Abstract
A pilot study was undertaken to assess the safety, activity, and immunogenicity of a polyvalent Wilms tumor gene 1 (WT1) peptide vaccine in patients with acute myeloid leukemia in complete remission but with molecular evidence of WT1 transcript. Patients received 6 vaccinations with 4 WT1 peptides (200 μg each) plus immune adjuvants over 12 weeks. Immune responses were evaluated by delayed-type hypersensitivity, CD4+ T-cell proliferation, CD3+ T-cell interferon-γ release, and WT1 peptide tetramer staining. Of the 9 evaluable patients, 7 completed 6 vaccinations and WT1-specific T-cell responses were noted in 7 of 8 patients. Three patients who were HLA-A0201-positive showed significant increase in interferon-γ–secreting cells and frequency of WT1 tetramer-positive CD8+ T cells. Three patients developed a delayed hypersensitivity reaction after vaccination. Definite related toxicities were minimal. With a mean follow-up of 30 plus or minus 8 months after diagnosis, median disease-free survival has not been reached. These preliminary data suggest that this polyvalent WT1 peptide vaccine can be administered safely to patients with a resulting immune response. Further studies are needed to establish the role of vaccination as viable postremission therapy for acute myeloid leukemia. This study was registered at www.clinicaltrials.gov as #NCT00398138.
Introduction
The Wilms tumor gene 1 (WT1) is one molecular marker that has become increasingly important in the biology and therapy of acute myeloid leukemia (AML).1-3 The WT1 gene encodes for a zinc finger transcription factor that is normally expressed in mesodermal tissues during embryogenesis. The putative role in leukemia biology and the continued low level expression in patients who would otherwise be considered to be without evidence of disease by conventional criteria make WT1 a potential target for therapeutic intervention. The finding of WT1 antibodies and WT1-specific cytotoxic T lymphocytes (CTLs) in patients across a variety of tumor types as well as the rejection of WT1 cancer cell challenges in mice immunized with WT1 peptides have provided a rationale for the development of immunotherapy targeting WT1.4-8 Clinical trials of vaccination with WT1 peptides have been undertaken, and both immunologic and clinical responses have been observed.9-12
We previously reported the feasibility of vaccinating patients with native as well as heteroclitic peptides for bcr-abl.13-15 We have now adapted a similar strategy in modifying WT1 peptides. Computer prediction analysis has allowed us to design several synthetic peptides capable of stabilizing major histocompatibility complex class I A0201 molecules better than native sequences and also able to elicit WT1-specific cytotoxic T-cell lymphocytes more effectively than native sequences. In addition, we developed human leukocyte antigen (HLA) class II peptides that have been shown to induce WT1-specific CD4+ responses in a broad range of HLA-DR.B1 haplotypes.16
Given the experience with disease status in allogeneic stem cell transplantation and numerous animal models, immune responses are much less probable to be effective in situations of high-volume disease. The chance for the successful application of such a modality may therefore be best when leukemia burden is minimal, so we chose to test the vaccine when patients are in complete remission (CR) but have measurable WT1 transcript. This manuscript reports the results of a pilot study in AML patients using a polyvalent WT1 vaccine composed of both CD4+ and CD8+ T-cell epitopes.
Methods
Trial design
This was a pilot study evaluating the safety and immunogenicity of a polyvalent WT1 peptide vaccine in 10 patients with AML. Patients were required to have histologic confirmation of the diagnosis at Memorial Sloan-Kettering Cancer Center (MSKCC) and to have WT1+ disease as assessed by a quantitative real-time reverse-transcription polymerase chain reaction assay (RT-PCR) for WT1 at the time of enrollment on study. All patients were required to be in CR and to have completed all planned chemotherapy (induction and postremission). The protocol was reviewed and approved by the Memorial Hospital Institutional Review Board and was conducted under a Food and Drug Administration investigational new drug application held by MSKCC. All patients gave written informed consent before enrolling in the study in accordance with the Declaration of Helsinki.
Treatment plan
Patients received 6 vaccinations (weeks 0, 4, 6, 8, 10, and 12) over a 12-week period. Vaccination sites were rotated between extremities. Injection sites were also prestimulated with 70 μg granulocyte-macrophage colony-stimulating factor (GM-CSF, Sargramostim, Bayer Healthcare Pharmaceuticals) injected subcutaneously on days −2 and 0 of each vaccination. Toxicity assessments were performed throughout the trial. Immune responses were evaluated after the third and sixth vaccinations and were assessed via delayed-type hypersensitivity (DTH), CD4+ T-cell proliferation, CD3+ T-cell interferon-γ (IFN-γ) release in ELISPOT assay, and WT1/HLA-A0201 tetramer staining for HLA-A0201-positive patients. Bone marrow aspirates were examined for morphology and were assessed after the third and sixth vaccinations. RT-PCR for WT1 in bone marrow was also used as a measure for minimal residual disease and evaluated at enrollment before vaccination and after the third and sixth vaccinations. Patients who had freedom from progression of disease and evidence of immunologic reactivity via one of the correlative assays or a decrease in measurable WT1 transcript were eligible to receive up to 6 more vaccinations (for a total of 12) administered approximately every month. Reevaluation of immune response was performed again after 12 vaccinations.
Vaccine formulation
The vaccine contains 1 WT1-derived peptide (WT1-A1) to stimulate CD8+ responses and 2 WT1 peptides (WT1-427 long, WT1-331 long) to stimulate CD4+ responses and one modified peptide (WT1-122A1) that could stimulate both CD4+ and CD8+ cells. The WT1-122A1-long peptide is a CD4+ epitope with a mutated amino acid R126Y; the sequence for the heteroclitic WT1-A1 peptide is embedded within the longer peptide. The amino acid sequences for the various peptides are given in Table 1. All peptides were manufactured under good manufacturing practices (GMP) conditions at the American Peptide Company and were synthesized using fluorenylmethoxycarbonyl chemistry and solid-phase synthesis. Purity was assessed by high-pressure liquid chromatography and amino acid sequence analysis by mass spectrometry.
Four peptides (200 μg each; total 800 μg) were mixed at a 1:1 ratio with Montanide ISA 51 UFCH (Seppic), an immune adjuvant, as an emulsion in phosphate-buffered saline (PBS) to a total volume of 1 mL. The dose of 200 μg per peptide was chosen because it is within the range found to be safe and active for other peptide vaccines.
DTH in patients
DTH tests were performed with a combination of the 4 peptides (15 μg per peptide) suspended in a 70 μL of PBS without Montanide at baseline and after the third vaccination. Positive control DTH tests included mumps or Candida (Allermed Laboratories) and were performed at baseline to test for anergy. A positive skin test reaction was defined as greater than 5-mm-diameter erythema and in duration of 48 hours after intradermal injection.
