Abstract
X-linked lymphoproliferative disease (XLP1), described in the mid-1970s and molecularly defined in 1998, and XLP2, reported in 2006, are prematurely lethal genetic immunodeficiencies that share susceptibility to overwhelming inflammatory responses to certain infectious triggers. Signaling lymphocytic activation molecule-associated protein (SAP; encoded by SH2D1A) is mutated in XLP1, and X-linked inhibitor of apoptosis (XIAP; encoded by BIRC4) is mutated in XLP2. XLP1 is a disease with multiple and variable clinical consequences, including fatal hemophagocytic lymphohistiocytosis (HLH) triggered predominantly by Epstein-Barr virus, lymphomas, antibody deficiency, and rarer consequences of immune dysregulation. To date, XLP2 has been found to cause HLH with and without exposure to Epstein-Barr virus, and HLH is commonly recurrent in these patients. For both forms of XLP, the only curative therapy at present is allogeneic hematopoietic cell transplantation. Beyond their common X-linked locus and their requirement for normal immune responses to certain viral infections, SAP and XIAP demonstrate no obvious structural or functional similarity, are not coordinately regulated with respect to their expression, and do not appear to directly interact. In this review, we describe the genetic, clinical, and immunopathologic features of these 2 disorders and discuss current diagnostic and therapeutic strategies.
Introduction
X-linked lymphoproliferative disease type 1 (XLP1) is 1 of 7 major X-linked genetically determined (primary) immunodeficiencies that affect humans through developmental and functional defects that generally cripple both innate and adaptive immunity. Although XLP1 has been recognized for more than 35 years,1 much remains to be understood about the role of the 128 amino acid signaling lymphocytic activation molecule (SLAM)–associated protein (SAP) adaptor molecule, which is ablated or functionally compromised by mutations that result in this disease. SAP is expressed during early immunologic development and is normally found in NK, NKT, and T cells. The harm from mutated SAP begins during the prenatal period and continues to influence effector functions after birth, with clinical consequences that appear variably and increase in number and severity over time.
In addition to XLP1, XLP phenotypes have also more recently been discovered to be associated with deficiency of X-linked inhibitor of apoptosis (XIAP).2 This clinical entity is sometimes referred to as XLP2. Although XLP1 is clearly associated primarily with immunologic defects (as discussed in “Immunopathology of XLP1/SAP deficiency”), it remains to be determined exactly how XLP2 can be explained as an immunodeficiency versus a cell intrinsic apoptosis abnormality not limited to the immune system.
In this review, we separately describe the genetic, clinical, and immunopathologic features of these 2 disorders and review current diagnostic and therapeutic strategies.
XLP1 (SAP deficiency)
History
The first description of what is now known as XLP1 is credited to Bar et al: “fulminating lymphoproliferative disease, initially believed to be infectious mononucleosis,”3 p363 which was fatal in 4 related males between the ages of 7 and 22 years. Circumstantial evidence for the association with Epstein-Barr virus (EBV) infection was noted. The earliest case in this family was observed in 1948 and initially carried the diagnosis of acute leukemia. In the other 3 cases where autopsies were performed, massive “lymphoreticular infiltrates” with variable stages of terminal lymphoid maturation were observed in lymphoid organs.
The report of Purtilo et al,1 describing “Duncan's syndrome” (after the kindred reported), identified many of the key clinical features of XLP. The X-linked inheritance of the findings was underscored by the presence of disease in half-brothers from separate fathers. The authors described fulminant lymphoproliferative disease with cytopenias, liver failure, hepatosplenomegaly, and erythrophagocytosis in lymph node and bone marrow samples. Some affected persons had hypogammaglobulinemia or dysgammaglobulinemia, with evidence of absent germinal centers at autopsy, and others developed lymphomas of the bowel, peritoneum, and brain. Although EBV was suspected as a cofactor in some cases, 1 affected male was found to have hypogammaglobulinemia without evidence of prior exposure to EBV. Several of the affected males had encountered varicella and rubella without adverse consequences.
Further adding to the clinical description of XLP1 was the report by Provisor et al in 1975.4 This report initiated the long held, but probably misleading, belief that hypogammaglobulinemia in XLP1 was a direct consequence of EBV infection.
Genetics
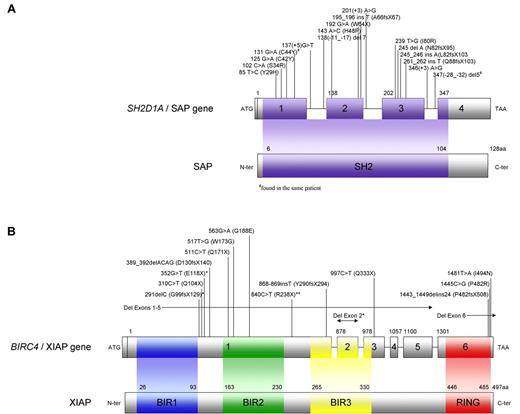
Skare et al (1987) localized the XLP1 gene to the Xq24-27 interval of the long arm of the X chromosome.5 Two years later, an interstitial deletion of Xq25 was reported in a male affected with XLP. The ensuing hunt to precisely identify the causal gene of XLP lasted nearly a decade, culminating in the formation of an international consortium of scientists, which yielded 3 reports in 1998.6-8 The investigators clarified that the SH2D1A sequence (previously termed DSHP, of unknown function), encoding a polypeptide of 128 amino acids containing a single SH2 domain, was genetically altered in patients with XLP1 (Figure 1A). The defective molecule in XLP1 is termed SAP.
