Abstract
Long-term survival is now an expected outcome after hematopoietic cell transplantation (HCT). However, the burden of morbidity long-term after HCT remains unknown. We examined the magnitude of risk of chronic health conditions reported by 1022 HCT survivors and their siblings (n = 309). A severity score (grades 1 [mild] through 4 [life-threatening]) was assigned to each health condition using the Common Terminology Criteria for Adverse Events, Version 3. Sixty-six percent of the HCT survivors reported at least one chronic condition; 18% reported severe/life-threatening conditions; comparable values in siblings were 39% and 8%, respectively (P < .001). The cumulative incidence of a chronic health condition among HCT survivors was 59% (95% confidence interval [CI], 56%-62%) at 10 years after HCT; for severe/life-threatening conditions or death from chronic health conditions, the 10-year cumulative incidence approached 35% (95% CI, 32%-39%). HCT survivors were twice as likely as siblings to develop a chronic condition (95% CI, 1.6-2.1), and 3.5 times to develop severe/life-threatening conditions (95% CI, 2.3-5.4). HCT survivors with chronic graft-versus-host disease were 4.7 times as likely to develop severe/life-threatening conditions (95% CI, 3.0-7.2). The burden of long-term morbidity borne by HCT survivors is substantial, and long-term follow-up of patients who received transplantation is recommended.
MedscapeCME Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians. Medscape, LLC designates this educational activity for a maximum of 1.0 AMA PRA Category 1 credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://cme.medscape.com/journal/blood; and (4) view/print certificate. For CME questions, see page 3377.
Disclosures
The authors, the Associate Editor Malcolm K. Brenner, and the CME questions author Charles P. Vega, University of California, Irvine, CA, declare no competing interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Distinguish disease and demographic factors that can influence the rate of chronic health conditions following hematopoietic cell transplantation (HCT)
Specify the risk factors for chronic health conditions and organ systems more commonly affected among recipients of HCT compared with their siblings
Introduction
Hematopoietic cell transplantation (HCT) is an established curative option for a variety of hematologic malignancies. Advances in transplantation techniques and supportive care strategies have resulted in a significant improvement in survival; more than 70% of those who survive the first 2 years after HCT are expected to become long-term survivors.1-3 However, cure or control of the underlying disease is not accompanied by full restoration of health. HCT survivors are at risk of developing long-term complications, such as endocrinopathies, musculoskeletal disorders, cardiopulmonary compromise, and subsequent malignancies.4-14
Previous reports have focused on specific complications, providing insight into their etiology and pathogenesis and identifying persons at increased risk for development of these outcomes, thus setting the stage for targeted surveillance and interventions to reduce morbidity and mortality. However, the burden of morbidity resulting from the cumulative impact of these individual complications has not been described. Understanding the burden of morbidity after HCT is important for a variety of reasons. It is important to the healthcare providers and policy makers in identifying and procuring resources for the long-term care of persons with a high burden of morbidity, to the researchers in identifying common etiologic pathways that lead to the overall morbidity, and to the HCT survivors in making an informed decision regarding the quality-of-life concerns long term after HCT. Using the resources offered by the Bone Marrow Transplant Survivor Study (BMTSS), we determined the prevalence and severity of chronic health conditions in HCT survivors, compared these outcomes with a healthy sibling cohort, and sought to identify subpopulations at increased risk.
Methods
Subjects
Eligible participants included persons who received HCT at City of Hope National Medical Center (COH) or the University of Minnesota (UMN) between 1974 and 1998 for a hematologic malignancy or severe aplastic anemia (SAA), survived at least 2 years after transplantation, were alive and 18 years of age or older at study participation, and had completed the questionnaire in English. The Human Subjects Committee at the participating institutions approved the protocol; informed consent was provided according to the Declaration of Helsinki.
A total of the 2175 patients underwent HCT at COH or UMN between 1974 and 1998 and survived the first 2 years after HCT; an additional 542 patients died after surviving the first 2 years. Of the 1633 survivors who were alive at study participation, 1468 (90%) were successfully contacted, and 1022 (70%) participated. Participants were older at HCT (mean age, 34 vs 29 years, P < .001), with a shorter follow-up after HCT (mean, 8.7 vs 10.4 years, P < .001). Non-Hispanic whites (65% vs 56%, P = .002), females (67% vs 60%, P = .005), and autologous HCT recipients (66% vs 60%, P = .01) were more likely to participate. Patients who had undergone HCT for SAA were less likely to participate compared with survivors with other diagnoses. Participation rate did not differ by risk of relapse at HCT or by transplanting institution. Comparison with a noncancer population was made possible by asking participating survivors to invite a nearest-age sibling to the study. A total of 309 siblings participated in this study.
