Abstract
The inflammatory response to ionizing radiation (IR) includes a proangiogenic effect that could be counterproductive in cancer but can be exploited for treating impaired wound healing. We demonstrate for the first time that IR stimulates hypoxia-inducible factor-1α (HIF-1α) up-regulation in endothelial cells (ECs), a HIF-1α–independent up-regulation of stromal cell–derived factor-1 (SDF-1), as well as endothelial migration, all of which are essential for angiogenesis. 5 Gray IR-induced EC HIF-1α and SDF-1 expression was greater when combined with hypoxia suggesting an additive effect. While small interfering RNA silencing of HIF-1α mRNA and abolition of HIF-1α protein induction down-regulated SDF-1 induction by hypoxia alone, it had little effect on SDF-1 induction by IR, demonstrating an independent pathway. SDF-1–mediated EC migra-tion in hypoxic and/or radiation-treated media showed IR induced strong SDF-1–dependent migration of ECs, augmented by hypoxia. IR activates a novel pathway stimulating EC migration directly through the expression of SDF-1 independent of HIF-1α induction. These observations might be exploited for stimulation of wound healing or controlling tumor angiogenesis.
Introduction
Recent reports have demonstrated a proangiogenic effect of ionizing radiation (IR) in both therapeutic and pathologic settings.1-3 Understanding and manipulating the affect of IR on angiogenesis in malignant disease is a crucial step in treating tumors, as 50% of all patients with solid tumors in the United States are treated with radiation therapy.2 A proangiogenic effect of IR therapy in these patients would be counterproductive. Conversely, identifying the mechanism by which IR stimulates neovascularization might provide a powerful new tool for treating the complex diseases associated with vascular insufficiency, ischemia, microvessel disease, and impaired wound healing.
Our understanding of new blood vessel formation has been altered significantly in the past decade. In addition to the classic model of angiogenesis, characterized by new blood vessel sprouting from resident endothelial cells (ECs), bone marrow–derived endothelial progenitor cells (EPCs) are recruited to form new blood vessels in a process known as adult vasculogenesis.4 Surprisingly, vasculogenesis is responsible for up to 35% of new blood vessel formation in adults.5 EPCs are recruited to sites of injury or ischemia in response to increased expression of the chemokine stromal cell-derived factor-1 (SDF-1 or CXCL12).6-10 SDF-1 is secreted by vascular ECs in response to hypoxic stimuli and binds to the chemokine receptor CXCR4 present on EPCs. SDF-1 is transcriptionally regulated by hypoxia-inducible factor-1α (HIF-1α) binding to hypoxia response elements on its promoter after HIF-1α translocation to the nucleus.11 HIF-1α is a master regulator of cellular response to hypoxia affecting expression of more than 60 target genes. The role of HIF-1α in stimulating neovascularization through up-regulation of proangiogenic stimuli such as vascular endothelial growth factor (VEGF) and SDF-1 is well known.12,13
Increasing evidence suggests that these angiogenic stimuli are modulated by IR.1,2,14-17 Conventional high-dose radiotherapy is known to lead to nuclear accumulation of HIF-1α and enhanced translation of HIF-1α regulated transcripts in tumor cells.2 In addition, the SDF-1 promoter region, which is regulated by HIF-1α in ECs, contains radiation-responsive sites.14 Stem cell trafficking has also been shown to be improved in the bone marrow in response to increased SDF-1 released after IR.16 In addition, VEGF release from mast cells and matrix metalloproteinase-9 (MMP9) up-regulation has been shown to improve tissue revascularization after low-dose IR.1 VEGF transcription is also known to be highly regulated by HIF-1α.12
Because these angiogenic factors are all intimately involved in vasculogenesis and are also modulated by IR, we hypothesized that a common pathway exists between IR and the molecular mediators of EPC recruitment and that this pathway is dependent on HIF-1α stabilization and up-regulation of SDF-1 in ECs.
In this study we demonstrate that low-dose IR leads to stabilization of HIF-1α in human ECs. Low-dose radiation results in up-regulation of the vasculogenic chemokine SDF-1 and subsequent improved EPC chemotaxis. Furthermore, this vasculogenic response is additive when low-dose IR is combined with hypoxic stimuli. Finally, we demonstrate that SDF-1 transcriptional up-regulation after IR is mediated by both HIF-1α–dependent and –independent mechanisms.
Methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) passages 2-5 (Clonetics) were grown in endothelial growth media-2 (Clonetics). Before all experiments, cells were starved in 0.5% fetal bovine serum media without growth factors for 8 hours. Cells were irradiated using a Varian 2300 Linear Accelerator using a dose energy of 6 Mv, dose rate 200 cGy/min, field size: 20 × 20 cm2, source to surface distance: 100 cm, on a 2-cm solid water (bolus). Cells were returned to full endothelial growth media-1 and placed in either normoxic (21% O2) or hypoxia (1% O2) conditions in a custom designed hypoxic incubator (Biospherix) maintained with a continuous infusion of a preanalyzed gas mixture (95% N2, 5% CO2).