Representations of SH2D1A/SAP and BIRC4/XIAP. (A) SH2D1A (SH2 domain protein 1A) has 4 exons and codes for a small protein, SAP, with only 128 amino acids that form 1 SH2 domain (amino acids 6-104). Previously unreported mutations observed in patients with XLP1 tested through the DCHI at Cincinnati Children's Hospital are shown. (B) BIRC4 (baculoviral IAP repeat-containing protein 4) is composed of 6 exons and codes for XIAP, which is 497 amino acids long. XIAP contains 3 BIR domains and 1 RING domain. Mutations observed in patients with XLP2 tested through the DCHI at Cincinnati Children's Hospital and by Rigaud et al2 (*) and Zhao et al50 (**) are shown.
Representations of SH2D1A/SAP and BIRC4/XIAP. (A) SH2D1A (SH2 domain protein 1A) has 4 exons and codes for a small protein, SAP, with only 128 amino acids that form 1 SH2 domain (amino acids 6-104). Previously unreported mutations observed in patients with XLP1 tested through the DCHI at Cincinnati Children's Hospital are shown. (B) BIRC4 (baculoviral IAP repeat-containing protein 4) is composed of 6 exons and codes for XIAP, which is 497 amino acids long. XIAP contains 3 BIR domains and 1 RING domain. Mutations observed in patients with XLP2 tested through the DCHI at Cincinnati Children's Hospital and by Rigaud et al2 (*) and Zhao et al50 (**) are shown.
As confirmatory evidence, the laboratories all sought to define whether SH2D1A mRNA was expressed differentially in lymphoid and nonlymphoid tissues. Discordance was seen among the 3 reporting groups of investigators, with 2 identifying SH2D1A transcripts in B cell–rich tissues and 1 not. However, all agreed that the gene was expressed in essentially all T cells and not in EBV-transformed B-cell lines.
Today, more than 70 mutations resulting in XLP1 have been reported to the Human Gene Mutation Database (HGMD), and more are probable to emerge. Between 2003 and 2009, SH2D1A mutational analysis has been performed on samples from 360 patients referred to the Diagnostic Center for Heritable Immunodeficiencies (DCHI) at Cincinnati Children's Hospital Medical Center because of a suspected diagnosis of XLP as determined by referring physicians. Mutations were found in 56 patients (16%) from 49 families, including 16 previously unreported mutations (Figure 1A). Gross deletions involving 1 or more exons were found in 15 patients from 13 families, accounting for nearly 25% of all SH2D1A mutations observed. The remaining mutations identified were nonsense, small insertion/deletion, missense, and splice site mutations expected to affect the SH2 domain. SAP protein analysis by flow cytometry was performed in a significant proportion of patients. Deletions and nonsense mutations were virtually always associated with absence of SAP, whereas missense and splice site mutations led to absent or decreased SAP expression.
There are no well-defined correlations between genotype and clinical phenotype. Clinical manifestations of patients often differ within families with multiple affected males, as illustrated in families identified through the DCHI (Table 1). Despite the lack of any clear-cut genotype-phenotype correlations, it is interesting to note that, in isolated instances, apparent genotype-phenotype correlation can be observed. For example, in 1 kindred affected by a splice site mutation, which has allowed variable amounts of SAP expression over time, affected identical twins remained healthy well into adulthood. One of the twins developed near-fulminant adenovirus infection at age 33 but fully recovered. He and his twin, who nursed him throughout that illness, both remain well at age 42.
Clinical phenotypes
The variable phenotypic expression of XLP1 was apparent in the first description of Duncan syndrome. Under the leadership of Dr Purtilo, the XLP registry was established at the University of Nebraska in 1980 to track and characterize the disease.9 A major emphasis was “to identify boys who had inherited what appeared to represent an inability to confront EBV” worldwide by identifying cases through word of mouth as well as through computerized literature search when this approach became more available. As reported in the publication of the registry in 1995, data on 272 boys from 80 families had been collected. Follow-up data were available for 87% of boys registered, of whom 75% had died, the majority before 10 years of age. Two males had survived to 40 years of age.9
Fulminant infectious mononucleosis (FIM) or hemophagocytic lymphohistiocytosis (HLH) related to EBV affected 58% of the cohort. This was the deadliest complication, with a 96% fatality rate during an era when effective therapies were not widely used. Only 5 patients survived, 4 of whom had been treated with etoposide. This dramatic clinical outcome led to the description of XLP1 as a disorder of “exquisite susceptibility” to EBV.
Approximately one-third of patients in the registry were reported to have lymphoproliferative disorders, and one-third were observed to have dysgammaglobulinemia. A total of 3% each of aplastic anemia and vasculitis/lymphoid granulomatosis were also identified.
Dysgammaglobulinemia was diagnosed in males both with and without prior evidence of seropositivity to EBV. Low IgG and elevated IgM were common patterns described.
More recently, patients with sporadic common variable immunodeficiency lacking other stigmata of XLP1 were tested for SAP expression and potential mutations in SH2D1A.10 Among 60 male patients with common variable immunodeficiency in the study, only 1 was found to have SAP deficiency; it was later discovered that he had an X-linked family history.
Malignant lymphomas arose overwhelmingly in extranodal sites, especially Burkitt type in the ileocecal region. Eighteen of 82 patients with lymphomas were documented to be EBV-seronegative.9
Aplastic anemia (either pancytopenia or pure red cell aplasia) was diagnosed after EBV infection, but it is not clear from the literature whether this was a consequence of FIM.