Clinical characteristics
Information regarding primary diagnosis, preparative regimens, stem cell source (autologous, sibling, or unrelated donor), graft type (bone marrow or peripheral blood stem cells), risk of relapse at HCT (standard or high risk), and prophylaxis and management of graft-versus-host disease (GVHD), was obtained from institutional databases. Patients transplanted in first or second complete remission after acute myeloid leukemia (AML) or acute lymphoid leukemia (ALL), and Hodgkin lymphoma or non-Hodgkin lymphoma (NHL), first chronic phase of chronic myeloid leukemia (CML), and patients with SAA were considered at standard risk for relapse; the remainder were considered at high risk.
Chronic health conditions
HCT survivors and siblings completed a 255-item BMTSS questionnaire, which covers the following general areas: questions regarding diagnosis by a healthcare provider of physical health conditions with age at diagnosis (endocrinopathies, central nervous system compromise, cardiopulmonary dysfunction, gastrointestinal and hepatic sequelae, musculoskeletal abnormalities, and subsequent malignancies), diagnosis and extent of chronic GVHD, access to and use of medical care, and sociodemographic characteristics (race/ethnicity, education, marital status, employment, household income, and insurance). The reliability and validity of the BMTSS questionnaire have been tested, and the responses have demonstrated a high level of sensitivity and specificity, confirming that survivors are able to report the occurrence of adverse medical conditions with accuracy.15
Chronic physical health conditions diagnosed after HCT were graded using the Common Terminology Criteria for Adverse Events, Version 3.0,16 an instrument used to grade acute and chronic conditions in persons with cancer, including cancer survivors, and distinguish grades 1 through 5 with unique clinical descriptions of the severity for each event (grade 1, mild; grade 2, moderate; grade 3, severe; grade 4, life-threatening/disabling; grade 5, death from chronic health conditions). The same scoring system was applied to responses from the sibling comparison group. A detailed description of the questions asked in the BMTSS questionnaire, the corresponding chronic health condition categories that were created from the responses, and the scoring of these conditions are presented in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This scoring system has been successfully used to describe the burden of morbidity resulting from chronic health conditions in childhood cancer survivors.17
Psychosocial sequelae and gonadal failure were not included as chronic health conditions in this analysis. However, psychologic health was evaluated in this cohort to evaluate its impact on chronic physical health conditions. This was accomplished using the Brief Symptom Inventory-18. Brief Symptom Inventory-18 measures psychologic distress experienced during the previous 7 days using 18 5-point Likert scale items (from 0 = “not at all” to 4 = “extremely”).18 The item responses were summed to determine the Global Severity Index; raw scores were converted to sex-specific T scores (mean ± SD; 50 ± 10) derived from a community sample of 1134 adults. Sex-specific T scores were dichotomized; those with a T score ≥ 63 were classified as having psychological distress.
Statistical analyses
Prevalence and predictors of chronic health conditions.
The prevalence of chronic health conditions was determined for participating HCT survivors (n = 1022) and the sibling comparison group (n = 309). Chronic health conditions were reported as 3 primary outcomes: presence of any chronic health condition (grades 1-4), as well as dichotomized as mild to moderate (grade 1 or 2) and severe/life-threatening/disabling (grade 3 or 4). For participants with more than one condition, the maximum grade was used in the analysis. Standard parametric and nonparametric techniques were used for comparisons between clinical and demographic subgroups.