Proliferation assay
HUVECs (n = 6) were grown for 24, 48, and 72 hours posttreatment. Six hours before harvest, cells were treated as previously described18 with 0.5 μCi/mL [3H]thymidine. Cells were then lysed, and thymidine incorporation was analyzed by liquid scintillation spectrometry (LS 1801; Beckman).
Apoptosis assay
After treatment for 24 and 48 hours, cells (n = 3) were harvested and analyzed using the annexin V–fluorescein isothiocyanate Apoptosis Detection Kit (Calbiochem). Samples were collected using a Becton Dickinson FACSCalibur flow cytometer. Cytometric analysis was conducted with FlowJo Version 8.0 (TreeStar).
In vitro immunocytochemistry
Treated cells (n = 6) were fixed with 4% paraformaldehyde and washed in phosphate-buffered saline. Primary antibody was HIF-1α (NB100-131A4; Novus) at 1:500 in glycine-buffered saline/phosphate-buffered saline. Secondary antibody was Alexa Fluor 594 goat anti–mouse 1:1000 (Molecular Probes). Nuclear staining was performed with Vectashield mounting media with DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories) and viewed on a BX51 epiflourescent microscope (Olympus) at 200× magnification.
HIF Western blotting
Cellular protein was extracted with the NE-PER Extraction kit (Pierce) and quantified using the BCA Protein Assay (Pierce). Protein (30 μg) was separated on 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel (Bio-Rad Laboratories), and transferred to a polyvinylidene fluoride membrane (Bio-Rad Laboratories). The membrane was blocked for 1 hour with casein in Tris-buffered saline (Pierce), and incubated in 1:250 monoclonal mouse anti–hHIF-1α (BD Transduction Laboratories) or 1:500 monoclonal mouse anti-hHIF-2α (Novus) for 12 hours. Anti-lamin A/C (1:5000; BD Transduction Laboratories) and 1:2500 anti–β-actin (Novus) were used as nuclear and cytoplasmic loading controls, respectively. All secondary antibodies used were horseradish peroxidase conjugates (Cell Signaling). Protein expression was detected on Hyperfilm (Amersham Biosciences) with enhanced chemiluminescence plus reagent (Amersham Biosciences). Bands were quantified by densitometry analysis using Kodak 1D software Version 3.5.
Transient transfection and reporter gene assay
The p2.1 plasmid used in the present study has been described previously and was generously donated by Dr Gregg Semenza.19 This reporter construct consists of the luciferase gene under the transcriptional control of the erythropoietin promoter region that contains 2 HIF binding sites or hypoxia response elements (HREs).19,20 Using 35-mm wells, 1 × 105 cells were plated (n = 6), allowed to grow to 60% confluence, washed twice with 2 mL of serum-free growth medium, and transfected as described above. Briefly, reporter plasmids were cotransfected with a DNA-liposome complex containing the Renilla luciferase construct (pHRL-TK; Promega) and our reporter construct mixed with 10 μL of Lipofectin Reagent (Invitrogen) in Opti-mem (Invitrogen) serum-free media for 6 hours, quenched with 2 mL of standard cell-culture medium with bovine serum and allowed to recuperate for 18 hours in normoxic conditions. Maximum luciferase transgene activity occurs 24 to 72 hours after initial transfection, therefore transfected cells were irradiated then incubated in either normoxic (21% O2) or hypoxic (1% O2) conditions for 48 hours. Next, cells were harvested and lysed using passive lysis buffer (Promega). After centrifugation at 14 000 rpm for 10 minutes to eliminate debris, cell lysate was mixed with luciferin containing buffer (Dual-Luciferase Assay Kit; Promega), and promoter activity was measured using a luminometer. Data were normalized to the Renilla luciferase baseline activity to account for differences in transfection activity and cell lysate recovery.
Quantitative real-time PCR
Total RNA was isolated using the RNeasy RNA isolation kit (QIAGEN). The RNA was assessed using an Agilent 2100 Bioanalyzer (Agilent), and 5 μg of total RNA were reverse-transcribed using Superscript II (Invitrogen) and oligo(dT) primers according to manufacturer's protocol. Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed using 1 ng of reverse-transcribed cDNA per sample in an ABI Prism 7900HT Sequence Detection System (Applied Biosystems). SYBR green was used to detect PCR products (SYBR Green PCR Master Mix; Applied Biosystems). All reactions were performed in triplicate in 4 different sets of RNA extractions (n = 4). The following primers were used: CXCL-12 forward 5′-CCAAACTGTGCCCTTCAGAT-3′, CXCL-12 reverse 5′-CCACTTTAGCTTCGGGTCAA-3′; EIF4B forward 5′-CCTGAGCCAAAGAAACCTGA-3′, EIF4B reverse 5′-GCAGCATACTTGCTTGCAGA-3′; standard curves were generated using EIF4B. Values are expressed as fold increases relative to the reference sample (untreated control).