CD4+ T-cell response
CD4+ T cells were purified from peripheral blood mononuclear cells (PBMCs) by standard magnetic bead isolation using anti-CD4 monoclonal antibody (mAb; Miltenyi Biotec). The cells (1 × 105/well) were incubated in 200 μL/well of RPMI 1640 supplemented with 5% pooled autologous plasma in 96-well round-bottomed microtiter plates for 5 days, in the presence or absence of peptides. A total of 1 μCi [3H]-thymidine was added to each well, and 20 hours later the cells were harvested with a Harvester Mach IIIM (Tomtec) and counted in a 1450 MicroBeta TriLux (Wallac). The measured counts per minute represented mean values of quadruplicate microwell cultures. BCR-ABL fusion protein-derived long peptide B2A2 was used as irrelevant peptide.15
CD8+ T-cell response
In vitro stimulation.
To reliably detect CD8+ T-cell responses, we performed 2 rounds of stimulations of CD3+ T cells in vitro. PBMCs from patients were obtained by Ficoll density centrifugation. CD14+ monocytes were isolated by positive selection using mAb to human CD14 coupled with magnetic beads (Miltenyi Biotec), and part of the cells were used for the first stimulation of T cells at a ratio of 10:1 (T/antigen-presenting cells [APCs]). The CD14-negative fraction of PBMCs was used for isolation of CD3+ T cells by negative immunomagnetic cell separation using a pan T-cell isolation kit (Miltenyi Biotec). Purified CD3+ T cells were stimulated with immunizing peptides WT1A1, 122A1, or with their native peptides WTA1 and 122A, respectively (20 μg/mL), to expand the WT1A-specific CD8 T cells. The cell cultures were carried out in RPMI 1640 supplemented with 5% autologous plasma, 1 μg/mL β2-microglobulin (Sigma-Aldrich) and 10 ng/mL interleukin-15 (IL-15; R&D Systems) for 7 days. Monocyte-derived dendritic cells (DCs) were generated from the remaining CD14+ cells by culturing the cells in RPMI 1640 medium supplemented with 1% autologous plasma, 500 units/mL recombinant IL-4, and 1000 units/mL GM-CSF. On days 2 and 4 of incubation, fresh medium with IL-4 and GM-CSF either was added or replaced half of the culture medium. For the 122A or 122A1 cultures, 20 μg/mL 122A or 122A1 peptides were added to the immature DCs on day 5 to allow for processing of long peptides. Maturation cytokine cocktail (IL-4, GM-CSF, 500 IU/mL IL-1, 1000 IU/mL IL-6, 10 ng/mL tumor necrosis factor-α, and 1 μg/mL prostaglandin E2) was added to all DC cultures on day 6. On day 7, mature DCs were used for secondary stimulation of CD3+ T cells at a ratio of 1:30, with the same condition for the first stimulation. Seven days later, IFN-γ secretion of the cells was examined by enzyme-linked immunospot (ELISPOT) assay and tetramer staining.
IFN-γ ELISPOT
HA-Multiscreen plates (Millipore) were coated with 100 μL of mouse antihuman IFN-γ antibody (10 μg/mL, clone1-D1K; Mabtech) in PBS, incubated overnight at 4°C, washed with PBS to remove unbound antibody, and blocked with RPMI 1640/10% autologous plasma for 2 hours at 37°C. T cells (105 cells) were incubated with autologous CD14+ cells (104 cells) in the presence or absence of 20 μg/mL of the test peptides. Negative control wells contained APCs with T cells alone or with irrelevant peptide (Ewing sarcoma-derived HLA-A0201-binding peptide, EW: QLQNPSYDK and JAK2-derived HLA-DR binding peptide: GVCVCGDENILVQEF). Positive control wells contained T cells, APCs, and 10 μg/mL phytohemagglutinin (Sigma-Aldrich). All conditions were done in quadruplicate. The cells were incubated overnight at 37°C, and the plates were developed the next day using a secondary antibody. The spots were developed as described,13 and spot numbers were automatically determined using a computer-assisted video image analyzer with KS ELISPOT Version 4.0 software (Carl Zeiss Vision).
Tetramer staining
WT1A-A0201 tetramers conjugated with phycoerythrin were constructed by the Sloan-Kettering Institute Tetramer core facility. CD3+ T cells were stained with WT1A/HLA-A0201 tetramer (1:50 dilution) and mAbs against CD3/CD4/CD8 or other markers, using a CYAN-ADP flow cytometer with Summit software Version 4.3 (Dako Cytomation). Analysis was performed using FlowJo software Version 8.1 (TreeStar).
Chromium-51 cytotoxicity
The presence of specific functional CTLs was measured in a standard 4-hour chromium release assay as previously described.16 Two HLA-A0201 cell lines were used as targets. The WT1-positive, ALL-derived cell line 697 was kindly provided by Hans J. Stauss (University College, London, United Kingdom), and the WT1-negative B-cell lymphoma cell line SKLY-16 was obtained from the ATCC. All cells were HLA typed by the laboratory of Bo Dupont (MSKCC).
Target cells used were pulsed or unpulsed with peptide. Briefly, an aliquot of target cells was pulsed with 20 μg/mL of synthetic peptides for 2 hours at 37°C, after which they were labeled with 50 μCi of Na251CrO4 (NEN Life Science Products) per 1 million cells. After extensive washing, target cells were incubated with T cells at effector/target ratios ranging from 75:1 to 8:1. All conditions were done in triplicate. Plates were incubated for 4 hours at 37°C in 5% CO2. Supernatant fluids were harvested, and radioactivity was measured in a γ-counter. Percentage specific lysis was determined from the following formula: 100 × [(experimental release − spontaneous release)/(maximum release − spontaneous release)]. Maximum release was determined by lysis of radiolabeled targets in 1% sodium dodecyl sulfate.
Measurement of WT1 transcript by RT-PCR
Total RNA was isolated from patient samples collected in ethylenediaminetetraacetic acid using a phenol/chloroform extraction method. RNA purity was confirmed by absorbance at 260 nm. The reverse transcription reaction was adapted from protocols supplied by Applied Biosystems and described elsewhere.16,17 Briefly, reaction conditions were 2 minutes at 50°C, 10 minutes at 95°C followed by 50 cycles of 15 seconds at 95°C and 60 seconds at 60°C. Each reaction was done in triplicate and discrepancies more than 1 Ct in one of the wells were excluded. The quantitative RT-PCR and fluorescence measurements were made on the Applied Biosystems 7500 Real-Time PCR System. ABL expression was used as the endogenous cDNA quantitative control for all samples.
Statistics
Statistical analyses were done on StatView software Version 3.0 (SAS Institute) using a 2-tailed unpaired t test, with the level of statistical significance set at .05. Clinical statistics, such as Kaplan-Meier survival curves, were calculated using GraphPad Prism Version 5 software.
