardio-toxic profile is manifested only after protracted exposure, which may not be problematic over the relatively short time frame needed to eradicate drug-resistant infections. However, COX2 inhibition only delayed mortality (Figure 5A), conferring protection up 50 hours after infection in our studies, after which time clinical signs of systemic inflammation (piloerection, reduced movement, shallow breathing) and mortality was equivalent to that of controls. This was surprising given that NS-398 improved the survival of mice over 7 days when challenged with Pseudomonas aeruginosa,44 but is consistent with our previous reports of a protective role for COX2 in the resolution of acute inflammation13,45-47 and those of others regarding a protective role for COX2-derived lipids as endogenous stop-signals of innate immune-mediated responses.48-50 We suspect that while COX2 inhibition protected animals in the early phase of infection (bacterial killing, conserved IL-10 release) it dampened pro-resolution pathways and exerted a resolution-toxic effect.
In conclusion, as NSAIDs not only possess antibacterial properties but also overcome drug resistance in a manner that does not interfere with antibiotic's mode of action, these data collectively support COX inhibition or strategies that disrupt PG signaling pathways as useful adjunctive therapies in treating persistent and multidrug-resistant infections.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Wellcome Trust and Medical Research Council, United Kingdom (to D.W.G.). D.W.G. is a Wellcome Trust Senior Research Fellow. M.J.S. was supported by a studentship from the Medical Research Council, United Kingdom.
Wellcome Trust
Authorship
Contribution: M.J.S., J.N., and C.J.H. designed and carried out experiments; S.S.A. and J.B. contributed valuable experimental material and expertise; and D.W.G. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Derek W. Gilroy, Centre for Clinical Pharmacology and Therapeutics, Division of Medicine, 5 University St, University College London, London WC1E 6JJ, United Kingdom; e-mail: d.gilroy@ucl.ac.uk.