siRNA HIF-1 knockdown
Cells were transfected with HIF-1α small interfering RNA (siRNA) (5′-TCCATGTGACCATGAGGAAA-3′, 3′-AAGCTTCGCTGTGTGTTTTG-5′) or HIF-2a siRNA (5′-CACCUACUGUGAUGACAGAtt-3′, 5′-UCUGUCAUCACAGUAGGUGaa-3′) using Lipofectamine 2000 (Invitrogen) for 24 hours. The media was then changed, and the cells were exposed to radiation or sham radiation followed by hypoxia or normoxia as described in the text. Control cells were treated with transfection reagent and nonsense siRNA only. All experiments were performed in triplicate from 4 different sets of cells (n = 4).
SDF and VEGF ELISA
Cell-culture supernates (n = 6) were collected over treated HUVEC monolayers. Protein concentration was normalized using the BCA Protein Assay kit (Pierce) and SDF and VEGF levels were quantified using the human SDF or VEGF Quantikine enzyme-linked immunosorbent assay (ELISA; R&D Systems). Absorbances were read on a Spectramax 340 plate reader (Molecular Devices), and data were analyzed using Softmax PRO software Version 2.04.
Migration assay
Using a ChemoTx transwell system (5.7 mm, 8-μm pore; Neuro Probe), EPCs were collected from normal healthy human donors as described21 after obtaining informed consent in accordance with the NYU School of Medicine Institutional Review Board. Institutional Review Board approval was obtained for the collection of blood from NYU. Cells (5 × 104) were seeded onto the membrane over conditioned media (EBM-2 plus 0.5% fetal bovine serum without growth factors) harvested from treated HUVEC monolayers. Cells (n = 6) were allowed to migrate for 6 hours before washing and fixing in 4% paraformaldehyde. Absorbent cells were stained with DAPI, viewed on a BX51 epiflourescent microscope at 100× magnification, and quantified with Kodak 1D software. Results represent 3 independent experiments. SDF-1 was used as a positive control. Anti-SDF-1 antibody was added to the conditioned media after normoxic/hypoxic conditioning to block SDF-1 signaling where indicated.
Statistical analysis
Data are expressed as the mean ± SEM. Data were analyzed with an unpaired 2-tailed Student t test or analysis of variance coupled with posthoc Tukey test for multiple pairwise comparisons. Probability values of less than .05 were considered to be statistically significant.
Results
Establishing dose for in vitro experiments
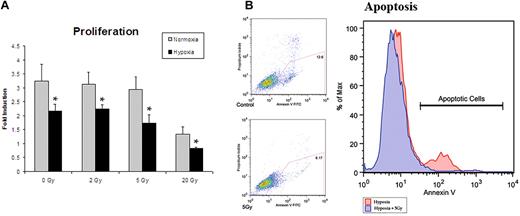
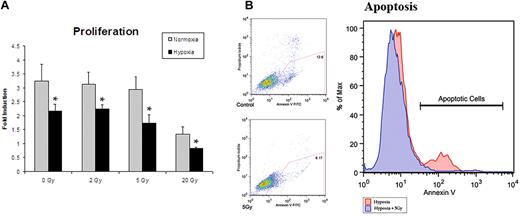
A wide range of radiation doses have been used for experimental purposes to investigate in vitro and in vivo effects. In vitro studies have used doses of under 5 Gray (5 Gy) for experiments with lower-dose IR therapy.1,22 In vivo radiation of microvascular free flaps (tissue beds containing their inherent vascular supply) showed no reduction in perfusion at doses as high as 8 Gy.23 We first established an optimal dose of IR to maximize cytokine production while minimizing cell death. We analyzed ECs treated with 0, 2, 5, and 20 Gy of irradiation in an attempt to find a dose that would be translatable to in vivo studies. All doses of radiation resulted in an absolute reduction in proliferation by 24 hours. After normalizing for initial cell death, doses of 2 Gy and 5 Gy were found to have no significant relative reduction in proliferation after 48 hours in normoxia or hypoxia (Figure 1A). Apoptosis was then assessed by annexin V expression measured by flow cytometry. Cells exposed to a single dose of 5 Gy radiation, and subsequently exposed to hypoxia, had decreased apoptosis suggesting a cytoprotective role (Figure 1B). Thus, a dose of 5 Gy radiation was used for all subsequent experiments.
Proliferation assays were undertaken in an attempt to ascertain the optimal dose of IR to maximize cytokine production while minimizing cell death. (A) Proliferation assays after radiation exposure demonstrated a dose of 5 Gy to yield the optimal setting of cytokine up-regulation with minimal cell death (n = 6). (B) Apoptotis of hypoxic cells with and without 5 Gy radiation exposure was assessed by annexin V expression. Cells exposed to 5 Gy radiation showed a decrease in apoptosis, suggesting a cytoprotective role. This dose was therefore used for all subsequent experiments (n = 3).