Results
Patient characteristics and clinical outcomes
A total of 10 patients were enrolled in the study; 9 were evaluable (Table 2). One patient was removed from the trial after a single vaccination because of noncompliance. The median age was 64 years (range, 22-76 years). Cytogenetic analysis at diagnosis revealed a normal karyotype in 7 patients, an inversion 16 in one patient, and a failure of karyotyping in one other patient. Although the chemotherapy varied among these patients, all had completed the planned AML therapy at the time of vaccination and were in first CR according to standard criteria. All had evidence of measurable WT1 transcript in cells from the bone marrow at the time of accrual onto the study. The median time spent in CR before receiving the vaccine was 10 months (range, 5-16 months).
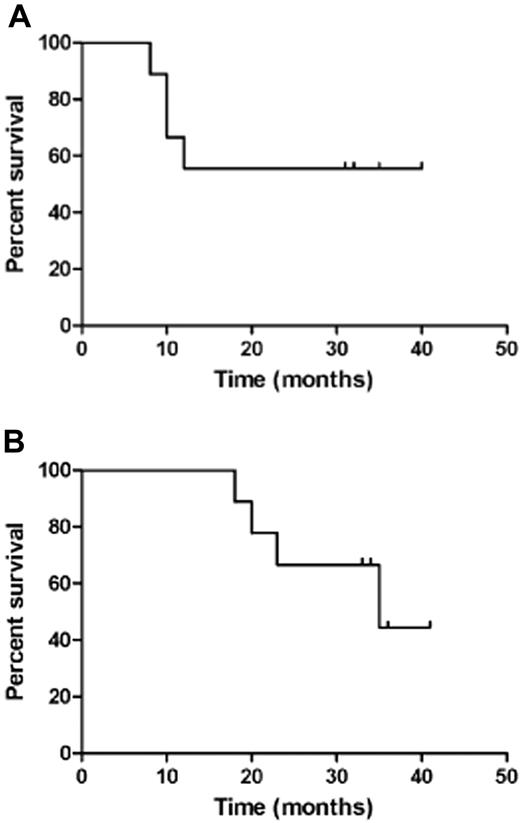
Of the 9 patients, 7 received the planned 6 vaccinations and 3 went on to complete all 12 vaccines (Table 3). One patient developed a delayed allergic reaction (see “Safety and toxicity”) after the fifth vaccine and was removed from study. Five of the 9 patients evaluated are alive in CR. All 4 patients who relapsed did so while receiving the vaccines (one after the third dose, 2 after the sixth dose, and one after the eighth dose). They discontinued therapy at the time of relapse. All of the patients with relapsed disease have died. The median disease-free survival has not been reached, whereas the median overall survival is 35+ months (Figure 1). The mean time for follow-up from diagnosis is 30 months (8 months; range, 18-41 months).
Survival curves for vaccinated patients. (A) Disease-free survival. (B) Overall survival.
Survival curves for vaccinated patients. (A) Disease-free survival. (B) Overall survival.
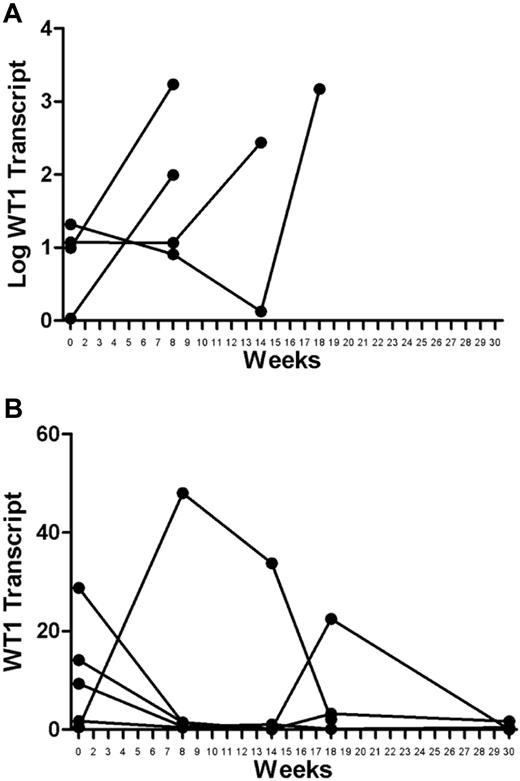
Baseline WT1 transcript measurements varied among the patients (reported as absolute copy number; range, 0.464-28.79; median, 9.87). Large increases in WT1 transcript levels were seen in relapsed patients measured at the time of or shortly before clinical relapse (Figure 2A). Although there was some variability among transcript levels measured serially in the patients who remain in continuous CR, the overall trend was either decreasing or stable transcripts at low levels (Figure 2B).
WT1 transcript levels in vaccinated patients. (A) Relapsed patients: increases in WT1 transcripts were large at the time of or just before relapse. The absolute changes occur over orders of magnitude and are shown using a logarithmic scale. (B) Remission patients: variations in transcript levels were comparatively small, either trending toward decrease or stable at very low levels. Minor variations are best appreciated using a linear scale.
WT1 transcript levels in vaccinated patients. (A) Relapsed patients: increases in WT1 transcripts were large at the time of or just before relapse. The absolute changes occur over orders of magnitude and are shown using a logarithmic scale. (B) Remission patients: variations in transcript levels were comparatively small, either trending toward decrease or stable at very low levels. Minor variations are best appreciated using a linear scale.
Safety and toxicity
Definite related toxicities were minimal (< grade 2) and generally consisted of local irritation, swelling, redness, tenderness, or pruritus at the site of vaccine administration for one to 3 days. One patient developed a delayed grade 2 allergic reaction to the vaccine consisting of generalized urticaria and a description of perceived laryngeal spasm approximately one to 2 hours after the administration of the fifth vaccine. She was treated with antihistamines and observation in the emergency room, and the symptoms quickly resolved. However, in the interest of patient safety, she received no further vaccinations, was taken off the study, and remains in CR 41+ months after her leukemia diagnosis. Another patient developed a localized hypersensitivity reaction to the GM-CSF adjuvant consisting of pain, erythema, and edema approximately 2 days after day −2 administration of the adjuvant before vaccine 9. The vaccine was held on day 0, and the patient had resolution of the symptoms within 2 weeks. However, a recurrence of the symptoms took place when the patient was rechallenged with GM-CSF at an alternative site, and the patient was therefore unable to continue with the therapy.
Immunologic responses
Vaccination induces WT1-specific CD4 T-cell response.