Four of 7 boys with vasculitis and/or pulmonary lymphomatoid granulomatosis reported to the XLP registry were EBV-seronegative. Subsequent reports of central nervous system vasculitis in XLP1 have also demonstrated lack of association with EBV.11 Expansion of CD8+ T cells contiguous with blood vessels, in some cases monotypic, is associated with areas of vascular necrosis on brain biopsies.12 This complication of XLP1 has been universally fatal despite the use of many immunosuppressive therapies.
Historically, the diagnosis of XLP1 was commonly considered in association with FIM and exposure to EBV based on early reports. It is possible that the true proportional distribution of clinical complications arising in XLP1 may be somewhat different from that described in the literature to date.
Immunopathology of XLP1/SAP deficiency
XLP1 and its murine model have provided an unprecedented opportunity to unravel the myriad functions that SAP-SLAM interactions regulate within the immune system. SAP is expressed in NK, NKT, and CD4+ as well as CD8+ T cells. The early observations of SAP expression in peripheral B cells have not been confirmed, even though SAP indirectly impacts some B-cell functions. It is important to note that, with the exception of missing NKT cells, the number and diversity of T, B, and NK cells in the blood of patients with XLP1 are normal.
Previously, SAP has been referred to as an immunologic “switch,” shuttling between other regulatory molecules (SLAMs) and affecting inhibitory as well as activating mechanisms.13 SAP serves as an adaptor molecule that controls signaling downstream of several SLAM family transmembrane receptors (SLAM-Rs) differentially expressed on hematopoietic cell subsets: SLAM (CD150, also measles receptor), Ly9 (CD229), 2B4 (CD244), CD84, and NTB-A (NK-, T-, and B-cell antigen).14-17 SLAM-Rs belong to the CD2 family of receptors. The association of SAP with CD150 is thought to be constitutive, although its interactions with other SLAM-Rs often depend on stimulus-induced phosphorylation of immunoreceptor tyrosine-based switch motifs (ITSMs) located within the receptor cytoplasmic tail.
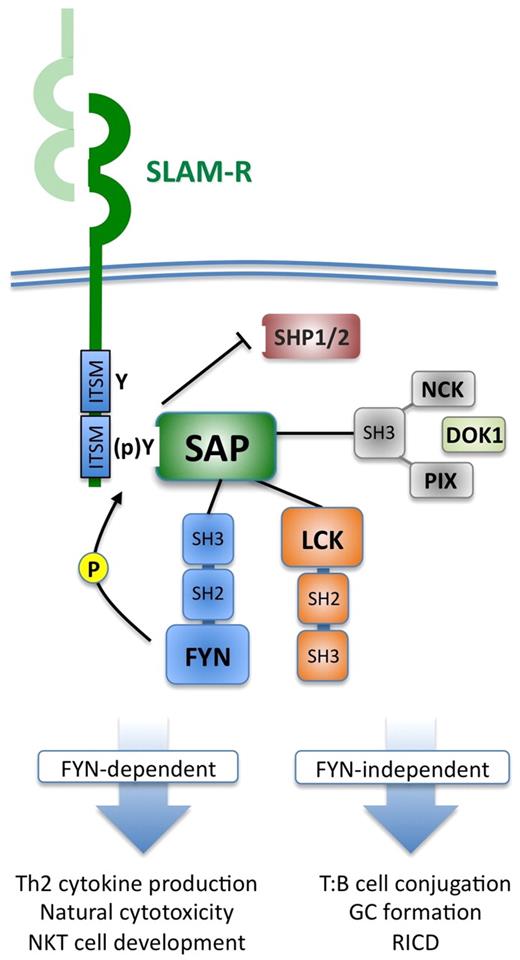
Some of the signaling outcomes mediated by SAP rely on the ability of SAP to interact with Src-family kinases (eg, FYN, LCK), which further phosphorylate SLAM-R tyrosines and other downstream signaling molecules.14 Binding of FYN (via its SH3 domain) is dependent on arginine 78 (R78) of SAP; mutation of R78 precludes FYN association and disrupts certain downstream functions, including Th2 cytokine production and cytotoxicity. Other functions attributed to SAP/SLAM-R signaling are FYN-independent (T-/B-cell conjugation, germinal center formation) and may be conveyed through demonstrated association of SAP with LCK, as well as recently described partners NCK, PIX, or DOK1, which influence certain signaling pathways downstream of the T-cell receptor (TCR), including extracellular signal-regulated kinase and nuclear factor of activated T-cell activation.18-21 Not surprisingly, SAP and certain SLAM-Rs (eg, SLAM, NTB-A) localize to the immunologic synapse during T-cell activation. SAP may also potentiate TCR and NK receptor signals by displacing inhibitory phosphatases, such as SHP-1 and SHP-2, bound to ITSMs before SLAM-R engagement. Figure 2 summarizes these multiple molecular interactions of SAP as currently understood.