Proliferation assays were undertaken in an attempt to ascertain the optimal dose of IR to maximize cytokine production while minimizing cell death. (A) Proliferation assays after radiation exposure demonstrated a dose of 5 Gy to yield the optimal setting of cytokine up-regulation with minimal cell death (n = 6). (B) Apoptotis of hypoxic cells with and without 5 Gy radiation exposure was assessed by annexin V expression. Cells exposed to 5 Gy radiation showed a decrease in apoptosis, suggesting a cytoprotective role. This dose was therefore used for all subsequent experiments (n = 3).
Hypoxia-dependent and -independent HIF-1 signaling activity after 5 Gy IR
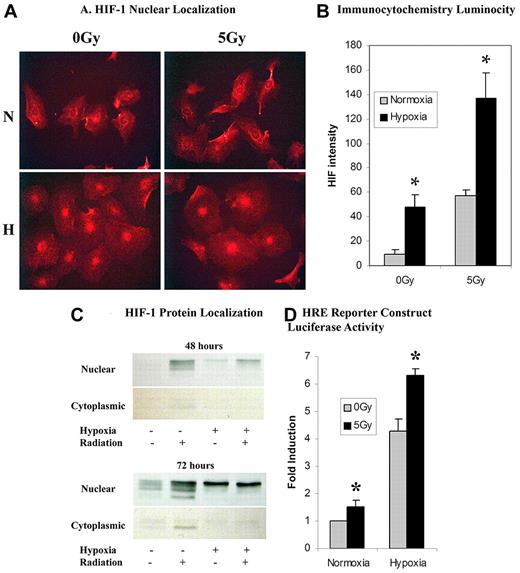
HIF-1α protein levels were significantly elevated after 5 Gy IR in both hypoxia and normoxia. Western blot and immunocytochemistry showed increased cytoplasmic and nuclear protein levels of HIF-1α. As expected, HIF-1α protein was first stabilized in the cytoplasm and then found to be elevated in the nucleus after translocation (Figure 2A-C). Functional cellular activity of nuclear HIF-1α was demonstrated through the luciferase reporter construct. HIF-1α readily bound the HRE on the promoter of the luciferase reporter construct leading to increased luciferase after 5 Gy IR in both hypoxia and normoxia (Figure 2D). There was no statistically significant difference in HIF-2α protein expression after 5 Gy IR (data not shown).
An analysis of HIF-1α expression through immunocytochemistry, protein expression, and functional assays. (A) Immunocytochemical analysis of celluar HIF-1α revealed a qualitative increase in nuclear HIF-1α presence in cells exposed to either hypoxia or 5 Gy radiation over normoxic and nonradiated controls, respectively. A combination of hypoxia and 5 Gy radiation dose showed a greater qualitative luminosity of HIF-1α staining over both hypoxia and radiation-exposed samples individually. (B) Quantification of in vitro HIF-1α luminosity by photometric analysis revealed a statistically significant increase in HIF-1α nuclear localization in hypoxic and radiated cells versus normoxic nonradiated controls. A combination of hypoxia and 5 Gy radiation revealed a statistically significant increase in HIF-1α nuclear localization over hypoxia or radiation alone (n = 6). (C) HIF-1α protein was assessed by Western blot at both 48 and 72 hours ± normoxia, hypoxia, or 5 Gy IR. Radiation was a strong stimulus for HIF-1α in both hypoxic and normoxic conditions. The strongest response was seen when hypoxia and radiation were combined. There is also increased cytoplasmic stabilization of HIF-1α starting at 48 hours. (D) Functional cellular activity of HIF-1α was determined through a luciferase reporter construct containing a HRE on the promoter. Statistically significant increases in luciferase activity were seen after 5 Gy IR in both hypoxia and normoxia (n = 6).
An analysis of HIF-1α expression through immunocytochemistry, protein expression, and functional assays. (A) Immunocytochemical analysis of celluar HIF-1α revealed a qualitative increase in nuclear HIF-1α presence in cells exposed to either hypoxia or 5 Gy radiation over normoxic and nonradiated controls, respectively. A combination of hypoxia and 5 Gy radiation dose showed a greater qualitative luminosity of HIF-1α staining over both hypoxia and radiation-exposed samples individually. (B) Quantification of in vitro HIF-1α luminosity by photometric analysis revealed a statistically significant increase in HIF-1α nuclear localization in hypoxic and radiated cells versus normoxic nonradiated controls. A combination of hypoxia and 5 Gy radiation revealed a statistically significant increase in HIF-1α nuclear localization over hypoxia or radiation alone (n = 6). (C) HIF-1α protein was assessed by Western blot at both 48 and 72 hours ± normoxia, hypoxia, or 5 Gy IR. Radiation was a strong stimulus for HIF-1α in both hypoxic and normoxic conditions. The strongest response was seen when hypoxia and radiation were combined. There is also increased cytoplasmic stabilization of HIF-1α starting at 48 hours. (D) Functional cellular activity of HIF-1α was determined through a luciferase reporter construct containing a HRE on the promoter. Statistically significant increases in luciferase activity were seen after 5 Gy IR in both hypoxia and normoxia (n = 6).