CD4+ T-cell response to immunizing HLA-DR peptides 331, 427, and 122A1 and its native peptide 122A was directly assessed by unprimed CD4+ T-cell proliferation. Eight patients were tested before and after 3 and 6 vaccinations. None of the patients showed any peptide-specific responses before vaccination. Except for patient 7, who did not respond to any of the peptides tested, 7 patients showed strong responses to the immunizing peptides (defined as an increase of more than twice the stimulation index: counts per minute in the test sample divided by counts per minute in the control) after 3 and 6 vaccinations (Table 3). All patients responded to peptide 331, 5 patients responded to 427, and 4 patients responded to 122A1. Patients 1, 6, and 10 responded to all 3 immunizing peptides. Figure 3A shows representative data of the CD4+ T-cell proliferation from patient 5. After 3 vaccinations, cell proliferation increased 54-fold to 331, 37-fold to 427, 4.2-fold to 122A1, and 2.6-fold to 122A peptides (P = .032) at a concentration of 50 μg/mL peptides tested. There was no significant dose dependency of the peptides, as shown by the 122-fold increase to 331, 57-fold to 427, 9.9-fold to 122A1, and 3.31-fold to 122A peptide (P = .016), when 20 μg/mL of peptides was used. Similar response was also seen after 6 vaccinations (data not shown). Although the responses varied between the peptides, they were sustained through the period of vaccination. Patient 6 completed 12 vaccinations, and the CD4+ T-cell responses were evaluated for all time points after vaccinations. Strong CD4+ T-cell responses were seen after 3 vaccinations that lasted until after 12 vaccinations (Figure 3B). Among the peptides tested, 331 seemed to be the most immunogenic, followed by 427 and 122A1. Although some patients showed a weak response to 122A peptide, except for patient 5, the responses were not statistically significant as assessed by Student t test.
CD4+ proliferation. (A) CD4+ T cells from pre (i), postvaccine 3 (ii), and postvaccine 6 (iii) vaccinations from patient 5 (A0201+) were incubated with indicated peptides at 20 μg/mL or 50 μg/mL for 5 days, and 1 μCi [3H]-thymidine was added to the cultures for 20 hours. The cell proliferation was determined by [3H]-thymidine incorporation. Data are mean ± SD from quadruplicate cultures. After 3 vaccinations, cell proliferation increased 54-fold to 331, 37-fold to 427, 4.2-fold to 122A1, and 2.6-fold to 122A (P = .032) at a concentration of 50 μg/mL peptides tested. There was no significant dose dependency of the peptides, and similar responses were also seen after 6 vaccinations. (B) Time course of CD4+ response: CD4+ T-cell responses of 3 patients who completed 12 vaccinations were calculated by the fold increase of the CD4+ T-cell proliferation against 331, 427, and 122A1 peptides over irrelevant peptide B2A2 long at a concentration of 50 μg/mL. Responses at T9 and T12 were not tested for patient 2 (A0201+) because of clinical relapse before those time points. CD4+ T-cell responses were elicited and maintained throughout vaccination, although the magnitude to each peptide with respect to vaccination times varied among patients.
CD4+ proliferation. (A) CD4+ T cells from pre (i), postvaccine 3 (ii), and postvaccine 6 (iii) vaccinations from patient 5 (A0201+) were incubated with indicated peptides at 20 μg/mL or 50 μg/mL for 5 days, and 1 μCi [3H]-thymidine was added to the cultures for 20 hours. The cell proliferation was determined by [3H]-thymidine incorporation. Data are mean ± SD from quadruplicate cultures. After 3 vaccinations, cell proliferation increased 54-fold to 331, 37-fold to 427, 4.2-fold to 122A1, and 2.6-fold to 122A (P = .032) at a concentration of 50 μg/mL peptides tested. There was no significant dose dependency of the peptides, and similar responses were also seen after 6 vaccinations. (B) Time course of CD4+ response: CD4+ T-cell responses of 3 patients who completed 12 vaccinations were calculated by the fold increase of the CD4+ T-cell proliferation against 331, 427, and 122A1 peptides over irrelevant peptide B2A2 long at a concentration of 50 μg/mL. Responses at T9 and T12 were not tested for patient 2 (A0201+) because of clinical relapse before those time points. CD4+ T-cell responses were elicited and maintained throughout vaccination, although the magnitude to each peptide with respect to vaccination times varied among patients.