SLAM-SAP intracellular signaling. On SLAM-R engagement (usually through homotypic interactions on an adjacent cell), SAP binds as an SH2-domain adaptor protein to ITSMs found in the cytoplasmic tail of SLAM-Rs. This interaction facilitates further recruitment of Src-family kinases, such as FYN (via its SH3 domain) and LCK (via its kinase domain), which further phosphorylate ITSM tyrosines in the SLAM-R as well as other downstream substrates.15,17 In docking to SLAM receptors, SAP may simultaneously displace phosphatases, such as SHP1/SHP2, which bind to SLAM-Rs before engagement, which potentiates cytotoxic function and TCR signal transduction by removing these inhibitory molecules. SAP can also associate directly with DOK1 and other SH3-containing proteins, such as NCK and PIX, which facilitates their activation and involvement in TCR-induced extracellular signal-regulated kinase and nuclear factor of activated T-cell activation, respectively.18-21 These molecular interactions allow SAP to regulate NK- and CTL-cell cytotoxicity and regulate several signaling outcomes entrained to the TCR, some of which are dependent on FYN binding (eg, Th2 cytokine secretion) and others that do not require FYN (eg, RICD).14,22
SLAM-SAP intracellular signaling. On SLAM-R engagement (usually through homotypic interactions on an adjacent cell), SAP binds as an SH2-domain adaptor protein to ITSMs found in the cytoplasmic tail of SLAM-Rs. This interaction facilitates further recruitment of Src-family kinases, such as FYN (via its SH3 domain) and LCK (via its kinase domain), which further phosphorylate ITSM tyrosines in the SLAM-R as well as other downstream substrates.15,17 In docking to SLAM receptors, SAP may simultaneously displace phosphatases, such as SHP1/SHP2, which bind to SLAM-Rs before engagement, which potentiates cytotoxic function and TCR signal transduction by removing these inhibitory molecules. SAP can also associate directly with DOK1 and other SH3-containing proteins, such as NCK and PIX, which facilitates their activation and involvement in TCR-induced extracellular signal-regulated kinase and nuclear factor of activated T-cell activation, respectively.18-21 These molecular interactions allow SAP to regulate NK- and CTL-cell cytotoxicity and regulate several signaling outcomes entrained to the TCR, some of which are dependent on FYN binding (eg, Th2 cytokine secretion) and others that do not require FYN (eg, RICD).14,22
By interacting with 1 or more of the SLAM receptors, SAP potentiates (1) the development of NKT cells, (2) T-cell help for the normal development of germinal centers leading to robust specific antibody production, and (3) the killing of EBV-transformed B cells by cytotoxic T and NK cells. More recently, it was also discovered that SAP is required for normal T-cell homeostasis mediated by reactivation-induced cell death (RICD),22 although the precise mechanism and details of SLAM receptor involvement remain to be elucidated.
NKT-cell development and function.
NKT cells, a minor T-cell population in the blood, are characterized by the coexpression of NK- and T-cell markers as well as an invariantly rearranged TCR consisting of TCRVα24 and TCRVβ11 chains, which recognize glycosphingolipid antigens presented by the CD1d molecule. Invariant NKT cells are capable of secreting a variety of cytokines and are thought to possess antitumor and antimicrobial activities as well as a regulatory function with respect to autoimmunity. SAP deficiency blocks NKT-cell ontogeny in mouse and humans.23,24 In both species, normal SAP, like FYN, has been shown to be absolutely required for NKT-cell development. The defect in NKT-cell development has been reversed in mice by molecular reconstitution of SAP in the hematopoietic compartment. Patients with XLP lack circulating NKT cells, whereas female carriers of XLP demonstrate skewed X-inactivation in the NKT cells toward normal SAP expression, in contrast to the typical pattern of bimodal SAP expression in T cells of XLP carriers.25 The implication of this latter observation is not yet obvious.
EBV-directed cytotoxicity.
SAP is also involved in NK-cell and CD8+ T-cell cytotoxicity in a very specific way that impacts the ability to kill EBV-infected B-cell targets in particular. SAP-deficient NK cells exhibit normal “global” cytotoxicity when stimulated by activating receptors not associated with SAP, such as CD16.16 However, NK- and CD8+ T-cell cytotoxicity is reduced with SAP deficiency when cells are stimulated via NTB-A or 2B4; their respective ligands (NTB-A and CD48) are both up-regulated on EBV-infected B cells.26-30 SAP-associated regulation of NK cytotoxicity is clearly demonstrated in mature human NK cells; in contrast, it appears that in immature NK cells and SAP-deficient NK cells 2B4 and NTB-A are preferentially linked to other adaptor molecules, such as EAT-2 or the inhibitor, SHP-1.
Evidence suggests that reduced cytotoxicity is not a function of reduced expression of cytotoxic proteins but rather a decreased capacity to form a polarized lytic synapse. It is hypothesized that failure of effective killing of EBV-infected cells leads to prolonged activation of the cytotoxic cells resulting in ongoing secretion of proinflammatory cytokines which, in turn, activates macrophages. The resultant scenario is very similar to that postulated with other genetic forms of HLH resulting from lack of perforin or the machinery needed to effectively deliver perforin and cytotoxic granule contents to the target cell.
Development of germinal centers and immunoglobulin production.
SAP is not constitutively expressed in B cells; however, the normal expression of SAP in CD4+ T cells is absolutely required for terminal B-cell maturation, germinal center formation, and effective antibody production. In work with murine models of XLP, SLAM-SAP-FYN signaling has been shown to be required for production of Th2 cytokines (interleukin-10 [IL-10], IL-4). In patients with XLP, B-cell development is largely intact until the stages of terminal differentiation into memory B cells and isotype switching. In vitro studies of B cells from patients with XLP confirm that the defect in terminal B-cell development is determined by ineffective interactions with XLP CD4+ T cells, which do not generate adequate amounts of IL-10.31 In contrast to the murine model of SAP deficiency, IL-4 production appears to be maintained in humans with XLP. However, development and maintenance of germinal centers are defective in both species with SAP deficiency.32,33 SAP-deficient T cells express reduced levels of inducible costimulator after TCR-mediated activation and fail to form sustained conjugates with B cells.31,33 Targeted depletion of SAP from either B or T cells has clearly identified these humoral immune defects to be T intrinsic.34,35
T-cell homeostasis.