Stimulation of SDF-1 signaling by 5 Gy IR
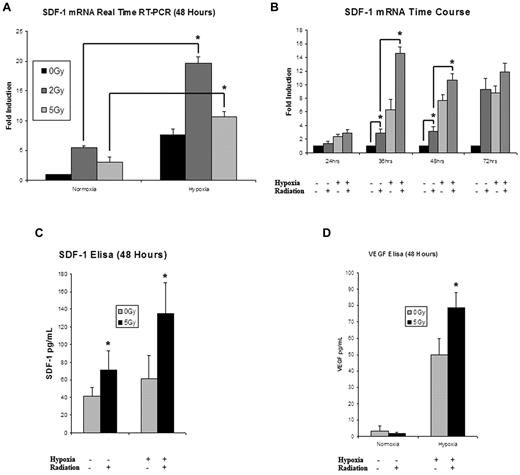
5 Gy IR up-regulated EC expression of SDF-1 mRNA both with and without an additional hypoxic stimulus. The addition of hypoxia after radiation induced a significant elevation in SDF-1 transcription greater than that seen with either stimulus alone (Figure 3A). When analyzed over time, 5 Gy IR significantly elevated SDF-1 expression at 24 hours after treatment, with a maximal expression of 4× greater than the 24-hour hypoxia/radiation stimulated activity occurring by 36 hours (Figure 3B). For all time points, the combination of stimuli was significantly elevated over hypoxia alone or 5 Gy IR alone. This regulation of SDF-1 was also observed at the translational level, as measured by SDF-1 protein ELISA (Figure 3C). These findings mimic the pattern of regulation seen with HIF-1α signaling discussed above.
An analysis of the effects of IR on cytokine mRNA and protein expression. (A) SDF-1 mRNA was significantly up-regulated in ECs exposed to radiation in both normoxic and hypoxic conditions (n = 4). (B) When examined over a time course ranging from 24 to 72 hours, 5 Gy IR significantly elevated SDF-1 mRNA expression over nonirradiated controls. The combination of 5 Gy IR and hypoxia resulted in the greatest up-regulation of SDF-1 mRNA expression at all time points. Maximal expression occurred at the 36-hour time point (n = 4). (C) SDF-1 protein expression was examined at 48 hours. Maximal expression was again seen with the combination of hypoxia and 5 Gy IR (n = 6). (D) To look at the effects of other proangiogenic HIF target genes, VEGF protein expression was examined at 48 hours showing maximal up-regulation with the combination of hypoxia and 5 Gy IR (n = 6).
An analysis of the effects of IR on cytokine mRNA and protein expression. (A) SDF-1 mRNA was significantly up-regulated in ECs exposed to radiation in both normoxic and hypoxic conditions (n = 4). (B) When examined over a time course ranging from 24 to 72 hours, 5 Gy IR significantly elevated SDF-1 mRNA expression over nonirradiated controls. The combination of 5 Gy IR and hypoxia resulted in the greatest up-regulation of SDF-1 mRNA expression at all time points. Maximal expression occurred at the 36-hour time point (n = 4). (C) SDF-1 protein expression was examined at 48 hours. Maximal expression was again seen with the combination of hypoxia and 5 Gy IR (n = 6). (D) To look at the effects of other proangiogenic HIF target genes, VEGF protein expression was examined at 48 hours showing maximal up-regulation with the combination of hypoxia and 5 Gy IR (n = 6).
Because IR is known to affect the regulation of multiple proangiogenic cytokines, we measured protein expression of VEGF, epidermal growth factor, fibroblast growth factor, and platelet-derived growth factor to measure patterns of regulation. VEGF displayed a strong pattern of increased expression after 5 Gy IR that was analogous to HIF-1α augmentation (Figure 3D). Platelet-derived growth factor showed a mild up-regulation after 5 Gy IR, while fibroblast growth factor and epidermal growth factor were both down-regulated (data not shown).