Comparisons between HCT survivors and siblings after adjusting for sex, age at study participation (treated as a continuous variable), race/ethnicity (non-Hispanic whites vs others), education (less than high school, high school/some college/training, or college graduate/postgraduate education), annual household income (< $20 000 per year, $20 000-$60 000 per year, and > $60 000 per year), and health insurance status (yes/no) were conducted using relative risk regression for common outcomes and were reported as relative risks (RR) with 95% confidence intervals (CIs).19 The analysis accounted for within-family correlations using sandwich SE estimates.20
Relative risk regression was also used for analyses restricted to HCT survivors. A fixed set of explanatory variables were selected a priori and were used to assess their simultaneous impact on the risk of chronic health conditions. These variables included stem cell donor type (autologous, related, and unrelated donor), stem cell source (peripheral blood stem cell [PBSC], bone marrow, cord blood), presence of chronic GVHD (for allogeneic HCT survivors only), sex, age at study participation (< 41 years, 41-55 years, and > 55 years), age at HCT (< 18 years, 18-< 45 years, ≥ 45 years), time since HCT (2-< 5 years, 5-< 10 years, ≥ 10 years), race (non-Hispanic whites, others), education, health insurance coverage, transplanting institution (COH, UMN), participants' residential characteristics (rural/urban; distance to participating institution [< 60 miles/≥ 60 miles]), risk of relapse at HCT, year of HCT (before or after 1990), exposure to total body irradiation (TBI), and primary cancer diagnosis.
Cumulative incidence of chronic health conditions among HCT survivors.
To provide an estimate of the magnitude of risk of chronic health conditions among HCT recipients with time from HCT, cumulative incidence of chronic health conditions was calculated. This analysis included patients who had undergone HCT and survived 2 or more years (including the 542 who died after having survived 2 years after HCT). For patients who were alive at study participation, self-reported chronic health conditions were used for the analyses (as described in “Chronic health conditions”). For the deceased patients, all deaths from chronic health conditions (n = 225) were considered grade 5; deaths from primary disease, accident, or suicide (n = 317) were not graded. Cumulative incidence calculations treated death from causes other than chronic health conditions as a competing risk according to the method describe by Gooley et al.21 Sensitivity analyses were conducted to create a lower boundary for the estimated cumulative incidence values by treating nonparticipants as having no chronic health condition.
Data were analyzed using SAS Version 9.1 (SAS Institute). All statistical tests were 2-sided, and P < .05 was considered statistically significant.
Results
Demographic and clinical characteristics of HCT survivors and siblings
The demographic and clinical characteristics of persons who completed the BMTSS questionnaire (HCT survivors, n = 1022; siblings, n = 309) are presented in Table 1. Although the age at study participation was comparable between siblings and HCT survivors (mean age, 44.8 vs 43.1 years), there was an overrepresentation of females (63% vs 45%), non-Hispanic whites (88% vs 81%), college graduates (56% vs 46%), and persons with higher household income (> $60 000, 64% vs 46%) among siblings. The proportion of persons with health insurance coverage was comparable between siblings and HCT survivors (95% vs 93%, P = .08). CML (23%), AML (24%), NHL (20%), ALL (10%), and Hodgkin lymphoma (9%) accounted for 85% of all primary diagnoses. TBI was used for 77%, and 35% of the survivors were at high risk for relapse at HCT. Fifty-five percent of the HCT survivors had received an allogeneic HCT, and approximately 53% developed chronic GVHD after HCT. The cohort had been followed for a median of 7.3 years after HCT.
Chronic health conditions among HCT survivors
Table 2 summarizes the prevalence of chronic health conditions reported by the HCT survivors. Of the 1022 HCT survivors who completed the BMTSS questionnaire, 66% reported at least one chronic health condition and 18% reported grade 3 (severe) or grade 4 (life-threatening) conditions. Furthermore, 50% of the HCT survivors reported at least 2 chronic health conditions, and 35% reported 3 or more conditions. The prevalence of chronic health conditions for those with and without post-HCT disease recurrence was comparable (any chronic health condition: 67% vs 65%, P = .6; grade 3 or 4 conditions: 19% vs 16%, P = .4, respectively). The prevalence of any chronic health condition was significantly higher among allogeneic HCT recipients compared with autologous HCT recipients (any chronic health condition: 71% vs 61%, P = .001; grade 3 or 4 conditions: 21% vs 16%, P = .04). The prevalence of chronic health conditions for allogeneic HCT recipients with and without chronic GVHD was comparable (any chronic health condition: 73% vs 68%, P = .2; grade 3 or 4 conditions: 23% vs 18%, P = .2; respectively). However, the prevalence of multiple health conditions was higher among allogeneic HCT recipients with chronic GVHD compared with those without (2 or more conditions: 62% vs 49%, P = .002; 3 or more conditions: 48% vs 23%, P < .001). Allogeneic PBSC products were used in 25 patients. There was no difference in the prevalence of chronic health conditions between those who received PBSC or bone marrow allogeneic grafts (P = .3). T cell–depleted products were used in 23 instances and, again, did not result in an increase in the prevalence of chronic health conditions (P = .3) compared with those who received unmanipulated grafts.