New studies of T cells from patients with XLP1 have also revealed an intrinsic defect in TCR RICD. After initial activation and IL-2-mediated clonal expansion, RICD constitutes a self-regulatory form of apoptosis that maintains immune homeostasis by restricting the size of the effector T-cell pool. Clues suggesting a role for SAP in apoptosis had been noted in T cells from SAP-deficient mice, which showed a survival advantage in the first few rounds of division compared with wild-type (normal) cells.36
In the absence of SAP, the induction of proapoptotic molecules, such as FASL and BIM, is impaired after TCR re-engagement, resulting in defective cell death of XLP1 patient T cells.22 Defective RICD of activated T cells was demonstrated in all 7 unrelated XLP patients tested in the study relative to normal donors, with apoptosis measuring significantly below the range of sensitivity noted for normal donors22 (Figure 3). Moreover, silencing SAP expression via RNA interference in normal donor T cells recapitulated this phenotype, implying that SAP is indeed required for RICD. Although the precise biochemical interplay of SAP with TCR signaling pathways remains to be elucidated, this SAP-dependent death signal appears to be conveyed through NTB-A. This requirement for SAP in potentiating TCR signal strength for RICD may explain excessive accumulation of activated T cells triggered by certain viral infections in both SAP-deficient mice and XLP1 patients.37 It is tantalizing to posit that the RICD defect associated with SAP deficiency helps to drive the massive, often lethal, accumulation of CD8+ T cells triggered by uncontrolled EBV infection. As noted in the paragraph above, SAP-deficient CD4+ T cells fail to form long-lived conjugates with B cells.33 EBV-infected B cells that persist in XLP1 patients probably serve as persistent weak antigen-presenting cells for CD8+ T cells as well, providing chronic T-cell restimulation that fails to trigger RICD. This newly recognized proapoptotic function for SAP in RICD and other programmed cell death pathways (ie, p53-response to DNA damage) is consistent with previous observations,38 including the in vitro persistence of interferon-γ high CD8 T cells from XLP patients in unstimulated cultures, an observation reminiscent of the descriptions of Bar et al in 1974.3
Laboratory studies that can be used to facilitate a diagnosis of XLP. (A) Flow cytometric detection of intracellular SAP in peripheral blood CD8+ T cells and NK cells from a normal control, a patient with SAP deficiency, and a maternal carrier. (B) Flow cytometric detection of intracellular XIAP in peripheral blood CD8+ T cells and NK cells from a normal control, a patient with SAP deficiency, and a maternal carrier. (C) Flow cytometric quantification of peripheral blood NKT cells of a normal control, a patient with SAP deficiency, and a patient with XIAP deficiency (NKT cells identified as CD3+, TCRVα24+, and TCRVβ11 copositive lymphocytes). (D) Sample scatter plots measuring percentage of viable T cells after induction of RICD of activated and expanded T cells of an adult control, a patient with SAP deficiency, and a patient with XIAP deficiency using the anti-CD3 monoclonal antibody OKT3 (500 ng/mL). (E) Graphical representation of T-cell death induced by RICD. Cell death was quantified as follows: % cell loss = (1 − (% viable cells, treated/% viable cells, untreated)) × 100.
Laboratory studies that can be used to facilitate a diagnosis of XLP. (A) Flow cytometric detection of intracellular SAP in peripheral blood CD8+ T cells and NK cells from a normal control, a patient with SAP deficiency, and a maternal carrier. (B) Flow cytometric detection of intracellular XIAP in peripheral blood CD8+ T cells and NK cells from a normal control, a patient with SAP deficiency, and a maternal carrier. (C) Flow cytometric quantification of peripheral blood NKT cells of a normal control, a patient with SAP deficiency, and a patient with XIAP deficiency (NKT cells identified as CD3+, TCRVα24+, and TCRVβ11 copositive lymphocytes). (D) Sample scatter plots measuring percentage of viable T cells after induction of RICD of activated and expanded T cells of an adult control, a patient with SAP deficiency, and a patient with XIAP deficiency using the anti-CD3 monoclonal antibody OKT3 (500 ng/mL). (E) Graphical representation of T-cell death induced by RICD. Cell death was quantified as follows: % cell loss = (1 − (% viable cells, treated/% viable cells, untreated)) × 100.
Diagnostic studies
The gold standard for confirmation of any genetic disorder is sequencing of the gene of interest. Sequencing of SH2D1A is available on a clinical basis in several laboratories. In addition, lymphocyte SAP expression can be measured by flow cytometry or Western blot analysis. Flow cytometric analysis of SAP is a clinically available screening test and offers the advantages of being rapid (results usually available in 1 day) and requiring a minimal amount of whole blood (< 1 mL). The method is simple and uses routine flow cytometric techniques. SAP can be readily detected in T cells and NK cells, and patients with SAP deficiency typically demonstrate markedly decreased or absent SAP expression.25,39 One patient has also uniquely been observed to possess bimodal expression of SAP in CD8+ T cells, possibly because this patient possesses an SH2D1A mutation at the splice acceptor site of exon 2, or, alternatively, possibly because of revertant mosaicism.25 Flow cytometric analysis of SAP can also be used for the detection of carrier status.25 Examples of flow cytometric detection of SAP are shown in Figure 3A. Rarely, males who exhibit no SAP expression in the context of a clinical XLP1 phenotype do not demonstrate genetic defects in the coding region of SH2D1A. The biologic basis of these cases is still unclear.