Vaccination induces WT1-specific CD8+ T-cell responses.
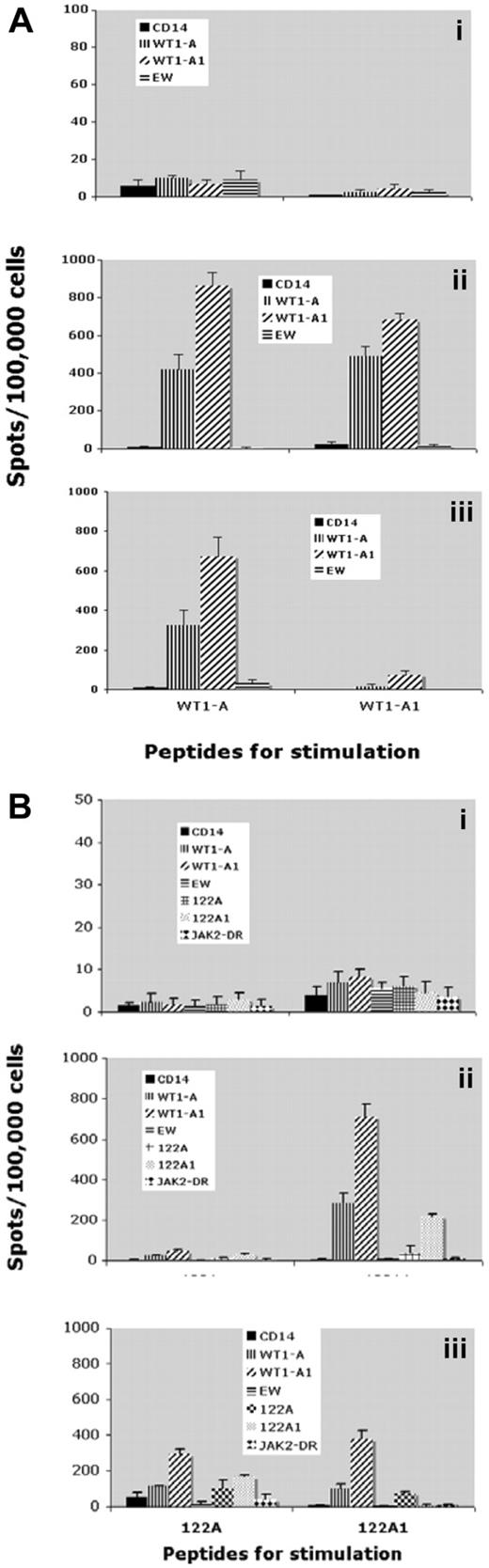
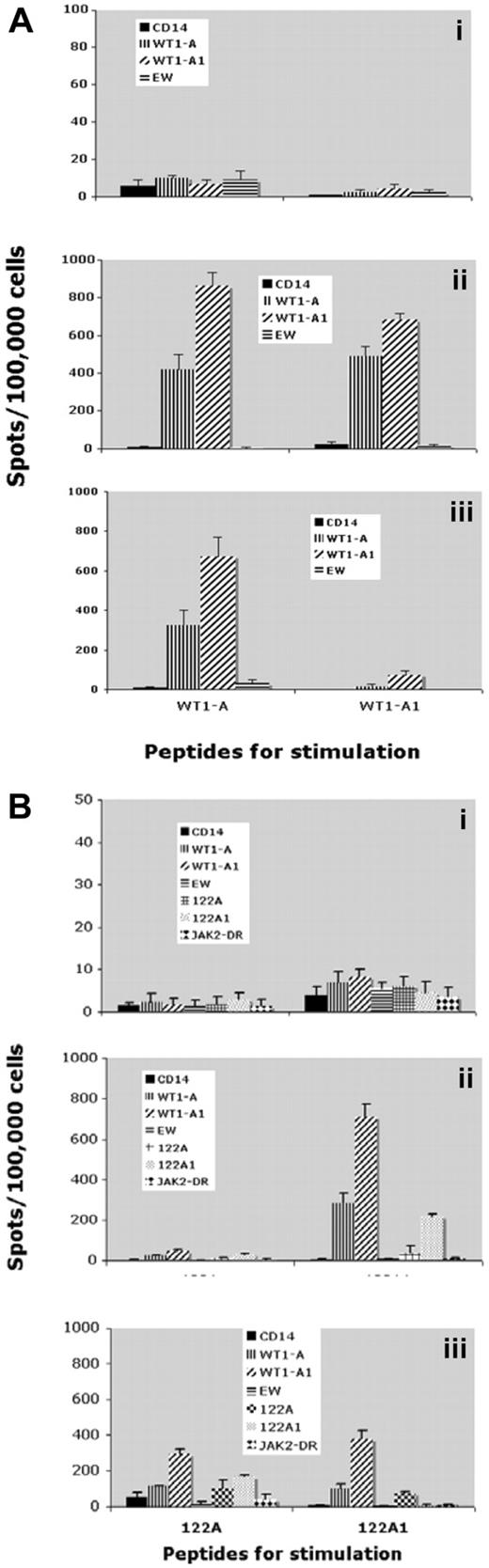
All 3 patients who were HLA-A0201–positive were tested for CD8+ T-cell response to HLA-A0201–restricted peptide WT1-A by IFN-γ ELISPOT assay and tetramer staining. This assay tests whether the peptides WTA1-A1 and 122A1, which contain analog WT1-A1 sequence, could generate immune responses against its native sequence, WT1-A. To reliably detect the peptide-specific response, CD3+ T cells were stimulated with immunizing peptides and their native sequences in vitro for 2 rounds to expand the frequency of the cells. A positive response to the vaccine was defined as a 2-fold increase in IFN-γ–secreting cells and in frequencies of CD8+WT1-A tetramer-positive cells, over the controls (irrelevant peptides). No immune responses were detected before vaccinations, but all 3 patients showed significant increase in IFN-γ–secreting cells and the frequency of WT1-A tetramer-positive CD8+ T cells, as early as after 3 vaccinations. IFN-γ secretion after vaccinations from patient 5 is illustrated in Figure 4. The stimulation with either native peptide WT1-A or its analog peptide WT1-A1 induced robust IFN-γ secretion, and the responses were cross-reactive to the native WT1-A peptide (Figure 4A). Moreover, analog long peptide 122A1 stimulation resulted in specific responses to both WT1-A and WT1-A1, in addition to long peptides 122A or 122A1. Peptide 122A1 seemed to be more efficient in inducing WT1-A–specific response than 122A, as shown after 3 vaccinations (Figure 4B). These results indicated that the WT1-A–specific response can be generated not only by HLA-A0201–restricted analog peptide, but also by the HLA-DR peptide, which contains the short sequence, demonstrating the processing and presentation of the WT1-A epitope.
IFN-γ secretion. CD3+ T cells from patient 5 were stimulated twice with WT1-A (native), WT1-A1 (analog) (A), 122A (native), or 122A1 (analog) (B) peptides. IFN-γ–secreting T cells were measured by ELISPOT assay after challenge with the indicated peptides. Controls were: no peptide (only CD14+ APCs) or with irrelevant Ewing sarcoma–derived peptide (EW) (A) or JAK-2 derived peptide (JAK-2 DR) (B). Data are mean ± SD from quadruplicate cultures from prevaccine (i), postvaccine 3 (ii), and postvaccine 6 (iii). Results indicate that a WT1-A–specific response can be generated not only by the HLA-A0201 restricted peptide but also by the HLA-DR peptide (WT1 122A1) that contains the embedded short sequence, demonstrating the processing and presentation of the WT1-A epitope.
IFN-γ secretion. CD3+ T cells from patient 5 were stimulated twice with WT1-A (native), WT1-A1 (analog) (A), 122A (native), or 122A1 (analog) (B) peptides. IFN-γ–secreting T cells were measured by ELISPOT assay after challenge with the indicated peptides. Controls were: no peptide (only CD14+ APCs) or with irrelevant Ewing sarcoma–derived peptide (EW) (A) or JAK-2 derived peptide (JAK-2 DR) (B). Data are mean ± SD from quadruplicate cultures from prevaccine (i), postvaccine 3 (ii), and postvaccine 6 (iii). Results indicate that a WT1-A–specific response can be generated not only by the HLA-A0201 restricted peptide but also by the HLA-DR peptide (WT1 122A1) that contains the embedded short sequence, demonstrating the processing and presentation of the WT1-A epitope.
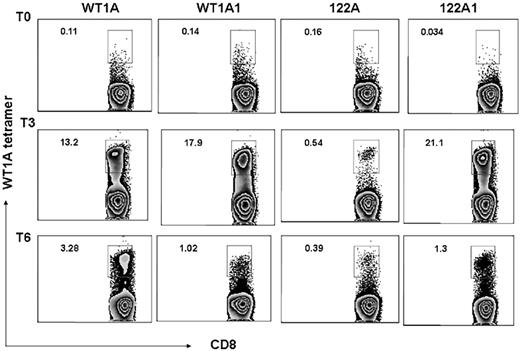
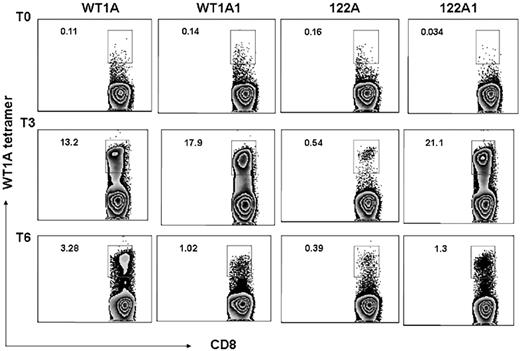
Frequency of the WT1-A–specific CD8+ T cells was also assessed by tetramer staining, and all 3 patients showed increased WT1-A tetramer–positive cells in the CD8+ population after vaccination. Representative data from the same patient (patient 5) are illustrated in Figure 5. Before vaccination, there were low percentages of tetramer-positive cells in CD8+ populations: 0.11%, 0.14%, 0.16%, and 0.034% with WT1-A, WT1-A1, 122A, or 122A1 stimulation, respectively. After vaccination, an increase in the percentage of WT1-specific CD8+ T cells was noted in cultures with WT1-A, WT1-A1, and 122A1 peptides. Peptide 122A stimulation induced a weak but significant response. These results indicated that analog peptides can induce stronger responses than the native peptide in most cases, and the processing and presentation of the native sequence, embedded within the long peptide, are not very efficient compared with its analog peptide 122A1.