A total of 817 long-term survivors visited transplantation centers during the 2 years before study participation. There was no significant difference in the prevalence of chronic health conditions between those who reported a recent visit to the transplantation center (66%) versus those who did not (67%, P = .6). No difference in the prevalence of chronic health conditions was observed by distance of residence from the transplantation center (P = .2) or by whether the survivors lived in a rural versus urban community (P = .7). Among the 1022 HCT survivors, 89 (9%) reported psychologic distress as indicated by the Brief Symptom Inventory-18 Global Severity Index scores. However, no differences were observed in the prevalence of chronic health conditions among those with psychologic distress (66%) versus those without (72%, P = .1).
Table 3 summarizes the predictors of chronic health conditions restricting the analysis to HCT survivors. Compared with autologous HCT recipients, allogeneic HCT survivors were more likely to report any chronic conditions (RR = 1.2; 95% CI, 1.1-1.4), grade 1 or 2 conditions (RR = 1.2; 95% CI, 1.0-1.5), and grade 3 or 4 conditions (RR = 1.7; 95% CI, 1.2-2.4). Females were more likely than males to report grade 1 or 2 conditions (RR = 1.2; 95% CI, 1.0-1.3), whereas patients with longer follow-up (≥ 5 years from HCT) were more likely to report grade 3 or 4 chronic health conditions, compared with those followed for less than 5 years. Survivors with higher education and those with health insurance coverage were more likely to report chronic health conditions. However, age at transplantation and use of TBI were not associated with reporting chronic health conditions. UMN patients were more likely to report chronic health conditions. These institutional differences held up even when the analysis was restricted to patients who reported a visit to their respective HCT centers in the 2 years before study participation. A higher prevalence of joint surgery, stroke, and intestinal surgery accounted for the institutional differences in the prevalence of chronic health conditions. Among allogeneic HCT recipients, there was no statistically significant difference in the prevalence of single chronic health conditions between those with chronic GVHD compared with those without. However, those with chronic GVHD were more likely to report multiple conditions (RR = 1.3; 95% CI, 1.1-1.6 for 2 or more conditions; RR = 1.6; 95% CI, 1.3-1.9, for 3 or more conditions).
Chronic health conditions: HCT survivors compared with siblings
Compared with the siblings, HCT survivors were significantly more likely to have a chronic health condition of any severity (66% vs 39%, P < .001), or of grade 3 or 4 severity (18% vs 8%, P < .001) (Table 2). Furthermore, HCT survivors were significantly more likely to have multiple conditions (2 or more: 50% vs 15%, P < .001; 3 or more: 35% vs 6%, P < .001). These differences between HCT survivors and the sibling comparison group retained statistical significance when the comparison was restricted to autologous or allogeneic HCT recipients, those with or without chronic GVHD, or those who did or did not have a recurrence after HCT (Table 2).
Table 4 summarizes the magnitude of risk of chronic health conditions for specific clinical subgroups of HCT survivors compared with siblings, adjusted for sex, age at study participation, race/ethnicity, education, household income, and health insurance status. The analyses were conducted for the entire cohort of HCT survivors, as well as stratified by presence or absence of chronic GVHD among allogeneic HCT survivors. Overall, HCT survivors were twice as likely as siblings to have a chronic health condition (95% CI, 1.6-2.1) of any severity, 1.9 times as likely to have a grade 1 or 2 condition (95% CI, 1.6-2.2), and 3.5 times as likely to have a grade 3 or 4 condition (95% CI, 2.3-5.4). Primary diagnoses with the highest risk for a grade 3 or 4 chronic health condition (compared with siblings) included ALL (RR = 4.9; 95% CI, 2.9-8.2), AML (RR = 4.1; 95% CI, 2.6-6.4), CML (RR = 3.7; 95% CI, 2.3-5.9), and NHL (RR = 3.3; 95% CI, 2.0-5.4). Survivors of unrelated donor HCT were at a 4.6-fold increased risk of having a grade 3 or 4 chronic health condition, compared with siblings (95% CI, 2.8-7.6). HCT survivors exposed to TBI were 3.9-fold more likely to have a grade 3 or 4 condition (95% CI, 2.6-6.0), whereas patients exposed to immunosuppressive therapy for GVHD management were at a 4.5-fold (95% CI, 3.0-6.9) increased risk. Furthermore, HCT survivors were more likely to report multiple conditions (RR = 3.6; 95% CI, 2.7-4.9, for 2 or more conditions; RR = 6.4; 95% CI, 3.9-10.4 for 3 or more conditions).