EPC chemotaxis after 5 Gy IR
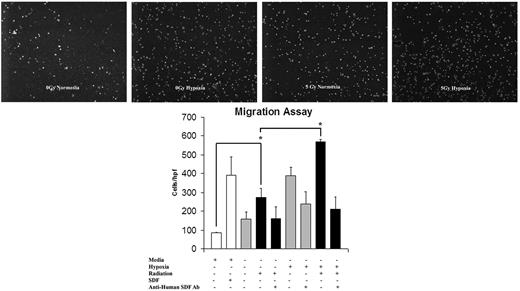
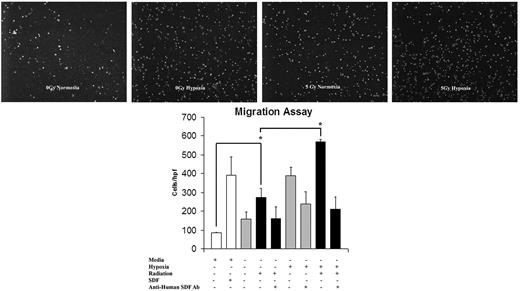
Conditioned media harvested from EC monolayers proved to be chemotactic for EPCs, as demonstrated by in vitro migration assay (Figure 4). The pattern of migration stimulation again fell into the recognizable pattern of SDF-1 up-regulation after 5 Gy IR, with the highest response occurring in reaction to the dually stimulated hypoxia plus radiation group. To confirm that SDF-1 is the specific chemokine involved in this migratory stimulus, anti–human SDF-1 monoclonal antibody was added to the media before migration. The addition of anti-SDF antibody abrogated the EPC response in all treatment groups.
The effect of IR on EC migration and its reversal using anti-SDF-1 antibody (Ab). Conditioned media from EC monolayers was harvested and assayed for EPC chemotactic potential. This migration pattern showed a similar up-regulation to SDF-1 up-regulation after 5 Gy IR stimulation. Maximal migration occurred with a combination of hypoxia and 5 Gy IR. The addition of SDF-1 to basal media resulted in a response similar to that of conditioned media harvested from IR/hypoxia-stimulated cells. The addition of anti-SDF-1 antibody blunted the migration response to media harvested from ECs exposed to 5 Gy IR, hypoxia, and the combination of both stimuli. This confirmed the causal relationship of increased SDF-1 expression and migration response (n = 6).
The effect of IR on EC migration and its reversal using anti-SDF-1 antibody (Ab). Conditioned media from EC monolayers was harvested and assayed for EPC chemotactic potential. This migration pattern showed a similar up-regulation to SDF-1 up-regulation after 5 Gy IR stimulation. Maximal migration occurred with a combination of hypoxia and 5 Gy IR. The addition of SDF-1 to basal media resulted in a response similar to that of conditioned media harvested from IR/hypoxia-stimulated cells. The addition of anti-SDF-1 antibody blunted the migration response to media harvested from ECs exposed to 5 Gy IR, hypoxia, and the combination of both stimuli. This confirmed the causal relationship of increased SDF-1 expression and migration response (n = 6).
mRNA and protein expression after HIF-1 silencing
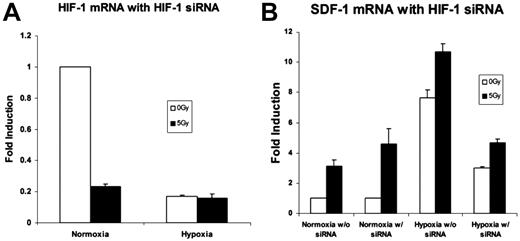
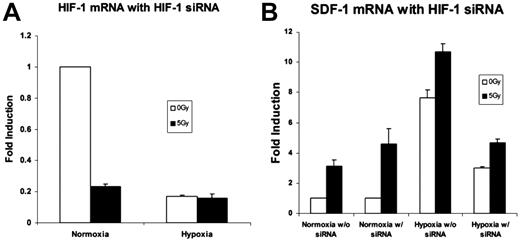
Silencing of the HIF-1α mRNA with siRNA was confirmed by real-time qRT-PCR. An 80%-85% reduction in HIF-1α expression was achieved (Figure 5A). SDF-1 mRNA expression after HIF-1α silencing demonstrated an overall reduction. Despite this decrease, up-regulation of SDF-1 was still observed after 5 Gy IR (Figure 5B).
An analysis of the effects of HIF-1α siRNA on genes up-regulated by low-dose radiation therapy. (A) HIF-1α mRNA expression was examined with real time qRT-PCR after silencing with HIF-1α siRNA to demonstrate effective HIF-1α knockdown. An 85% reduction in HIF-1α mRNA expression was achieved (n = 4). (B) SDF-1 mRNA levels with and without HIF-1α silencing. There was an overall reduction in SDF-1 expression in hypoxia. However, 5 Gy IR exposure still effected a relative increase versus nonradiated samples despite HIF-1α blockade (n = 4).
An analysis of the effects of HIF-1α siRNA on genes up-regulated by low-dose radiation therapy. (A) HIF-1α mRNA expression was examined with real time qRT-PCR after silencing with HIF-1α siRNA to demonstrate effective HIF-1α knockdown. An 85% reduction in HIF-1α mRNA expression was achieved (n = 4). (B) SDF-1 mRNA levels with and without HIF-1α silencing. There was an overall reduction in SDF-1 expression in hypoxia. However, 5 Gy IR exposure still effected a relative increase versus nonradiated samples despite HIF-1α blockade (n = 4).