Tetramers. CD3+ T cells from the same culture described in Figure 3 were stained with WT1-A/HLA-A0201 tetramer with mAbs to CD8 and other T-cell markers. Percentage of tetramer-positive CD8+ T cells (number shown in upper left corner of each histogram) were gated on CD3+ events after passing through the small lymphocyte gate. Cells from prevaccine, postvaccine 3, and postvaccine 6 are shown as T0, T3, and T6, respectively. The data are representative staining from triplicate cultures. After vaccination, a robust increase in percentage of WT1-specific CD8+ T cells was noted in cultures with WT1-A, WT1-A1, and 122A1 peptides (top axis). Peptide 122A stimulation induced a weak but significant response.
Tetramers. CD3+ T cells from the same culture described in Figure 3 were stained with WT1-A/HLA-A0201 tetramer with mAbs to CD8 and other T-cell markers. Percentage of tetramer-positive CD8+ T cells (number shown in upper left corner of each histogram) were gated on CD3+ events after passing through the small lymphocyte gate. Cells from prevaccine, postvaccine 3, and postvaccine 6 are shown as T0, T3, and T6, respectively. The data are representative staining from triplicate cultures. After vaccination, a robust increase in percentage of WT1-specific CD8+ T cells was noted in cultures with WT1-A, WT1-A1, and 122A1 peptides (top axis). Peptide 122A stimulation induced a weak but significant response.
Vaccinations induce CTLs that kill WT1+ target cells.
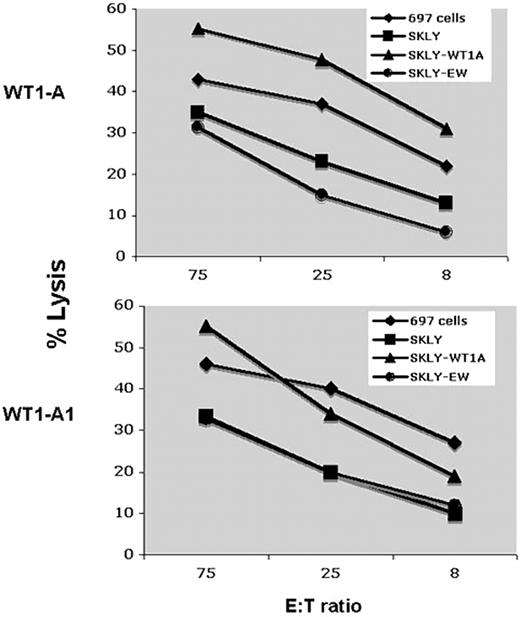
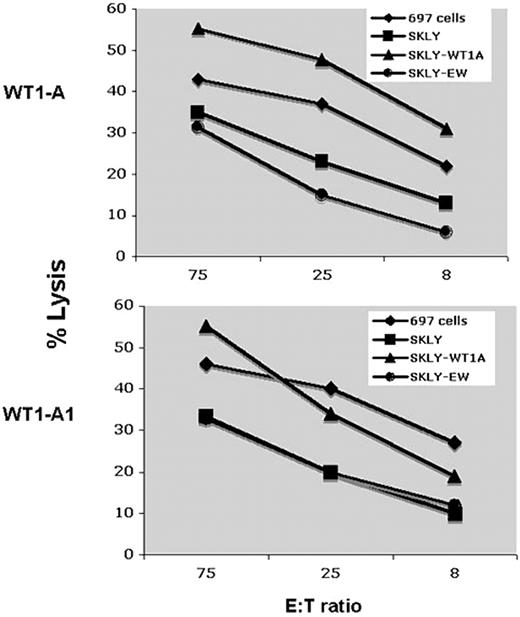
Positive results in an IFN-γ ELISPOT assay are not always associated with functional killing. Therefore, we tested the ability of T cells obtained from patient 5 (A0201+) after 6 vaccinations to kill WT1+ HLA-matched leukemia/lymphoma cell lines. As shown in Figure 6, CD3+ T cells stimulated with either WT1 A or WT1-A1 peptides were able to kill the WT1+ 697 cell line but not the WT1− cell line SKLY-16, unless the cells were pulsed with WT1-A peptide, demonstrating WT1-specific killing. These data show that vaccination with the heteroclitic WT1-A1 peptide induced HLA-A0201–restricted cytotoxicity against target cells that express WT1 native protein.
Cytotoxicity assay. CD3+ T cells from patient 5 were stimulated with WT1-A or WT1A1 peptides twice as described in Figure 5. Target cells used included the ALL derived 697 cell line (A0201+; WT1+) and the B-cell lymphoma cell line SKLY-16 (A0201+; WT1−). The cytotoxicity of the T cells was measured using a standard 51Cr release assay. The SKLY-16 cells pulsed with WT1-A (SKLY-WT1A) or an irrelevant Ewing sarcoma–derived HLA-A0201 binding peptide (SKLY-EW) were used as positive and negative controls for the specificity of killing. Effector/target (E:T) ratios are indicated on the x-axis. Data demonstrate T cell–specific killing against WT1 plus HLA-matched targets.
Cytotoxicity assay. CD3+ T cells from patient 5 were stimulated with WT1-A or WT1A1 peptides twice as described in Figure 5. Target cells used included the ALL derived 697 cell line (A0201+; WT1+) and the B-cell lymphoma cell line SKLY-16 (A0201+; WT1−). The cytotoxicity of the T cells was measured using a standard 51Cr release assay. The SKLY-16 cells pulsed with WT1-A (SKLY-WT1A) or an irrelevant Ewing sarcoma–derived HLA-A0201 binding peptide (SKLY-EW) were used as positive and negative controls for the specificity of killing. Effector/target (E:T) ratios are indicated on the x-axis. Data demonstrate T cell–specific killing against WT1 plus HLA-matched targets.
Induction of DTH responses.
Tests for DTH reactions against the administered peptides were used for detecting induction of antigen-specific CD4+ T-cell immunity. Although before vaccination there were no positive DTH reactions to the WT1 peptides/GM-CSF in any of the patients, significant DTH activity was observed in 3 patients (patients 1, 5, and 6) after 3 vaccinations. One of the patients with a reactive test was found to have a sustained positive reaction at week 14.