In addition to genetic sequencing and evaluation of SAP expression, other laboratory testing can support a diagnosis of XLP1. NKT cells are absent in humans and mice with SAP deficiency,23,24 and the observation of an absence of NKT-cell populations in suspected patients can indicate a possible diagnosis of XLP1 (Figure 3C). Evaluation of whole blood NKT-cell populations can be performed by routine flow cytometric techniques in experienced laboratories.
The observation of defective RICD can also suggest a diagnosis of XLP1,22 although the study of RICD is time and labor intensive, and not yet available on a clinical basis (Figure 3D-E). Defective RICD has been seen in disorders of, as yet, unknown cause, in addition to XLP1 (R.A.M. and A.H.F., unpublished observation, 2010).
Therapy of XLP1
Therapy of patients with XLP1 depends in part on the clinical scenario. Patients with hypogammaglobulinemia or antibody deficiency probably benefit from IgG replacement, although this has not been shown to prevent acquisition of primary EBV infection (D. Purtilo, personal communication to A.H.F., circa 1990). Rituximab (anti-CD20 monoclonal antibody) has been reported to abort new-onset EBV infection in patients with known XLP1 who are closely monitored prospectively.40 In patients with active HLH, treatment with standard-of-care therapies, such as steroids and etoposide, is often used.
Definitive correction of XLP1 to date has only been achieved with allogeneic hematopoietic cell transplantation (HCT), which was first described in 1986.41 Historically, overall survival of patients with XLP undergoing allogeneic HCT has been estimated at 71%.42 More recently, reduced-intensity conditioning regimens have been used for patients with XLP1.43-45 Because of the limited number of patients with XLP1 treated with RIC-HCT thus far, the overall success of this method is difficult to estimate at this time.
Gene therapy has only been tested, to date, in vitro. In 2004, retroviral gene transfer of SH2D1A was reported to restore normal anti-EBV responses in T cells from patients with XLP1.46
XLP2 (XIAP deficiency)
History
The XIAP/BIRC4 gene was initially characterized in 1996.47-49 However, the association with human disease was not discovered until 2006 when Rigaud et al found that mutations of XIAP/BIRC4 were associated with XLP phenotypes in 12 patients from 3 kindreds who lacked SH2D1A mutations.2 Since that time, limited further clinical information has been collected regarding this newly recognized primary immune deficiency. A recent case report described a Japanese boy with XIAP deficiency and recurrent HLH,50 and experience with an additional 10 American patients from 8 families has also been summarized.51 The majority of patients display recurrent HLH. However, there is a general lack of understanding as to why a deficiency of XIAP leads to HLH. XIAP is primarily known for its antiapoptotic functions, which are not specific to the immune system. XIAP is also implicated in a number of signaling pathways and other functions as discussed below in “Immunopathology of XLP2/XIAP deficiency.” Thus, the majority of the history of XIAP deficiency remains to be written.
Genetics
The BIRC4 gene is located on the X chromosome (in curious proximity to SH2D1A) and consists of 6 exons (Figure 1B). To date, 12 mutations have been reported in the literature in patients with the XIAP deficiency. Mutations observed include large whole exon deletions, nonsense, small insertion, and missense mutations, which result in truncated, decreased, or absent XIAP expression (Figure 1B).2,50,51
At the DCHI at Cincinnati Children's Hospital Medical Center, BIRC4 mutational analyses have been performed in 130 patients, who had previously been determined to be normal for SH2D1A/SAP by either mutational or protein analysis. Mutations in XIAP were found in 15 patients (12%) from 13 families (Figure 1B). Similar to SAP, more than two-thirds of the patients have deleterious mutations, including gross deletions, small deletion/insertions, and nonsense mutations. Missense mutations were found in 5 patients from 4 families. Good correlation was observed between the type of mutation and the level of XIAP expression detected by flow cytometry. As with XLP1, thus far, no clear genotype-phenotype correlations have been observed.
Clinical phenotypes
XIAP deficiency has been reported in 23 patients (Table 2). The original cohort of patients with XIAP deficiency presented with phenotypes consistent with XLP.2 Specifically, all patients were members of families with multiple affected males who generally presented with HLH, often with a history of EBV infection.2 However, there was an intrinsic selection bias in this cohort, as patients were first diagnosed with XLP on the basis of XLP phenotypes, before the discovery of XIAP deficiency as the causative defect in these patients. Splenomegaly was often noted, and colitis was also observed in 2 patients.
More recently, XIAP deficiency has been reported in patients who lack the classic clinical phenotype of XLP, and more readily fit the definition of familial HLH.50,51 These patients commonly presented in infancy or early childhood with HLH, in many cases without EBV infection. Recurrent HLH and splenomegaly were often observed.