Discussion
A growing body of evidence supports the proangiogenic role of low-dose radiation therapy. These reports have evaluated factors such as VEGF, MMP-9, and nitric oxide, which are involved in the processes of both classic angiogenesis and progenitor cell recruitment in adult vasculogenesis.1,3,24,25 HIF-1α up-regulation after radiation therapy has been characterized in tumor cells.2 We demonstrate for the first time, the up-regulation of HIF-1α as well as SDF-1 by 5 Gy IR in normal human ECs. In addition, we demonstrate that SDF-1 up-regulation occurs after IR in part through a HIF-1α–independent fashion.
The increase in HIF-1α signaling that is seen after 5 Gy IR occurs in both normoxic and hypoxic conditions. Furthermore when combined, hypoxia and IR lead to greater HIF-1α signaling than is seen with either stimulus alone. The increase in HIF-1α signaling after 5 Gy IR could explain many of the angiogenic effects of radiation. The mechanism of HIF-1α stimulation by 5 Gy IR, however, is likely unique from that of hypoxia as demonstrated by their individual and synergistic up-regulation.
HIF-1α protein levels were significantly elevated after 5 Gy IR in both hypoxia and normoxia. The increased cytoplasmic and nuclear protein levels of HIF-1α as demonstrated by Western blot analysis and immunocytochemistry were shown to have functional activity through the use of the luciferace reporter assay. This luciferase construct contains the hypoxia response element in its promoter region, which when bound to HIF-1α leads to increased luminescence.19,20 Increased HIF-1α binding in the transfected cell line led to increased luciferase expression after exposure to radiation in the absence of hypoxia. Radiation also augmented luciferase activity in hypoxia leading to a more robust response than that of hypoxia alone (Figure 2D). This functional assay demonstrates the increased HIF signaling that is seen in ECs after 5 Gy IR both with and without hypoxia. The increase in luminosity is analogous to increased HIF-1α target gene activation. Two of these HIF target genes, SDF-1 and VEGF, were measured and displayed patterns of up-regulation consistent with the HIF-1α up-regulation seen on Western blot and luciferase assay.
Although we identified HIF-1α activation in this cell system, both HIF-1α and HIF-2α are expressed in ECs. In addition, there is evidence demonstrating that HIF-2α imparts radiation resistance in head and neck tumors as well as renal cell carcinoma cell lines.26,27 We therefore examined HIF-2α protein expression in this cell system as well. However, we found no statistically significant difference in HIF-2α protein expression.
Although they have a partly overlapping set of target genes, mounting evidence suggests that HIF-1α and HIF-2α activate a distinct subset of hypoxically induced genes. Both bind to endogenous HREs found within the promoters of target genes. Binding, however, is not sufficient for target gene activation. Cooperation with other transcription factors may be necessary for cell type-specific up-regulation of HIF target genes. For example, HIF-2α is known to regulate the chemokine receptor CXCR4, but it is unclear if it plays an important role in regulating the ligand of this receptor, SDF-1.26,28-31 It is known, however, that HIF-1α does regulate expression of SDF-1.11 In this study, our findings demonstrate no HIF-2α protein increase in response to IR.
There is significant evidence that HIFs are predominantly regulated in a posttranslational fashion by hypoxia-dependent protein stabilization.32,33 In normoxic conditions, hydroxylation of proline residues leads to ubiquination and subsequent proteasomal degradation. Accordingly, there were no differences in HIF-1α or HIF-2α mRNA abundance as measured by qRT-PCR after 5 Gy IR (data not shown34 ).
Immunoblot analysis demonstrated a distinct conformational difference in cytoplasmic HIF-1α protein after 5 Gy IR. The HIF-1α protein band that was up-regulated by radiation alone was different from that seen after hypoxia (Figure 2C). Immunocytochemistry studies demonstrated increased cytoplasmic HIF-1α localized at the nuclear periphery after 5 Gy IR (Figure 2A). Analysis of posttranslational control mechanisms of HIF-1α regulation, as well as protein conformational studies after radiation exposure, are currently under way. We hypothesize that 5 Gy IR leads to a conformational change in the HIF-1α protein within the cytoplasm, which is associated with decreased HIF-1α degradation.
After analyzing HIF-1α activity, we examined the effect of 5 Gy IR on SDF-1. SDF-1 is one of numerous downstream effectors activated by HIF-1α. Recent studies have shown that radiation increases the expression of the CXC family of chemokines and specifically the chemokine SDF-1/CXCL12.14,16,17 Increased stem cell trafficking in the bone marrow has been demonstrated after RT.16 5 Gy IR has been shown to augment vasculogenesis, but this effect has been attributed to mast cell release of VEGF and up-regulation of MMP-9.1 Here we demonstrate that 5 Gy IR does up-regulate SDF-1 release from ECs. We also demonstrate for the first time that IR induces a HIF-1α independent stimulation of SDF-1 and subsequent EPC activation.