Discussion
WT1 has been recognized as an oncogene and has been implicated in leukemogenesis.18,19 The ability to generate a WT1-specific immune response has been demonstrated by the low level of detectable WT1 IFN-γ–secreting T cells in the peripheral blood of 50% of patients with AML and IFN-γ mRNA in T cells stimulated with WT1 peptides in 50% of healthy persons and in 60% of patients with chronic myelogenous leukemia.4,5 Several studies have attempted to build on the finding of naturally occurring WT1-specific immunity by administering WT1 analog peptide vaccines. A phase 1 study by Oka et al9 injected escalating doses of an HLA-A2402–restricted peptide and observed responses in patients with leukemia and myelodysplastic syndrome. In some patients, the generated WT1-specific CTL in the peripheral blood correlated with a reduction in bone marrow blasts and a decreased WT1 level in the bone marrow.9 Rezvani et al reported results of a phase 1 trial where WT1 peptide and proteinase 3–derived PR1 peptides were administered to 8 patients with myeloid malignancies.11 CD8+ T cells against either PR1 or WT1 were detected in 8 of 8 patients and were associated with a decrease in WT1 mRNA expression.11 Keilholz et al administered an HLA-A2–restricted WT1 peptide vaccine to 10 patients with active AML and myelodysplastic syndrome and demonstrated stabilization of disease in 10 patients and hematologic improvement in 2 others.12
Although the initial experience with these peptide vaccines has demonstrated immunologic effects, there are several potential obstacles to using WT1 as a target for a clinically effective immunotherapy. WT1 is a self-antigen and may be poorly immunogenic. Immunologic tolerance to self-proteins can inhibit the development of an effective immunologic response to cancer-associated self-antigens. Some of the responses reported in the hosts found to have endogenous immunity consisted only of low-avidity CD8+ T cells, which could be expected to contribute to partial or total tolerance of high-avidity CTLs. Weak immunogens may therefore be detrimental to promoting an effective immune therapeutic strategy, and efforts have been undertaken to modify the peptides and amplify the response.
We have previously described a method to bypass tolerance by creating several analog peptides derived from native WT1 sequences, which contained enhanced antigenicity by virtue of the improvement of their binding affinity and major histocompatibility complex/peptide complex stability.13,16,20 These peptides generated cytotoxic responses and cross-reacted with native sequences, suggesting the feasibility of incorporating such peptides in a vaccine strategy.
To date, most cancer vaccines have been designed to induce only a cytotoxic CD8+ T-cell response. The induction and maintenance of long-lasting CD8+ CTL response, however, require CD4+ T-cell help as CD4+ T cells recognize peptides bound to HLA class II molecules on APCs and help sustain the activation and survival of cytotoxic T cells.21,22 Activated CD4+ T cells have also been shown to induce tumor cell death by direct contact with the tumor cell or apoptosis pathway.23 Direct recognition and killing of leukemia cells by CD4+ T cells stimulated with WT1 337-347 in an HLA-DP5–restricted manner have been reported.24 Mesothelioma tumor cells are able to process and present antigens in the context of HLA class I and II molecules, and it has been demonstrated that CD8+ T cells, with the help of CD4+ cells, can eradicate mesothelial tumors in mice.16,25
In this manuscript, we describe the results of a pilot clinical trial using combination heteroclitic peptides to vaccinate patients who are in CR after receiving chemotherapy for AML. The heteroclitic WT1A1 peptide is a class I targeted molecule restricted to defined HLA class I subtypes. Each of the 3 long peptides was chosen for inclusion in the vaccine because of their predicted binding to HLA class II molecules. Class II molecules have a more permissive binding pocket than class I molecules, and adding amino acid residues to the N and C terminals of these peptides increases their predicted affinity to a broader range of class II molecules potentially covering a larger percentage of the population. The WT1 122A1 long peptide is a longer version of a published peptide that also has a single amino acid change to allow increased binding of the CD8 peptide YMFPNAPYL embedded within it providing the potential to stimulate both a CD8+ and CD4+ response.26,27 Therefore, by virtue of the designed changes to the peptides and the polyvalent nature of the vaccine, there was potential reactivity across a wide spectrum of HLA subtypes, and no HLA subtype restriction was placed on the eligibility criteria for the trial.
DTH responses were elicited in 3 of the 9 patients tested, suggesting successful presentation of the peptide in the context of effector cells in these patients. These 3 patients were also noted to have a CD4+ proliferative response. Overall, CD4+ proliferative responses were seen in 7 of the 8 patients (87.5%) tested, suggesting that the peptides were able to induce WT1-specific CD4+ T-cell responses across HLA subtypes. Responses occurred after the third vaccination and were sustained throughout the period of vaccination. The patient with a negative CD4+ response had no evidence of an immunologic effect of the vaccine as the DTH was negative as well. This patient relapsed after 6 vaccinations.
Three of the patients treated were of the HLA-A0201 subtype, and CD8+ responses were tested in these patients. All 3 patients were shown to have WT1-specific CD8+ T-cell responses as demonstrated by IFN-γ ELISPOT. Two of the 3 are long-term survivors as they are 35+ months and 40+ months out from the diagnosis of AML. In addition, 2 of the 3 were shown to have an increase in WT1A tetramer–positive cells after vaccination indicative of the generation of antigen-specific CTLs. In addition to the immune response generated in vitro, the effectors induced in a selected A0201 patient were able to demonstrate functional killing activity in a chromium-51 release assay. WT1 transcript levels remained relatively low in vaccinated patients who continued in CR, suggesting that there may have been some activity against minimal residual disease. There also may have been evidence of a clinical immunologic effect as 2 of the patients with HLA-A0201 subtypes had to prematurely discontinue the vaccine because of hypersensitivity to the adjuvant (GM-CSF) or the vaccine itself. Both events occurred late (after 5 and 7 vaccinations), and both of these patients are alive without evidence of disease at 36+ and 41+ months after diagnosis. The third HLA-A0201 patient relapsed early after 3 vaccinations; and although there was evidence of in vitro generation of a CD8+ response, there may not have been sufficient exposure to the vaccine to allow the generation of an immune response of sufficient magnitude to mount clinically significant activity against residual disease.
It is difficult to assess clinical efficacy in a small group of patients, particularly within the confines of a pilot study whose primary endpoints are the generation of in vitro immune response. However, several clinical observations can be made from the study group. Five of the 9 patients vaccinated on this study continue to do well without evidence of disease at the time of writing this manuscript. The median disease-free survival has not been reached, whereas the median overall survival for the entire study group is 35+ months. In contrast, Appelbaum et al reported a median overall survival of 18.8 months in patients younger than 56 years, 9.0 months in patients 56 to 65 years of age, and 6.9 months in patients 66 to 75 years of age.28 The median age of patients treated on this study was 64 years, so the outcomes for this study group compare favorably with published data from the youngest cohort who have the best outcomes. Caution, however, needs to be exercised in interpreting these results as there may be a bias with regard to patient selection in the study group. These patients not only successfully achieved CR after induction therapy but had a median period of 10 months in continuous CR before undergoing vaccination. Patients with early death or relapse would therefore be excluded from the study, so it is uncertain whether vaccination would have any effect (immunologic or clinical) in this poor-risk group.