Severe or life-threatening/disabling chronic health conditions
The prevalence of specific severe or life-threatening (grade 3 or 4) chronic health conditions and the associated adjusted RRs for HCT survivors compared with siblings are summarized in Table 5. These analyses were performed for all HCT survivors, as well as stratified by type of transplantation and among allogeneic recipients by the presence of chronic GVHD. Overall, HCT survivors were more likely to have gastrointestinal (RR = 4.3; 95% CI, 1.0-18.1), musculoskeletal (RR = 5.1; 95% CI, 1.2-21.1), and cardiovascular problems (RR = 2.9; 95% CI, 1.3-5.9), compared with siblings after adjustment for age, sex, and race/ethnicity. Autologous HCT recipients had a higher prevalence of cardiovascular problems (RR = 2.7; 95% CI, 1.2-5.9) compared with siblings. Compared with the siblings, allogeneic recipients were more likely to report cardiovascular problems (RR = 3.0; 95% CI, 1.4-6.4), auditory or visual impairment (RR = 3.7; 95% CI, 1,1-12.2), gastrointestinal problems (RR = 6.0; 95% CI, 1.4-26.5), and musculoskeletal problems (RR = 7.1; 95% CI, 1.6-31.7).
Cumulative incidence of chronic health conditions among HCT recipients
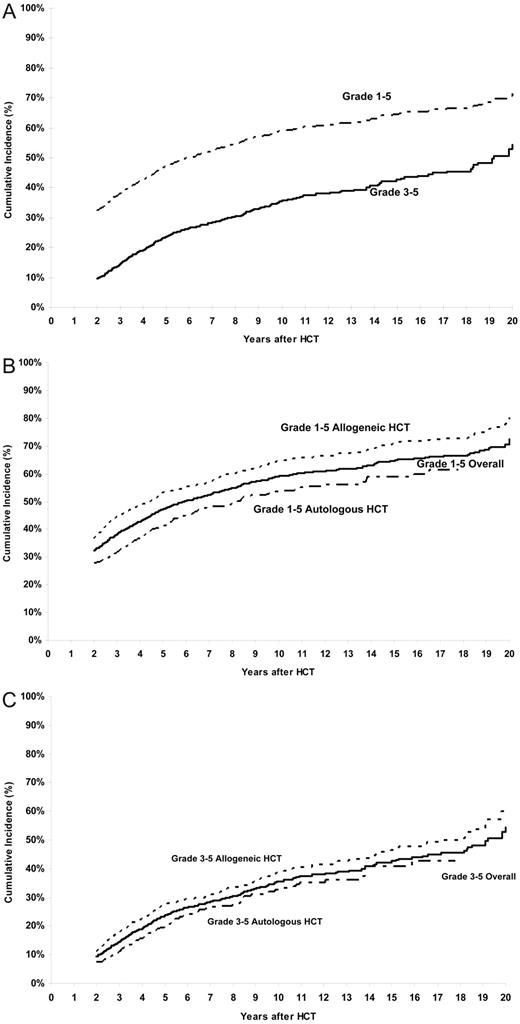
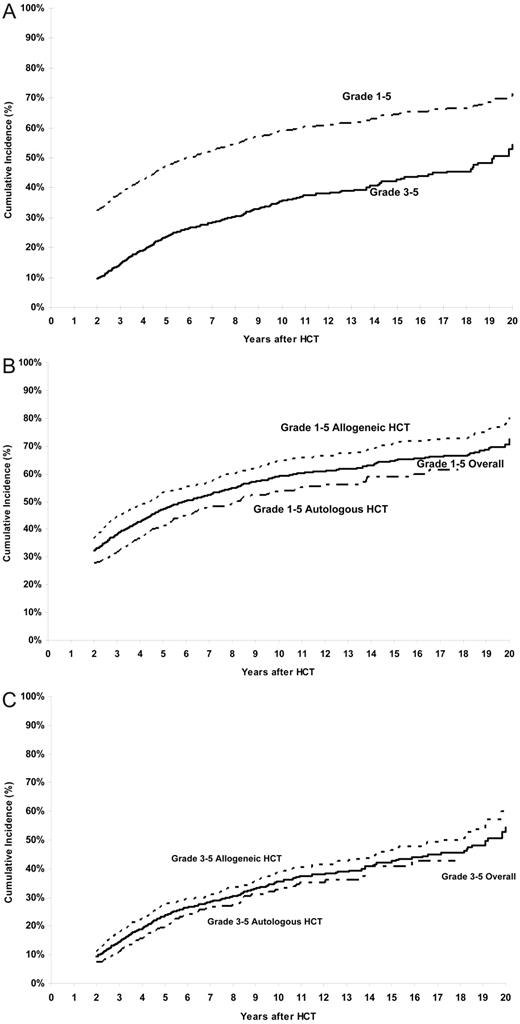
The cumulative incidence of a chronic health condition (grades 1-5) among patients who had survived the first 2 years after HCT, and including those who died subsequently, was 32% (95% CI, 30%-35%) and 59% (95% CI, 56%-62%) at 2 and 10 years after HCT, respectively (Figure 1A). For grades 3-5 chronic health conditions, the cumulative incidence was 9% (95% CI, 8%-11%) at 2 years, approaching 35% (95% CI, 32%-39%) at 10 years (Figure 1A). Restricting the analysis to those in complete continuous remission after HCT (n = 1187) revealed the cumulative incidence to be 67% for any chronic health condition and 41% for grades 3-5 conditions at 10 years after HCT.
Cumulative incidence of chronic health conditions after HCT. (A) Cumulative incidence of any chronic health conditions (grades 1-5) and severe or life-threatening chronic health conditions (grade 3 or 4) or death from a chronic health condition (grade 5) after HCT. (B) Cumulative incidence of any chronic health conditions (grades 1-5) in allogeneic and autologous HCT recipients. (C) Cumulative incidence of chronic health conditions (grades 3-5) in allogeneic and autologous HCT recipients.
Cumulative incidence of chronic health conditions after HCT. (A) Cumulative incidence of any chronic health conditions (grades 1-5) and severe or life-threatening chronic health conditions (grade 3 or 4) or death from a chronic health condition (grade 5) after HCT. (B) Cumulative incidence of any chronic health conditions (grades 1-5) in allogeneic and autologous HCT recipients. (C) Cumulative incidence of chronic health conditions (grades 3-5) in allogeneic and autologous HCT recipients.