SDF-1 up-regulation after 5 Gy IR was measured at both the transcriptional level by real-time qRT-PCR and the translational level by ELISA. The pattern of up-regulation is seen in both normoxia and hypoxia and is similar to HIF-1α up-regulation. These findings suggest that 5 Gy IR acts alone and in synergy with hypoxia to increase SDF-1 signaling.
It is known that the SDF-1 promoter has hypoxia response elements that bind HIF-1α leading to increased SDF-1 transcription.11 In addition, recent evidence has suggested that CXC chemokine promoter regions also have specific radiation-responsive elements.14 We therefore sought to determine whether the SDF-1 chemokine was activated by IR in the absence of HIF-1α signaling. To examine this possibility, HIF-1α activity was silenced by siRNA. HIF-1α silencing was confirmed by qRT-PCR detection of HIF-1α mRNA levels and immunoblot analysis of nuclear HIF-1α protein. ECs treated with HIF-1α siRNA were then exposed to hypoxia and radiation as before. SDF-1 activity after 5 Gy IR continued to demonstrate a significant increase compared with controls. These data confirm that SDF-1 transcriptional up-regulation after 5 Gy IR acts in a HIF-1α–independent fashion in addition to its known HIF-1α–mediated mechanism.
To further examine the provasculogenic effect of 5 Gy IR, we next looked at EPC activity in vitro to assess cell function. EPC migration was analyzed using a modified Boyden chamber and conditioned media harvested from EC monolayers exposed to 5 Gy IR. IR exposure of 5 Gy was found to be chemotactic for EPCs (Figure 4). The pattern of increased migration was again consistent with that seen in both HIF-1α and SDF-1 regulation after 5 Gy IR. The greatest migration rate of EPCs was seen in response to media from ECs exposed to radiation combined with hypoxia. To confirm that SDF-1 was responsible for this increased migration, monoclonal anti–human SDF-1 antibody was used to block SDF-1 activity in the conditioned media before assay. SDF-1 inhibition abrogated EPC migration in all treatment groups. Thus, 5 Gy IR alone is sufficient to induce a powerful vasculogenic signal and increase EPC migration. When combined with hypoxia, 5 Gy IR stimulation resulted in even greater signal activation and EPC migration, which is necessary for the trafficking of EPCs and vasculogenesis.
In summary, a single 5-Gy dose of radiation up-regulates vasculogenic signaling and EPC activity through HIF-1α– and SDF-1–mediated pathways. The affect of IR on these vasculogenic signaling mediators is both independent of and augmented by hypoxia. In addition, 5 Gy IR up-regulates SDF-1 through both HIF-1α–dependent and –independent pathways. These data offer new insight into the proangiogenic and provasculogenic effects of IR.
Much focus has been placed on inhibiting angiogenesis in the fight against cancer. Much current research is focused on finding additional upstream targets, such as HIF-1α, to inhibit in the angiogenic pathway.35,36 Our findings suggest that blocking the effect of HIF-1α in the tumor milieu may not prevent the release of SDF-1. In fact, SDF-1 release after radiation may provide an escape mechanism for tumor vasculature to survive conventional radiation therapy by signaling EPC homing. While targeting HIF-1α as an upstream master regulator of neovascularization is a crucial step toward controlling tumor growth, additional effort should be placed on inhibiting alternative pathways such as SDF-1–mediated vasculogenesis.
Alternatively, these data also shed new light on possible therapies in the fight against ischemic diseases. By stimulating mediators of both classic angiogenesis (HIF-1α, VEGF) and adult vasculogenesis (SDF-1), IR activates 2 unique pathways that are essential for neovascularization and tissue repair in ischemic processes such as peripheral vascular disease, ischemic cardiomyopathy, or ischemic brain injury. Regardless of its final application however, the mechanisms involved in this processes need to be further elucidated in order that they be more readily controlled.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
We acknowledge the National Foundation for Facial Reconstruction for their generous support of this and other projects in our laboratory.
Authorship
Contribution: O.Z.L., R.J.S., S.C.F., P.B.S., and J.P.L. designed the research; O.Z.L., M.R.G., V.D.T., S.P.S., N.S., and D.J.B. performed the research; R.J.S. and S.C.F. contributed vital new reagents or analytical tools; M.R.G., V.D.T., S.P.S., N.S., and D.J.B. collected data; O.Z.L., M.R.G., V.D.T., S.P.S., C.C.C., N.S., D.J.B., R.J.S, S.C.F., P.B.S., and J.P.L. analyzed and interpreted data; O.Z.L., M.R.G., V.D.T., S.P.S., and C.C.C. performed statistical analysis; and O.Z.L., M.R.G., S.P.S., and J.P.L. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jamie P. Levine, Director of Microvascular Surgery, Institute of Reconstructive Plastic Surgery, 560 First Ave, Suite 8Y, NYU Medical Center, New York, NY 10016; e-mail: jamie.levine@nyumc.org.
References
Author notes
O.Z.L. and M.R.G. contributed equally to this study.