We used several strategies to potentially improve the immunologic efficacy of the peptide vaccine, including (1) use of an HLA class I heteroclitic peptide (WT1-A1) to induce stronger CD8+ responses, (2) use of long peptides to induce CD4+ responses that could provide help for long-lasting CD8+ T-cell responses across several HLA types, (3) use of a class II peptide containing an imbedded WT1-A1 heteroclitic sequence to induce both CD4+ and CD8+ T-cell responses from the same peptide, and (4) vaccinating patients with minimal disease burden (CR status). However, this study cannot distinguish between the effects of the several novel design strategies we incorporated into the multivalent vaccine, nor can any weight be attributed to any individual strategy. Although the in vitro data suggest the ability to induce effectors that are capable of specific killing of leukemia cells, such findings cannot be directly related to the observed clinical outcome. The results are, however, intriguing enough to warrant further study in a larger clinical trial examining the role of vaccination as a viable treatment for AML.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Bo Dupont and Alice Yeh (MSKCC) for HLA genotyping.
This work was supported by the National Institutes of Health (PO1 23766), the Experimental Therapeutics Center of MSKCC, the Lymphoma Foundation, and the Glades and Tudor foundations.
National Institutes of Health
Authorship
Contribution: P.G.M. designed and performed research, collected, analyzed, and interpreted data, and wrote the manuscript; T.D. performed research, collected, analyzed, and interpreted data, and wrote the manuscript; L.M.K. designed research; S.C. collected, analyzed, and interpreted data; T.K., V.Z., R.Z., and J.Y. contributed vital analysis; J.D.W. analyzed and interpreted data; J.P.-I. designed research and contributed vital new reagents; E.B., M.W., J.J., and M.G.F. performed research; and D.A.S. designed research, contributed vital new reagents, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: L.M.K. received research funding from Innovive Pharmaceuticals. J.P.-I. has filed patents on the peptides and has received research funding from Innovive Pharmaceuticals. D.A.S. has filed patents on the peptides. MSKCC owns the rights to the vaccine. The remaining authors declare no competing financial interests.
Correspondence: Peter G. Maslak, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: maslakp@mskcc.org.


![Figure 3. CD4+ proliferation. (A) CD4+ T cells from pre (i), postvaccine 3 (ii), and postvaccine 6 (iii) vaccinations from patient 5 (A0201+) were incubated with indicated peptides at 20 μg/mL or 50 μg/mL for 5 days, and 1 μCi [3H]-thymidine was added to the cultures for 20 hours. The cell proliferation was determined by [3H]-thymidine incorporation. Data are mean ± SD from quadruplicate cultures. After 3 vaccinations, cell proliferation increased 54-fold to 331, 37-fold to 427, 4.2-fold to 122A1, and 2.6-fold to 122A (P = .032) at a concentration of 50 μg/mL peptides tested. There was no significant dose dependency of the peptides, and similar responses were also seen after 6 vaccinations. (B) Time course of CD4+ response: CD4+ T-cell responses of 3 patients who completed 12 vaccinations were calculated by the fold increase of the CD4+ T-cell proliferation against 331, 427, and 122A1 peptides over irrelevant peptide B2A2 long at a concentration of 50 μg/mL. Responses at T9 and T12 were not tested for patient 2 (A0201+) because of clinical relapse before those time points. CD4+ T-cell responses were elicited and maintained throughout vaccination, although the magnitude to each peptide with respect to vaccination times varied among patients.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/2/10.1182_blood-2009-10-250993/4/m_zh89991054410003.jpeg?Expires=1765962367&Signature=MNdYj9u7Pz5UlzRSt3foWej6yCPmQsG73v4r9DfV0P14vYr4gfx1jkmPtxrSqncFNzOyP-Htd2f1F0tSnoslmxcfIilJhffTupfi4kI4mc2sC8l3ybgz7om-ZSHssPQDOMiFhInhLVDLXj2zzUwBGK1ZifPVv-2EA3g00reeoIGbOmgdYvl0vH9z1b111r0I6-c8uu3ahsEnvXUIDp~f7DziIKbopo10JUo3i9TGe6GSOr09dqbVgUuxSyzOVp12RF9PU7KJDNRocfzAkyXp1P8xClO0ziGvlKsay716yZOH4nkYQ7Rs~QXTSOVPB24MBw8mriZzD6D7rMvZDtgU4g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 3. CD4+ proliferation. (A) CD4+ T cells from pre (i), postvaccine 3 (ii), and postvaccine 6 (iii) vaccinations from patient 5 (A0201+) were incubated with indicated peptides at 20 μg/mL or 50 μg/mL for 5 days, and 1 μCi [3H]-thymidine was added to the cultures for 20 hours. The cell proliferation was determined by [3H]-thymidine incorporation. Data are mean ± SD from quadruplicate cultures. After 3 vaccinations, cell proliferation increased 54-fold to 331, 37-fold to 427, 4.2-fold to 122A1, and 2.6-fold to 122A (P = .032) at a concentration of 50 μg/mL peptides tested. There was no significant dose dependency of the peptides, and similar responses were also seen after 6 vaccinations. (B) Time course of CD4+ response: CD4+ T-cell responses of 3 patients who completed 12 vaccinations were calculated by the fold increase of the CD4+ T-cell proliferation against 331, 427, and 122A1 peptides over irrelevant peptide B2A2 long at a concentration of 50 μg/mL. Responses at T9 and T12 were not tested for patient 2 (A0201+) because of clinical relapse before those time points. CD4+ T-cell responses were elicited and maintained throughout vaccination, although the magnitude to each peptide with respect to vaccination times varied among patients.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/2/10.1182_blood-2009-10-250993/4/m_zh89991054410003.jpeg?Expires=1765962368&Signature=12qj-LR-7UuqEkfTcbY72vNRk0Gn4rOyrH-iOskdUn6CYXdiIFgsevpWy8V0Q1aGzH5fm0QpDptVmEZR6NJ1JMSz-tFSRGXklR3Yc5is~2K5mEuT5QoU1UgXt~r26Y971-Q0nUaxUz8Z3bYnR6y~HHSwZTRN6Ih6GXSw92LvgFeCdyW8qqWBKPVC3qh-fCdjeP23fokOoslndDm8WOCIzOtk1R-lGWgtR-zttrqNa0oD57ZdbvbQlvSnAu-7LF6YnKwjIO61jBbSGgX8BWr84LeZDT4bGVg87EVzT9Ls~LMBz46grKYYj1xlEtXDJ54joBDI6KJxgSFx~ZQUY4MkZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)