Patients with XIAP deficiency may or may not develop hypo/dysgammaglobulinemia. When this finding is observed, it may be transient and secondary to immune-mediated destruction of a previously intact humoral immune system or secondary to immunosuppression (steroids, etoposide) in the setting of HLH. In addition, whereas SAP deficiency can be observed in males presenting with common variable immunodeficiency, XIAP deficiency has not been observed in such patients.52
Thus far, no cases of lymphoma have been observed in patients with XLP2, further differentiating XIAP deficiency from SAP deficiency. Given these clinical differences, it has recently been suggested that XIAP deficiency may be best classified as a cause of X-linked FHLH rather than XLP.51
Immunopathology of XLP2/XIAP deficiency
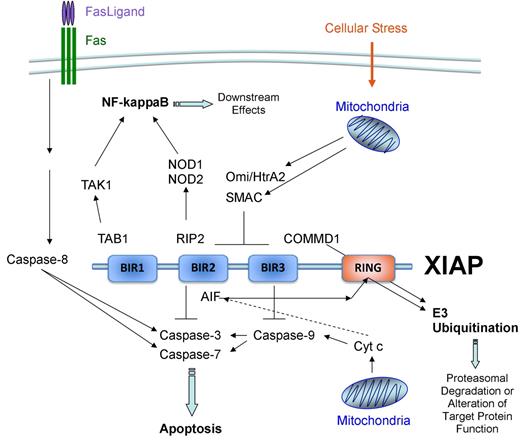
XIAP is a 497-amino acid member of the inhibitor of apoptosis (IAP) family of proteins that was discovered in 1996 (Figure 1B).47-49 IAPs are defined by their inclusion of 1 or more baculovirus IAP repeat (BIR) domains, which are named as such because the first IAP sequence identified was a baculoviral gene product capable of inhibiting apoptosis.53 BIR domains span approximately 70 amino acids and contain a conserved sequence of cysteines and histidine that coordinate an atom of zinc.54 BIR domains are primarily involved with mediating protein-protein interactions. XIAP contains 3 BIR domains. BIR2 and its N-terminal linker region inhibit caspase-3 and caspase-7, whereas BIR3 inhibits caspase-9.55-58 These antiapoptotic attributes of XIAP are the most well-studied functions of the protein. Inhibition of caspases by XIAP can be relieved by second mitochondria-derived activator of caspases (Smac), Omi/HtrA2, and ARTS, which are released by the mitochondria after proapoptotic stimuli.59-62 The BIR regions of XIAP can also interact with noncaspase proteins, such as RIP2 and TAB1.63-65 These and other XIAP interactions mediate signaling pathways involving nuclear factor-κB, c-Jun N-terminal kinase, Smad, NOD1, and NOD2, and the bone morphogenetic protein receptors.63-68 Well-described protein interactions are illustrated in Figure 4.
Protein interactions of XIAP. Well-described XIAP interactions are illustrated. BIR2 and its N-terminal linker region interact with and inhibit caspase-3 and caspase-7, whereas BIR3 inhibits caspase-9.55-58 Inhibition of caspases by XIAP can be relieved by SMAC and Omi/HtrA2, which are released by the mitochondria after proapoptotic stimuli and interact with BIR2 and BIR3.59-61 BIR1 and BIR2 also interact with TAB1 and RIP2, respectively.63-65 The C-terminal RING finger domain of XIAP possesses E3 ubiquitin ligase function.69,70 XIAP-mediated ubiquitination of proteins, such as COMMD1, results in the subsequent targeting of the protein for proteasomal degradation.71 Ubiquitination of other proteins, such as apoptosis-inducing factor, may serve to alter the function of the protein.72
Protein interactions of XIAP. Well-described XIAP interactions are illustrated. BIR2 and its N-terminal linker region interact with and inhibit caspase-3 and caspase-7, whereas BIR3 inhibits caspase-9.55-58 Inhibition of caspases by XIAP can be relieved by SMAC and Omi/HtrA2, which are released by the mitochondria after proapoptotic stimuli and interact with BIR2 and BIR3.59-61 BIR1 and BIR2 also interact with TAB1 and RIP2, respectively.63-65 The C-terminal RING finger domain of XIAP possesses E3 ubiquitin ligase function.69,70 XIAP-mediated ubiquitination of proteins, such as COMMD1, results in the subsequent targeting of the protein for proteasomal degradation.71 Ubiquitination of other proteins, such as apoptosis-inducing factor, may serve to alter the function of the protein.72
In addition to its BIR domains, XIAP also contains a C-terminal RING domain. The RING domain possesses E3 ubiquitin ligase function, although knowledge regarding the specific functions of XIAP's RING domain is limited.69,70 XIAP-mediated ubiquitination often results in the subsequent targeting of the protein for proteosomal degradation, such as in the case of COMMD1 (copper metabolism MURR1 domain-containing protein 1).71 More interestingly, XIAP-mediated ubiquitination does not always result in targeting to the proteasome. Ubiquitination of other proteins, such as apoptosis-inducing factor, may serve to alter the function of the protein.72
Unlike SAP, whose expression appears to be limited to T and NK cells, XIAP is widely expressed. XIAP was detected in all originally evaluated human tissues and has also been found to be present in all cells of hematopoietic origin.2,49,73 Although best known for its antiapoptotic properties, it is not clear whether the lack of XIAP's normal caspase-inhibitory function is the reason for HLH. Deficiency of XIAP has been observed to result in increased sensitivity of T cells to undergo RICD.2,51 It has been postulated that this increased sensitivity of lymphocytes to undergo apoptosis may result in ineffective viral (microbial) clearance and predispose to the development of HLH. However, this seems counterintuitive given that HLH is a condition characterized by excessive lymphocyte overactivation, proliferation, and accumulation.