The 10-year cumulative incidence of grades 3-5 chronic health conditions was 35% after CML and ALL, and 40% after AML and NHL. The cumulative incidence of a chronic health condition among allogeneic HCT survivors was 64% at 10 years and approached 71% at 15 years after HCT, whereas that for autologous HCT recipients was 54% at 10 years and 59% at 15 years (P < .001) (Figure 1B). For grades 3-5 chronic health conditions, the cumulative incidence approached 39% at 10 years among allogeneic HCT recipients and 33% among the autologous HCT recipients (P < .001; Figure 1C). The 10-year cumulative incidence of grades 3-5 chronic health conditions was significantly higher among those with chronic GVHD compared with those without (50% vs 26%, P < .001). The 10-year cumulative incidence was significantly higher after exposure to TBI (62% vs 52%, P = .002 for any chronic health condition; 36% vs 33%, P < .001 for grades 3-5 conditions).
Sensitivity analyses for cumulative incidence calculations
Although we had data on chronic health conditions for all 542 deceased patients (from data on cause of death files from medical records and National Death Index1,2 ) and the 1022 survivors who participated by completing the questionnaire, we did not have details regarding chronic health conditions for the 611 HCT survivors who did not participate. To alleviate potential problems related to incomplete data, sensitivity analyses were performed in which all survivors with missing data were censored as of their last contact date. It was assumed that those without information on chronic health conditions did not develop a chronic health condition. With these extremely conservative assumptions, a lower boundary was placed on the reported estimates. The results of the sensitivity analyses are presented in Table 6.
Discussion
HCT is now the treatment of choice for several life-threatening diseases.22 Among those who survive the first 2 years after HCT, 80% of allogeneic HCT recipients1,3 and 70% of autologous HCT recipients2 are expected to become long-term survivors. The high intensity of therapeutic exposures, coupled with the occurrence of chronic GVHD, has a negative impact on the health of HCT survivors, increasing the likelihood of premature death.1,2 Studies examining the burden of morbidity would help make decisions regarding appropriation of resources, both in terms of quantity and quality, for the long-term care of HCT survivors.