The finding of decreased numbers of NKT cells has also been postulated to contribute to the development of HLH in patients with XLP2 as well as XLP1.74 However, there is no direct evidence that a lack of NKT cells contributes to the development of HLH in patients with XLP1. In addition, in contrast to XLP1, many patients with XLP2 possess numerically normal NKT populations,75 although studies of NKT-cell function have not yet been reported.
Murine studies have also failed to reveal a clear mechanism for HLH development thus far. Curiously, mice deficient in XIAP were originally described to possess normal lymphocyte apoptosis induced by a variety of means.76 Embryonic fibroblasts were noted to have increased levels of 2 related IAP family proteins, cIAP-1 and cIAP-2, and it was postulated that this may offer some compensation for the lack of XIAP. XIAP-deficient mice have also been described to possess normal NKT-cell populations and no humoral defects,77 a sharp contrast to SAP-deficient mice. XIAP-deficient mice do have increased susceptibility to infections with Listeria monocytogenes and Chlamydia pneumoniae.78,79 Lack of XIAP during these infections leads to alterations in proinflammatory cytokine production and cell survival.78,79
Diagnostic studies
Diagnosis of XLP2 requires genetic sequencing of BIRC4/XIAP gene, ideally complemented by analysis of XIAP expression. As with XLP1 (and several other primary immune deficiencies), flow cytometric techniques can be used for rapid screening of XLP2.73 Thus far, patients with BIRC4 mutations have demonstrated decreased or absent expression of XIAP when measured by flow cytometry (Figure 3B).50,73
In contrast to XLP1, measurement of NKT-cell populations in XLP2 does not reveal significant abnormalities in many patients.75 Whereas the original European cohort of patients with XLP2 was observed to possess decreased NKT-cell populations,2 an American cohort of patients with XIAP deficiency was found to have numerically normal populations of NKT cells compared with a large pediatric and adult control group.75 This is in line with the observation of numerically normal NKT-cell populations observed in XIAP-deficient mice.77 It is possible that the decreased NKT cells observed in the first report of the disorder may have been a secondary phenomenon related to the disease status of the patients at the time of evaluation. In addition, NKT cells can constitute as little as 0.008% of peripheral blood T cells in normal persons.75 This makes it difficult to interpret low levels of NKT cells unless there is truly an absence of this unique cell population (as in XLP1; Figure 3C).
Similarly, the evaluation of RICD is not a robust screening test for XLP2. Patients with XLP2 have been observed to possess increased sensitivity to RICD in vitro when using both plate-bound2 and soluble51 anti-CD3 antibodies. However, there can be significant overlap with the sensitivity of normal controls to RICD depending on the methodology used (Figure 3E).
Therapy of XLP2
Given the relative paucity of collective clinical experience with XLP2, the optimal treatment of this disorder remains to be defined. It is clear that patients with XLP2 develop HLH and recurrent HLH on a frequent basis. Although the occurrence of HLH always carries a significant mortality risk, the mortality related to any given episode of HLH in patients with XLP2 appears to be less than that associated with the first occurrence of EBV-HLH in patients with XLP1. Indeed, all patients in an American cohort survived HLH,51 whereas historical survival of XLP1 patients with EBV-HLH is less than 5%.9 Established therapies for HLH, such as dexamethasone/methylprednisolone and etoposide, should be used as needed. In addition, rituximab can be of benefit in the setting of EBV-HLH, and alemtuzumab has been successfully used as salvage therapy in 1 patient with XLP2 and recalcitrant HLH.51
Accurate mortality rates for XLP2 are currently not available because of the lack of significant cumulative clinical experience with this disease. A very rough estimate can be gleaned from the original report of the disease. A total of 25% of patients were observed to die between the ages of 6 months and 41 years resulting from HLH, and an additional patient died from complications of HLH and HCT at age 11.2
The decision to pursue allogeneic HCT can be difficult in the setting of a novel disorder, such as XIAP deficiency. It is the authors' opinion that HCT should be considered for patients with XLP2 for whom an appropriately matched bone marrow donor is available. In support of this, early experience with RIC-HCT in 5 patients with XLP2 treated at Cincinnati Children's Hospital Medical Center suggests that this approach to HCT can be successful without any apparent complications related specifically to deficiency of XIAP.
In conclusion, this review has highlighted the historic, genetic, and clinical features of XLP1 and XLP2, reviewed the current understanding of the immunopathology caused by deficiency of SAP and XIAP, and discussed current diagnostic and therapeutic strategies. It is apparent that, although SAP deficiency and XIAP deficiency are similar with regard to causing HLH in male patients, very little else appears to be related between these 2 primary immunodeficiencies, suggesting that these 2 disorders are not brothers as initially presumed, but more distant cousins within a family of diseases that predispose to the development of HLH.
Acknowledgments
The authors thank Colin Duckett for critical reading of portions of the manuscript and helpful discussions.
This work was supported in part by the Histiocytosis Association of America and the National Institutes of Health (grant R03 1R03AI079797-01). A.L.S. is supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
National Institutes of Health
Authorship
Contribution: A.H.F. researched the literature and wrote much of the paper; K.Z. contributed to the sections of Genetics of XLP1 and 2 and Figure 1; A.L.S. contributed the description of RICD data for XLP1 and prepared Figure 2; and R.A.M. prepared portions of the manuscript dealing with XLP2, produced Figures 3 and 4, and assisted with editing the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexandra H. Filipovich, Division of Bone Marrow Transplantation and Immunodeficiency, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: Lisa.Filipovich@cchmc.org.