The current study of long-term HCT survivors demonstrates that the prevalence of any chronic health condition is high: 66.4% have at least one chronic health condition, 18.3% have a severe or life-threatening condition, more than one-half have 2 or more conditions, and more than one-third have 3 or more conditions. The prevalence of at least one chronic health condition reported by survivors of childhood cancer participating in the Childhood Cancer Survivor Study (CCSS) was 62.3%, rates that are comparable with those reported in the current study.17 However, the prevalence of severe or life-threatening conditions reported by the childhood cancer survivors (27.5%) was higher than those that described in the current study. The cumulative incidence of severe or life-threatening chronic health conditions or death from a chronic condition (grades 3-5) exceeds 35% at 10 years from HCT in the current study and was 42% at 30 years from primary cancer diagnosis in the CCSS cohort. Thus, although the magnitude of risk of chronic health conditions in the current study is similar to CCSS, the curve is shifted to the left in the current study, possibly reflecting the older age of the patient population, the high intensity of therapeutic exposures used for conditioning in patients undergoing HCT coupled with exposure to various intensities of pre-HCT chemotherapy and radiation, and the occurrence of chronic GVHD. Unlike the CCSS, the current study did not include hypogonadism as a chronic health condition (a condition rated as grade 3 by CCSS). Inclusion of hypogonadism would have resulted in a much higher prevalence of chronic health conditions in the current study because of the high prevalence of hypogonadism (> 90%) among HCT survivors.23
The increment in cumulative incidence for any chronic health conditions among HCT recipients is 12% from 5 years to 10 years, whereas that for severe/life-threatening conditions or deaths from chronic health conditions is very similar, 11%. Similarly, identical increments are observed in the cumulative incidence of chronic health conditions among allogeneic and autologous HCT recipients from 10 years to 15 years after HCT. This is evident visually from the cumulative incidence curves that show that the curves are parallel. These observations suggest that the major differences in the cumulative incidence rates for patients with grade 1 or 2 conditions versus grades 3-5 conditions, or between allogeneic HCT recipients and autologous HCT recipients, are established in the first 2 years and that the rate of occurrence of chronic health conditions is similar over time, with no evidence of a plateau through the period of follow-up available for the current study. Thus, attention needs to focus on events occurring in the first 2 years that allowed the establishment of these differences in chronic health conditions, as well as long-term follow-up of the HCT survivors to institute surveillance for early detection of these adverse outcomes.
Overall, HCT survivors were 3.5 times more likely to develop a severe or life-threatening health condition compared with the siblings. Among these severe or life-threatening conditions, the risk was particularly increased for gastrointestinal symptoms (4.3 times as likely as among siblings) and musculoskeletal problems (5 times as likely). These results quantify the burden of morbidity borne by HCT survivors and identify those at risk, emphasizing the need for close monitoring of these vulnerable populations.
Chronic GVHD contributed significantly to the increased risk of severe or life-threatening conditions, and more importantly, to the development of multiple conditions. Among allogeneic HCT survivors, nearly three-fourths of patients with chronic GVHD reported at least one chronic health condition, nearly one-fourth had severe or life-threatening conditions, and more than one-half had 2 or more conditions. The 10-year cumulative incidence of grades 3-5 chronic health conditions approached 50% among those with chronic GVHD and was twice that of those without chronic GVHD (26%). Furthermore, patients exposed to immunosuppressive therapy for GVHD management were at a 4.5-fold increased risk compared with siblings. Finally, HCT survivors with chronic GVHD were 4-fold to 11-fold more likely to report severe or disabling endocrine sequelae, gastrointestinal complications, and musculoskeletal problems.
Chronic GVHD is a relatively common complication after allogeneic HCT, with several series reporting an incidence of 40% to 70%.13 Chronic GVHD and its treatment are a leading cause of nonrelapse mortality in HCT survivors1,3 and a significant contributor to functional impairments.8 The incidence of chronic GVHD will probably increase in the future, secondary to the increasing use of HCT in older patients, utilization of unrelated and mismatched related donors, and PBSC transplantation.13,24,25 The significant impact of chronic GVHD on the health burden borne by HCT survivors shown in this study emphasizes the critical need for a multidisciplinary approach to the management of chronic GVHD.
An interesting observation in this study was the association between higher education (more than high school) and the increased risk of chronic health conditions. Although this association has not been reported before, it might reflect the ability of those with higher education to self-report chronic health conditions better than those who are less well educated. However, HCT survivors without a high school education constituted a very small fraction of our cohort (17%) and therefore could not have contributed significantly to the overall prevalence rates. Furthermore, a previously published validation study has shown high levels of sensitivity and specificity and kappa statistics between medical record data and self-reported outcomes used as part of this questionnaire.15 Nonetheless, this observation does underscore the need to develop effective methods of communication between patients and healthcare providers, so that patients can serve as advocates of their own health.
Another observation that deserves attention is the association between health insurance coverage and the risk of reporting chronic health conditions. This finding was independent of education and income. This observation can be interpreted in the context of availability of adequate health care to identify chronic health conditions and therefore the ability of the HCT survivors to report them. This finding, coupled with the observation that the risk of chronic health conditions increases with time from HCT underscores the importance of adequate long-term follow-up of the HCT survivors to decrease the morbidity associated with these chronic health conditions.
This study describes the burden of morbidity carried by HCT survivors to assist the healthcare providers and policy makers with the scope of the problem, such that appropriate resources can be identified and procured for the long-term care of HCT survivors. In our previous publications, we have demonstrated that HCT survivors continue to have premature deaths long after HCT; and although a substantial proportion of this is the result of primary disease, a sizeable part is attributed to chronic health conditions acquired after HCT.1,2 It is therefore imperative that the healthcare providers and policy makers understand the need for life-long follow-up and provision of proactive care for the HCT survivors.
Identification and allocation of the resources to care for the burgeoning population of HCT survivors usually vary by institution. We have demonstrated that, although a significant proportion of HCT survivors continue to receive long-term care at their transplanting centers, many are discharged to the care provided by the primary care physicians in the community.26 Clear communications between the transplanting center and physicians in the community regarding the specifics of the long-term care of this vulnerable population become critical. Successful models exist in the pediatric cancer survivor community, where exposure-related, risk-based, life-long care is provided to cancer survivors using consensus-based guidelines (www.survivorshipguidelines.org).27 Similar models need to be explored and tested in the HCT population.
The results of the study must be interpreted in the context of potential limitations. Participation rate was 63% of all eligible subjects and 70% of those successfully contacted; we addressed this limitation by conducting sensitivity analyses and hence creating a conservative lower boundary for the cumulative incidence of chronic health conditions in the current study. This exercise demonstrates that the cumulative incidence reported in this study could not possibly be lower than the lower boundary created by the sensitivity analyses and that, even at its most conservative estimate, the cumulative incidence of chronic health conditions is elevated.
This study was not designed to capture details regarding the burden of morbidity from similarly treated patients who had not received HCT. Instead, the comparison group consisted of unaffected siblings who completed an identical questionnaire and were therefore able to serve as a healthy control group. The use of siblings is an effective comparison group that is associated with high participation rates, ease of access, and general uniformity of socioeconomic status and level of health awareness.
The current cohort included patients who were alive at study participation. Thus, patients who could have died of chronic health conditions with relatively short latency and high fatality (such as therapy-related leukemia) were potentially missed when describing the morbidity after HCT. Although this study was not designed to describe the magnitude of risk of individual chronic health conditions (many prior studies have done so in great detail), it does lend itself perfectly to assessing the cumulative contribution of a variety of chronic health conditions to the overall burden of morbidity among long-term HCT survivors.
These limitations notwithstanding, this study represents the first assessment of its kind to describe the burden of morbidity in a large population of long-term HCT survivors. The study demonstrates unequivocally that HCT survivors have a high rate of illness because of chronic health conditions, that those with chronic GVHD are particularly vulnerable, that the incidence of these outcomes continues to increase with follow-up, and that attention needs to focus on instituting systematic and targeted follow-up of those at high risk.28
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Institutes of Health (grant R01 CA078938, S.B.; grant P01 CA 30206, S.J.F.; grant K23 CA85503-01, K.S.B.) and the Leukemia & Lymphoma Society (2192, S.B.).
National Institutes of Health
Authorship
Contribution: C.-L.S. analyzed data and wrote the paper; L.F. performed research; T.K. helped analyze data; W.L. provided critical insight in analyzing the data; L.L.R., K.S.B., D.J.W., and S.J.F. provided critical insight in the interpretation of data; and S.B. designed the study, supervised and performed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Smita Bhatia, City of Hope National Medical Center, 1500 E. Duarte Rd, Duarte, CA 91010-3000; e-mail: sbhatia@coh.org.